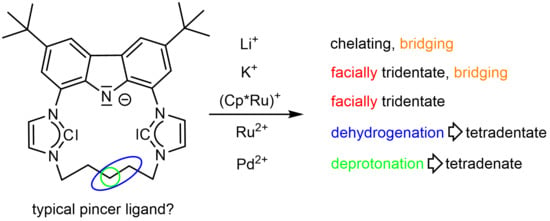
The Fascinating Flexibility and Coordination Modes of a Pentamethylene Connected Macrocyclic CNC Pincer Ligand
Abstract
1. Introduction
2. Results and Discussion
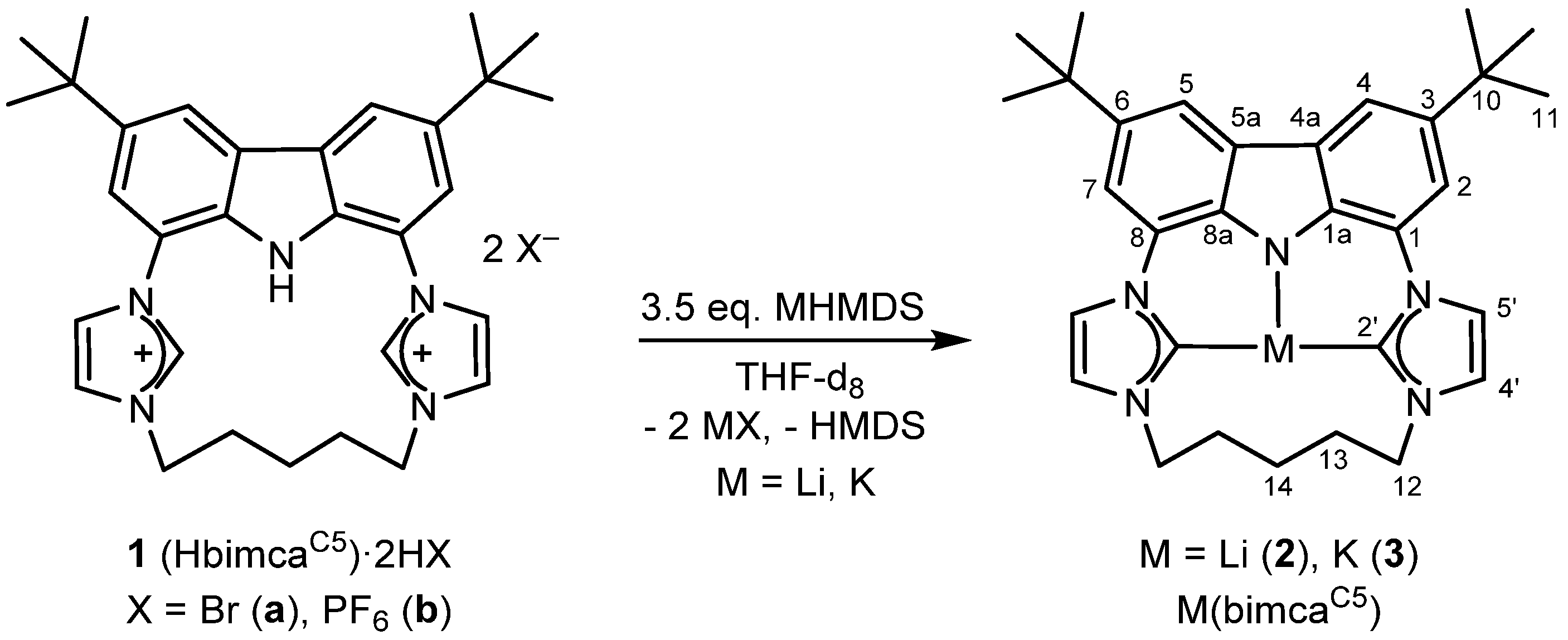
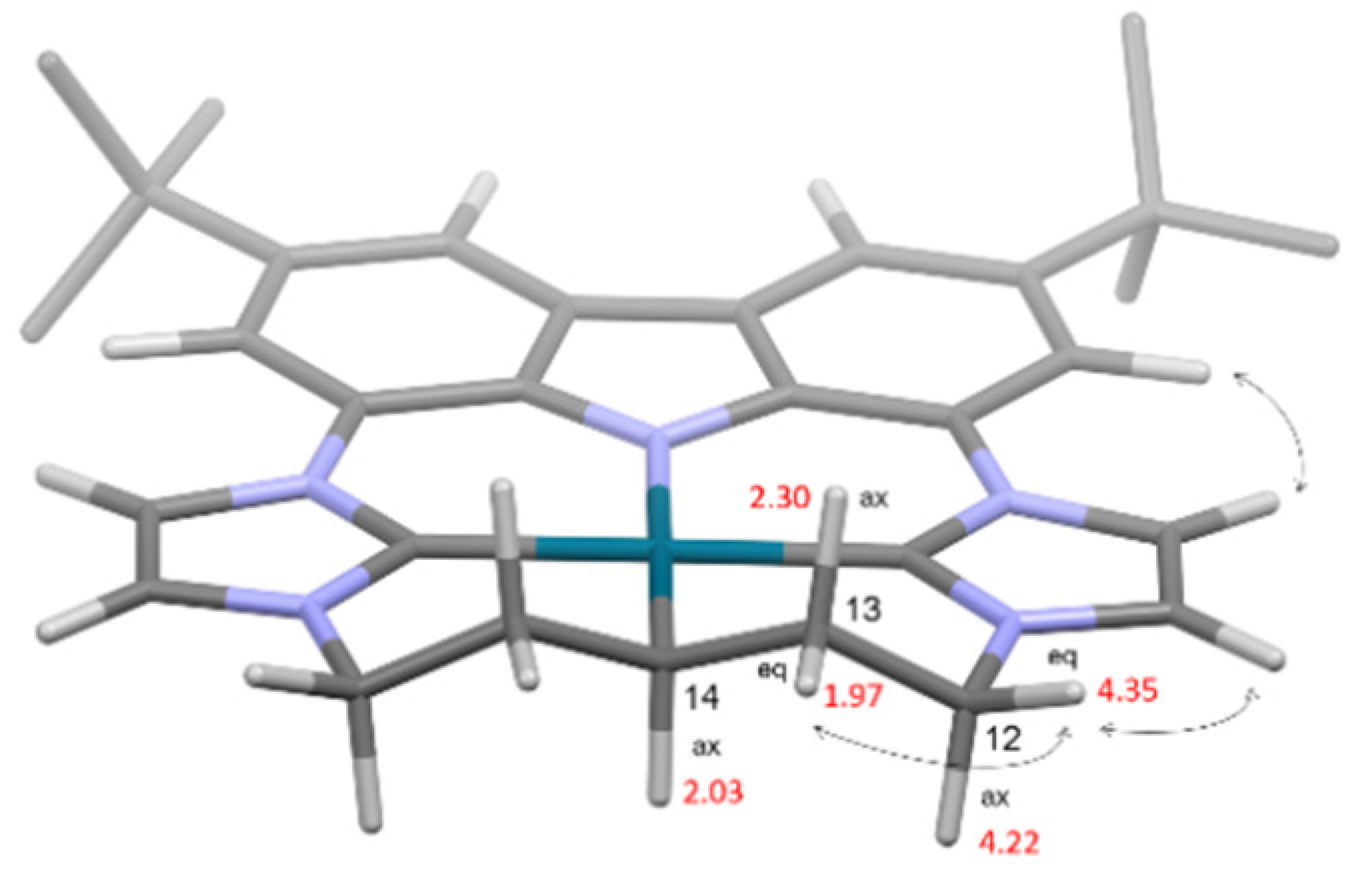
2.1. Alkali Metal bimcaC5 Complexes
2.2. Ruthenium(II) bimcaC5 Complexes
2.2.1. Facial Coordination of the bimcaC5 Ligand
2.2.2. Complex Formation under Dehydrogenation of bimcaC5
2.3. A macrocyclic Palladium(II) bimcaC5 Complex by C-H Activation
3. Materials and Methods
3.1. Preparation of (HbimcaC5)·2HPF6 (1b)
3.2. General Procedure for the Generation of Alkali Metal bimcaC5 Complexes
3.3. Synthesis of the Ru(II) Sandwich Complex 4 (Ru(bimcaC5)Cp*)
3.4. Synthesis of the Macrocyclic Ru(II)(bimcaC5) Complex 5
3.5. Preparation of the Macrocyclic Pd(II) Complex 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hood, T.M.; Gyton, M.R.; Chaplin, A.B. Synthesis and rhodium complexes of macrocyclic PNP and PONOP pincer ligands. Dalton Trans. 2020, 49, 2077–2086. [Google Scholar] [CrossRef]
- Leforestier, B.; Gyton, M.R.; Chaplin, A.B. Synthesis and group 9 complexes of macrocyclic PCP and POCOP pincer ligands. Dalton Trans. 2020, 49, 2087–2101. [Google Scholar] [CrossRef]
- Andrew, R.E.; Chaplin, A.B. Synthesis, structure and dynamics of NHC-based palladium macrocycles. Dalton Trans. 2014, 43, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.E.; Chaplin, A.B. Synthesis and reactivity of NHC-based rhodium macrocycles. Inorg. Chem. 2015, 54, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.E.; Storey, C.M.; Chaplin, A.B. Well-defined coinage metal transfer agents for the synthesis of NHC-based nickel, rhodium and palladium macrocycles. Dalton Trans. 2016, 45, 8937–8944. [Google Scholar] [CrossRef] [PubMed]
- Storey, C.M.; Gyton, M.R.; Andrew, R.E.; Chaplin, A.B. Terminal Alkyne Coupling Reactions through a Ring: Mechanistic Insights and Regiochemical Switching. Angew. Chem. Int. Ed. 2018, 57, 12003–12006. [Google Scholar] [CrossRef]
- Leforestier, B.; Gyton, M.R.; Chaplin, A.B. Oxidative Addition of a Mechanically Entrapped C(sp)-C(sp) Bond to a Rhodium(I) Pincer Complex. Angew. Chem. 2020, 59, 23500–23504. [Google Scholar] [CrossRef]
- Storey, C.M.; Gyton, M.R.; Andrew, R.E.; Chaplin, A.B. Terminal Alkyne Coupling Reactions Through a Ring: Effect of Ring Size on Rate and Regioselectivity. Chem. Eur. J. 2020, 26, 14715–14723. [Google Scholar] [CrossRef]
- Biffis, A.; Cipani, M.; Bressan, E.; Tubaro, C.; Graiff, C.; Venzo, A. Group 10 Metal Complexes with Chelating Macrocyclic Dicarbene Ligands Bearing a 2,6-Lutidinyl Bridge: Synthesis, Reactivity, and Catalytic Activity. Organometallics 2014, 33, 2182–2188. [Google Scholar] [CrossRef]
- Alcalde, E.; Ramos, S.; Perez-Garcia, L. Anion Template-Directed Synthesis of Dicationic [14]Imidazoliophanes. Org. Lett. 1999, 1, 1035–1038. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, G.; Jiang, Z.-L.; You, J.-S.; Zhou, Z.-Y.; Yuan, D.-Q.; Xie, R.-G. Synthesis and selective anion recognition of imidazolium cyclophanes. Tetrahedron 2002, 58, 8993–8999. [Google Scholar] [CrossRef]
- Radloff, C.; Gong, H.-Y.; Schulte to Brinke, C.; Pape, T.; Lynch, V.M.; Sessler, J.L.; Hahn, F.E. Metal-Dependent Coordination Modes Displayed by Macrocyclic Polycarbene Ligands. Chem. Eur. J. 2010, 16, 13077–13081. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, E.; Buys, K.N.; Schmidt, A.-T.; Furfari, S.K.; Cole, M.L.; Moser, M.; Rominger, F.; Kunz, D. Optimised synthesis of monoanionic bis(NHC)-pincer ligand precursors and their Li-complexes. New J. Chem. 2016, 40, 9160–9169. [Google Scholar] [CrossRef]
- Lu, T.; Yang, C.-F.; Steren, C.A.; Fei, F.; Chen, X.-T.; Xue, Z.-L. Synthesis and characterization of Ag(I) and Au(I) complexes with macrocyclic hybrid amine N-heterocyclic carbene ligands. New J. Chem. 2018, 42, 4700–4713. [Google Scholar] [CrossRef]
- Moser, M.; Wucher, B.; Kunz, D.; Rominger, F. 1,8-Bis(imidazolin-2-yliden-1-yl)carbazolide (bimca): A New CNC Pincer-Type Ligand with Strong Electron-Donating Properties. Facile Oxidative Addition of Methyl Iodide to Rh(bimca)(CO). Organometallics 2007, 26, 1024–1030. [Google Scholar] [CrossRef]
- Evans, K.J.; Campbell, C.L.; Haddow, M.F.; Luz, C.; Morton, P.A.; Mansell, S.M. Lithium Complexes with Bridging and Terminal NHC Ligands: The Decisive Influence of an Anionic Tether. Eur. J. Inorg. Chem. 2019, 2019, 4894–4901. [Google Scholar] [CrossRef]
- Simler, T.; Karmazin, L.; Bailly, C.; Braunstein, P.; Danopoulos, A.A. Potassium and Lithium Complexes with Monodeprotonated, Dearomatized PNP and PNC NHC Pincer-Type Ligands. Organometallics 2016, 35, 903–912. [Google Scholar] [CrossRef]
- Nesterov, V.; Reiter, D.; Bag, P.; Frisch, P.; Holzner, R.; Porzelt, A.; Inoue, S. NHCs in Main Group Chemistry. Chem. Rev. 2018, 118, 9678–9842. [Google Scholar] [CrossRef]
- Tapu, D.; Dixon, D.A.; Roe, C. 13C NMR spectroscopy of “Arduengo-type” carbenes and their derivatives. Chem. Rev. 2009, 109, 3385–3407. [Google Scholar] [CrossRef] [PubMed]
- Arduengo, A.J., III; Gamper, S.F.; Tamm, M.; Calabrese, J.C.; Davidson, F.; Craig, H.A. A Bis(carbene)-Proton Complex: Structure of a C-H-C Hydrogen Bond. J. Am. Chem. Soc. 1995, 117, 572–573. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Runte, O.; Artus, G. Synthesis and structure of an ionic beryllium-“carbene” complex. J. Organomet. Chem. 1995, 501, C1–C4. [Google Scholar] [CrossRef]
- Simler, T.; Danopoulos, A.A.; Braunstein, P. N-Heterocyclic carbene-phosphino-picolines as precursors of anionic ‘pincer’ ligands with dearomatised pyridine backbones; transmetallation from potassium to chromium. Chem. Commun. 2015, 51, 10699–10702. [Google Scholar] [CrossRef] [PubMed]
- Seyboldt, A.; Wucher, B.; Hohnstein, S.; Eichele, K.; Rominger, F.; Törnroos, K.W.; Kunz, D. Evidence for the Formation of Anionic Zerovalent Group 10 Complexes as Highly Reactive Intermediates. Organometallics 2015, 34, 2717–2725. [Google Scholar] [CrossRef]
- Jürgens, E.; Kunz, D. A Rigid CNC Pincer Ligand Acting as a Tripodal Cp Analogue. Eur. J. Inorg. Chem. 2017, 2017, 233–236. [Google Scholar] [CrossRef]
- Brookhart, M.; Green, M.L.H.; Parkin, G. Agostic interactions in transition metal compounds. Proc. Natl. Acad. Sci. USA 2007, 104, 6908–6914. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, W.; Ito, J.; Yamashita, M. CNC-pincer iron complexes containing a bis(N-heterocyclic carbene)Amido ligand: Synthesis and application to catalytic hydrogenation of alkenes. J. Organomet. Chem. 2020, 923, 121436. [Google Scholar] [CrossRef]
- Gusev, D.G.; Lough, A.J. Double C−H Activation on Osmium and Ruthenium Centers: Carbene vs Olefin Products. Organometallics 2002, 21, 2601–2603. [Google Scholar] [CrossRef]
- Chatt, J.; Watson, H.R. 491. Complexes of zerovalent transition metals with the ditertiary phosphine, Me2P·CH2 ·CH2 ·PMe2. J. Chem. Soc. 1962, 2545–2549. [Google Scholar] [CrossRef]
- Chatt, J.; Davidson, J.M. 154. The tautomerism of arene and ditertiary phosphine complexes of ruthenium(0), and the preparation of new types of hydrido-complexes of ruthenium(II). J. Chem. Soc. 1965, 843–855. [Google Scholar] [CrossRef]
- Kakiuchi, F.; Murai, S. Catalytic C-H/Olefin Coupling. Acc. Chem. Res. 2002, 35, 826–834. [Google Scholar] [CrossRef]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-catalyzed C-H bond activation and functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C-H bond functionalizations: Mechanism and scope. Chem. Rev. 2011, 111, 1315–1345. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. Organometallic alkane CH activation. J. Organomet. Chem. 2004, 689, 4083–4091. [Google Scholar] [CrossRef]
- Zhou, X.; Malakar, S.; Zhou, T.; Murugesan, S.; Huang, C.; Emge, T.J.; Krogh-Jespersen, K.; Goldman, A.S. Catalytic Alkane Transfer Dehydrogenation by PSP-Pincer-Ligated Ruthenium. Deactivation of an Extremely Reactive Fragment by Formation of Allyl Hydride Complexes. ACS Catal. 2019, 9, 4072–4083. [Google Scholar] [CrossRef]
- Gruver, B.C.; Adams, J.J.; Warner, S.J.; Arulsamy, N.; Roddick, D.M. Acceptor Pincer Chemistry of Ruthenium: Catalytic Alkane Dehydrogenation by (CF3PCP)Ru(cod)(H). Organometallics 2011, 30, 5133–5140. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, W.; Fang, H.; Hu, A.; Huang, Z. Catalytic alkane dehydrogenations. Sci. Bull. 2015, 60, 1316–1331. [Google Scholar] [CrossRef]
- Chatt, J.; Hart, F.A.; Watson, H.R. 490. Complex compounds of ditertiary phosphines and arsines with nickel(0) and palladium(0). J. Chem. Soc. 1962, 2537–2545. [Google Scholar] [CrossRef]
- Al-Salem, N.A.; Empsall, H.D.; Markham, R.; Shaw, B.L.; Weeks, B. Formation of large chelate rings and cyclometallated products from diphosphines of type But2P(CH2)n PBut2(n = 5–8) and Ph2P(CH2)5PPh2 with palladium and platinum chlorides: Factors affecting the stability and conformation of large chelate rings. J. Chem. Soc. Dalton Trans. 1979, 1972–1982. [Google Scholar] [CrossRef]
- Rousseaux, S.; Gorelsky, S.I.; Chung, B.K.W.; Fagnou, K. Investigation of the mechanism of C(sp3)-H bond cleavage in Pd(0)-catalyzed intramolecular alkane arylation adjacent to amides and sulfonamides. J. Am. Chem. Soc. 2010, 132, 10692–10705. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Recent developments in SHELX. Acta Crystallogr. A Found. Crystallogr. 2013, 69, s74. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Platon Squeeze: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B Condens. Matter 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. TmoleX—A graphical user interface for TURBOMOLE. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef]
- University of Karlsruhe and Forschungszentrum Karlsruhe GmbH. TURBOMOLE. 2011. Available online: https://www.turbomole.org/ (accessed on 21 January 2021).
- Treutler, O.; Ahlrichs, R. Efficient molecular numerical integration schemes. J. Chem. Phys. 1995, 102, 346–354. [Google Scholar] [CrossRef]
- von Arnim, M.; Ahlrichs, R. Performance of parallel TURBOMOLE for density functional calculations. J. Comput. Chem. 1998, 19, 1746–1757. [Google Scholar] [CrossRef]
- van Wüllen, C. Shared-memory parallelization of the TURBOMOLE programs AOFORCE, ESCF, and EGRAD: How to quickly parallelize legacy code. J. Comput. Chem. 2011, 32, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Deglmann, P.; Furche, F.; Ahlrichs, R. An efficient implementation of second analytical derivatives for density functional methods. Chem. Phys. Lett. 2002, 362, 511–518. [Google Scholar] [CrossRef]
- Deglmann, P.; Furche, F. Efficient characterization of stationary points on potential energy surfaces. J. Chem. Phys. 2002, 117, 9535–9538. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Armbruster, M.K.; Weigend, F.; van Wüllen, C.; Klopper, W. Self-consistent treatment of spin-orbit interactions with efficient Hartree-Fock and density functional methods. Phys. Chem. Chem. Phys. 2008, 10, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Middendorf, N.; Weigend, F.; Reiher, M. An efficient implementation of two-component relativistic exact-decoupling methods for large molecules. J. Chem. Phys. 2013, 138, 184105. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials (Chem. Phys. Letters 240 (1995) 283–290). Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- Deglmann, P.; May, K.; Furche, F.; Ahlrichs, R. Nuclear second analytical derivative calculations using auxiliary basis set expansions. Chem. Phys. Lett. 2004, 384, 103–107. [Google Scholar] [CrossRef]
- Weigend, F. A fully direct RI-HF algorithm: Implementation, optimised auxiliary basis sets, demonstration of accuracy and efficiency. Phys. Chem. Chem. Phys. 2002, 4, 4285–4291. [Google Scholar] [CrossRef]
- Sierka, M.; Hogekamp, A.; Ahlrichs, R. Fast evaluation of the Coulomb potential for electron densities using multipole accelerated resolution of identity approximation. J. Chem. Phys. 2003, 118, 9136–9148. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. Theory Comput. Modeling (Theor. Chim. Acta) 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordan, R.; Kunz, D. The Fascinating Flexibility and Coordination Modes of a Pentamethylene Connected Macrocyclic CNC Pincer Ligand. Molecules 2021, 26, 1669. https://doi.org/10.3390/molecules26061669
Jordan R, Kunz D. The Fascinating Flexibility and Coordination Modes of a Pentamethylene Connected Macrocyclic CNC Pincer Ligand. Molecules. 2021; 26(6):1669. https://doi.org/10.3390/molecules26061669
Chicago/Turabian StyleJordan, Ronja, and Doris Kunz. 2021. "The Fascinating Flexibility and Coordination Modes of a Pentamethylene Connected Macrocyclic CNC Pincer Ligand" Molecules 26, no. 6: 1669. https://doi.org/10.3390/molecules26061669
APA StyleJordan, R., & Kunz, D. (2021). The Fascinating Flexibility and Coordination Modes of a Pentamethylene Connected Macrocyclic CNC Pincer Ligand. Molecules, 26(6), 1669. https://doi.org/10.3390/molecules26061669