Triterpenes and Phenolic Compounds from the Fungus Fuscoporia torulosa: Isolation, Structure Determination and Biological Activity
Abstract
1. Introduction
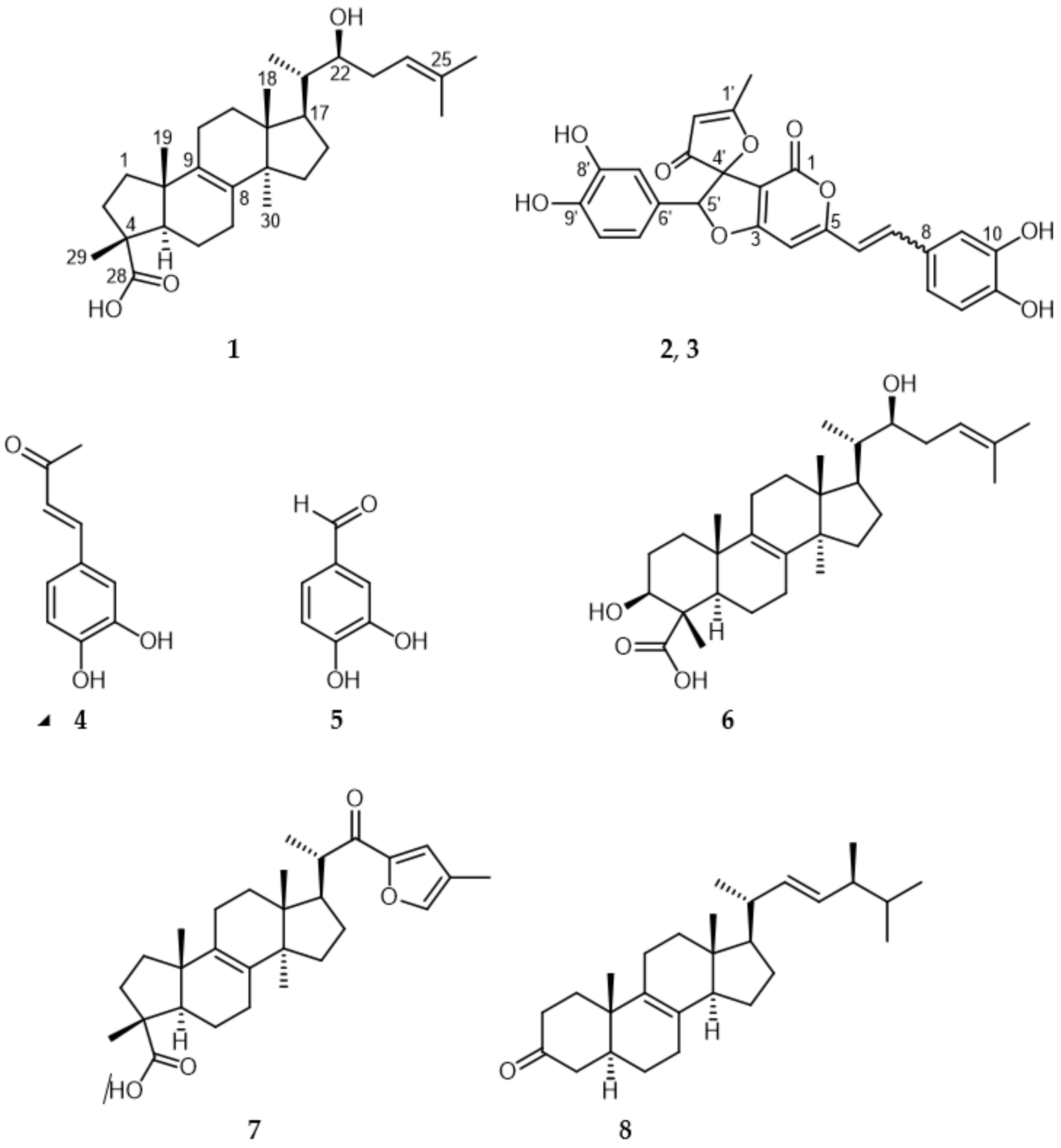
2. Results and Discussion
3. Materials and Methods
3.1. Mushroom Material
3.2. Extraction and Isolation
3.3. Cell Culture
3.4. Assay for Cytotoxic Effect
3.5. Checkerboard Combination Assay
3.6. Rhodamine 123 Accumulation Assay
3.7. Bacterial Strains
3.8. Determination of Minimum Inhibitory Concentrations by Microdilution Method
3.9. DPPH Assay
3.10. ORAC Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dai, Y.-C. Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 2010, 45, 131–343. [Google Scholar] [CrossRef]
- Kovács, B.; Zomborszki, Z.P.; Orbán-Gyapai, O.; Csupor-Löffler, B.; Liktor-Busa, E.; Lázár, A.; Papp, V.; Urbán, E.; Hohmann, J.; Ványolos, A. Investigation of antimicrobial, antioxidant, and xanthine oxidase-inhibitory activities of Phellinus (Agaricomycetes) mushroom species native to Central Europe. Int. J. Med. Mushrooms 2017, 19, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.R.; Deshmukh, S.K. Advances in Macrofungi; CRC Press: Boca Raton, FL, USA, 2019; pp. 277–303. [Google Scholar]
- Sárközy, A.; Kúsz, N.; Zomborszki, Z.P.; Csorba, A.; Papp, V.; Hohmann, J.; Vanyolos, A. Isolation and characterization of chemical constituents from the poroid medicinal mushroom Porodaedalea chrysoloma (Agaricomycetes) and their antioxidant activity. Int. J. Med. Mushrooms 2020, 22, 125–131. [Google Scholar] [CrossRef]
- Dai, Y.-C.; Zhou, L.-W.; Cui, B.-K.; Chen, Y.-Q.; Decock, C. Current advances in Phellinus sensu lato: Medicinal species, functions, metabolites and mechanisms. Appl. Microbiol. Biotechnol. 2010, 87, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- He, M.-Q.; Zhao, R.-L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; Sánchez-Ramírez, S. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Chen, Q. Global diversity and phylogeny of Fuscoporia (Hymenochaetales, Basidiomycota). Mycosphere 2020, 11, 1477–1513. [Google Scholar] [CrossRef]
- Du, P.; Chen, Q.; Vlasák, J. Fuscoporia ambigua Sp. Nov., a new species from America and China. Phytotaxa 2020, 456, 175–185. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid Fungi of Europe; Fungiflora: Oslo, Norway, 2014. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P.; Arras, L.; Facchini, M.; Porcu, G.; Trichies, G. Polypores of the Mediterranean Region; Romar: Segrate, Italy, 2020; p. 904. [Google Scholar]
- González, A.G.; Expósito, T.S.; Toledo Marante, F.J.; Pérez, M.J.M.; Tejera, E.B.; Bermejo Barrera, J. Lanosterol derivatives from Phellinus torulosus. Phytochemistry 1994, 35, 1523–1526. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Duru, M.E.; Öztürk, M. Isolation, characterization, and bioactivities of compounds from Fuscoporia torulosa mushroom. J. Food Biochem. 2019, 43, e13074. [Google Scholar] [CrossRef] [PubMed]
- Khadhri, A.; Aouadhi, C.; Aschi-Smiti, S. Screening of bioactive compounds of medicinal mushrooms collected on Tunisian territory. Int. J. Med. Mushrooms 2017, 19, 127–135. [Google Scholar] [CrossRef]
- Duru, M.E.; Tel-Çayan, G.; Deveci, E. Evaluation of phenolic profile, antioxidant and anticholinesterase effects of Fuscoporia torulosa. Int. J. Second. Metab. 2019, 6, 79–89. [Google Scholar] [CrossRef]
- Covino, S.; D’Ellena, E.; Tirillini, B.; Angeles, G.; Arcangeli, A.; Bistocchi, G.; Venanzoni, R.; Angelini, P. Characterization of biological activities of methanol extract of Fuscoporia torulosa (Basidiomycetes) from Italy. Int. J. Med. Mushrooms 2019, 21, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-K.; Tsai, T.-H.; Chang, T.-T.; Chou, C.-J.; Lin, L.-C. Lanostane triterpenoids from the fungus Phellinus gilvus. Phytochemistry 2009, 70, 558–563. [Google Scholar] [CrossRef]
- González, A.G.; Expósito, T.S.; Barrera, J.B.; Castellano, A.G.; Marante, F.J.T. The absolute stereochemistry of senexdiolic acid at C-22. J. Nat. Prod. 1993, 56, 2170–2174. [Google Scholar] [CrossRef]
- Kim, J.-P.; Yun, B.-S.; Shim, Y.K.; Yoo, I.-D. Inoscavin A, a new free radical scavenger from the mushroom Inonotus xeranticus. Tetrahedron Lett. 1999, 40, 6643–6644. [Google Scholar] [CrossRef]
- Bagno, A.; Rastrelli, F.; Saielli, G. Toward the complete prediction of the 1H and 13C-NMR spectra of complex organic molecules by DFT methods: Application to natural substances. Chem. Eur. J. 2006, 12, 5514–5525. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Gomez-Paloma, L.; Duca, D.; Silvestri, A.; Riccio, R.; Bifulco, G. Structure validation of natural products by quantum-mechanical GIAO calculations of 13C-NMR chemical shifts. Chem. Eur. J. 2002, 8, 3233–3239. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Smith, S.G.; Goodman, J.M. Assigning stereochemistry to single diastereoisomers by GIAO NMR calculation: The DP4 probability. J. Am. Chem. Soc. 2010, 132, 12946–12959. [Google Scholar] [CrossRef]
- Mo, S.; Wang, S.; Zhou, G.; Yang, Y.; Li, Y.; Chen, X.; Shi, J. Phelligridins C-F: Cytotoxic pyrano [4,3-c][2]benzopyran-1,6-dione and furo[3,2-c]pyran-4-one derivatives from the fungus Phellinus igniarius. J. Nat. Prod. 2004, 67, 823–828. [Google Scholar] [CrossRef]
- Lee, I.K.; Seok, S.J.; Kim, W.K.; Yun, B.S. Hispidin derivatives from the mushroom Inonotus xeranticus and their antioxidant activity. J. Nat. Prod. 2006, 69, 299–301. [Google Scholar] [CrossRef]
- Batta, A.K.; Rangaswami, S. Crystalline chemical components of Fomes senex and structure of senexdiolic acid and related compounds. J. Chem. Soc. Perkin Trans. 1 1975, 5, 451–455. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, S.K. The isolation of lanosta-7,9(11),24-trien-3β,21-diol from the fungus Ganoderma australe. Phytochemistry 1984, 23, 686–687. [Google Scholar] [CrossRef]
- Wang, X.; Bao, H.; Bau, T. Investigation of the possible mechanism of two kinds of sterols extracted from Leucocalocybe mongolica in inducing HepG2 cell apoptosis and exerting anti-tumor effects in H22 tumor-bearing mice. Steroids 2020, 163, 108692. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, S.U.; Noh, H.J.; Zee, O.; Lee, K.R. Cytotoxic ergosterol derivatives from the mushroom Naematoloma fasciculare. Nat. Prod. Sci. 2014, 20, 76–79. [Google Scholar]
- Njue, A.W.; Omolo, J.O.; Cheplogoi, P.K.; Waweru, A.W. Cytotoxic triterpenoids from the mushroom Clavulina cinerea (Bull) J. Schroet (Cantharellaceae). Int. J. Biol. Chem. Sci. 2017, 11, 865–873. [Google Scholar] [CrossRef]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A High-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Gajdács, M.; Spengler, G.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenoesters and selenoanhydrides as novel multidrug resistance reversing agents: A confirmation study in a colon cancer MDR cell line. Bioorg. Med. Chem. Lett. 2017, 27, 797–802. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium compounds as novel adjuvants of chemotherapy drugs—a promising approach to fight cancer drug resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kincses, A.; Szabó, S.; Rácz, B.; Szemerédi, N.; Watanabe, G.; Saijo, R.; Sekiya, H.; Tamai, E.; Molnár, J.; Kawase, M.; et al. Benzoxazole-based metal complexes to reverse multidrug resistance in bacteria. Antibiotics 2020, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Miser-Salihoglu, E.; Akaydin, G.; Caliskan-Can, E.; Yardim-Akaydin, S. Evalution of antioxidant activity of various herbal folk medicines. J. Nutr. Food Sci. 2013, 3, 222. [Google Scholar]
- Mielnik, M.B.; Rzeszutek, A.; Triumf, E.C.; Egelandsdal, B. Antioxidant and other quality properties of reindeer muscle from two different Norwegian regions. Meat Sci. 2011, 89, 526–532. [Google Scholar] [CrossRef] [PubMed]
| Position | δ13C ppm | δ1H ppm | Multiplicity (J in Hz) |
|---|---|---|---|
| 1 α | 36.0 | 1.49 | m |
| 1 β | 1.60 | m | |
| 2 α | 36.9 | 2.48 | dd (13.7, 8.3) |
| 2 β | 1.67 | m | |
| 4 | 48.2 | ||
| 5 | 52.9 | 2.06 | m |
| 6 α | 1.63 | m | |
| 6 β | 18.6 25.9 | 1.72 | m |
| 7 | 2.12 | m | |
| 8 | 134.1 | ||
| 9 | 135.0 | ||
| 10 | 45.6 | ||
| 11 α | 22.5 | 2.02 | m |
| 11 β | 30.4 | 2.13 | m |
| 12 α | 1.76 | m | |
| 12 β | 1.69 | m | |
| 13 | 45.3 | ||
| 14 | 48.9 | ||
| 15 α | 30.6 | 1.20 | m |
| 15 β | 27.3 | 1.60 | m |
| 16 α | 1.82 | m | |
| 16 β | 47.1 | 1.44 | m |
| 17 | 1.57 | m | |
| 18 | 15.5 | 0.76 | s |
| 19 | 19.3 | 0.98 | s |
| 20 | 41.6 | 1.80 | m |
| 21 | 12.7 | 0.96 | d (6.6) |
| 22 | 73.4 | 3.68 | m |
| 23 | 29.1 | 2.05 | m |
| 24 | 121.3 | 5.19 | m |
| 25 | 135.2 | ||
| 26 | 26.0 | 1.75 | s |
| 27 | 18.0 | 1.66 | s |
| 28 | 185.3 | ||
| 29 | 21.3 | 1.24 | s |
| 30 | 24.4 | 0.89 | s |
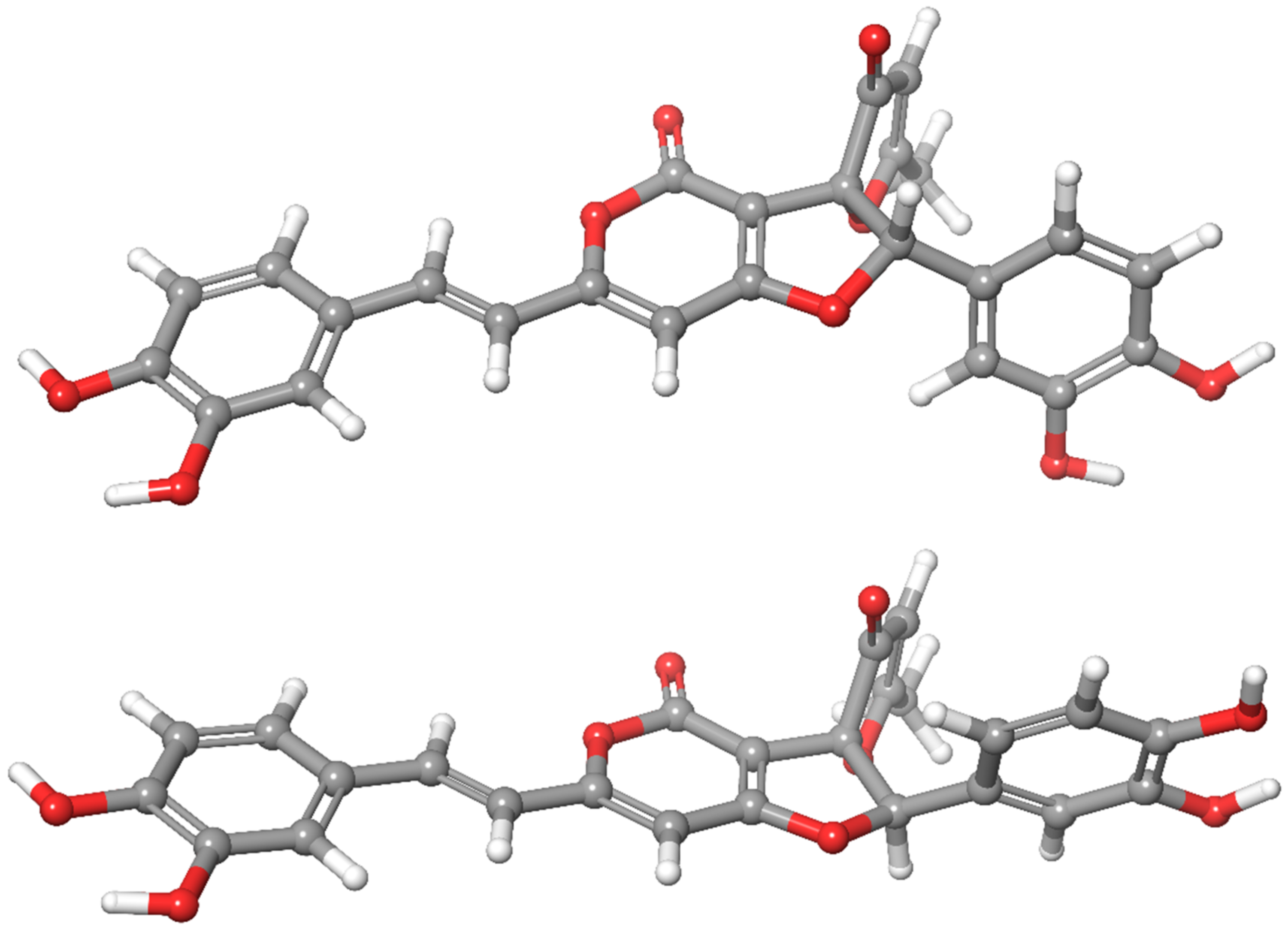
| No. | Atom No. in Figure 1 | δexp Experimental Shift (ppm) | SS Boltzmann Averaged Shielding | RS Boltzmann Averaged Shielding | δcalc SS Uncaled Shift (ppm) * | δs SS Scaled Shift (ppm) | δcalc RS Unscaled Shift (ppm) * | δs RS Scaled Shift (ppm) |
|---|---|---|---|---|---|---|---|---|
| C1 | Me | 16.8 | 165.1 | 164.5 | 16.2 | 16.3 | 16.8 | 16.3 |
| C2 | 1′ | 193.1 | −18.1 | −18.5 | 195.1 | 195.1 | 195.5 | 195.8 |
| C3 | 2′ | 105.3 | 74.1 | 74.8 | 105.1 | 105.2 | 104.4 | 104.3 |
| C4 | 3′ | 203.3 | −25.1 | −24.1 | 202.0 | 202.0 | 201.0 | 201.3 |
| C6 | 4′ | 94.4 | 82.5 | 80.5 | 96.9 | 97.0 | 98.8 | 98.7 |
| C8 | 5′ | 96.0 | 83.0 | 85.5 | 96.4 | 96.5 | 93.9 | 93.7 |
| C10 | 3 | 177.0 | 0.6 | 0.8 | 176.9 | 176.9 | 176.6 | 176.9 |
| C11 | 2 | 99.6 | 80.7 | 79.5 | 98.6 | 98.7 | 99.8 | 99.7 |
| C12 | 1 | 160.8 | 19.3 | 18.9 | 158.6 | 158.7 | 159.0 | 159.1 |
| C15 | 5 | 167.2 | 9.0 | 9.2 | 168.6 | 168.7 | 168.4 | 168.6 |
| C16 | 4 | 95.7 | 84.1 | 83.8 | 95.3 | 95.4 | 95.6 | 95.4 |
| C17 | 6 | 116.8 | 62.3 | 62.5 | 116.6 | 116.6 | 116.4 | 116.4 |
| C18 | 7 | 140.7 | 36.6 | 36.8 | 141.7 | 141.8 | 141.5 | 141.6 |
| C19 | 8 | 128.6 | 48.5 | 48.8 | 130.0 | 130.1 | 129.8 | 129.8 |
| C20 | 9 | 115.2 | 64.5 | 64.3 | 114.4 | 114.5 | 114.7 | 114.6 |
| C21 | 10 | 147.1 | 31.2 | 31.2 | 146.9 | 147.0 | 147.0 | 147.0 |
| C22 | 11 | 149.6 | 30.3 | 30.4 | 147.9 | 147.9 | 147.7 | 147.8 |
| C23 | 12 | 116.1 | 63.7 | 63.7 | 115.3 | 115.3 | 115.2 | 115.2 |
| C24 | 13 | 122.8 | 54.3 | 54.5 | 124.4 | 124.5 | 124.2 | 124.2 |
| C27 | 6′ | 123.3 | 52.1 | 51.4 | 126.6 | 126.7 | 127.2 | 127.2 |
| C28 | 7′ | 115.6 | 64.0 | 62.9 | 114.9 | 115.0 | 116.0 | 115.9 |
| C29 | 8′ | 146.4 | 31.6 | 32.0 | 146.6 | 146.7 | 146.2 | 146.3 |
| C30 | 9′ | 148.0 | 31.9 | 32.1 | 146.3 | 146.4 | 146.1 | 146.2 |
| C31 | 10′ | 116.8 | 65.2 | 65.3 | 113.8 | 113.9 | 113.7 | 113.6 |
| C32 | 11′ | 120.4 | 59.2 | 57.8 | 119.6 | 119.7 | 121.0 | 121.0 |
| H35 | 5′ | 5.68 | 26.27 | 25.92 | 5.56 | 5.51 | 5.90 | 5.75 |
| H36 | 6 | 6.75 | 24.88 | 24.98 | 6.90 | 6.80 | 6.80 | 6.67 |
| H37 | 7 | 7.47 | 23.99 | 23.93 | 7.75 | 7.61 | 7.80 | 7.71 |
| Me | 2.00 | 29.95 | 29.54 | 2.03 | 2.13 | 2.43 | 2.20 | |
| H41 | 2′ | 5.60 | 26.33 | 26.65 | 5.51 | 5.46 | 5.20 | 5.03 |
| H42 | 4 | 6.53 | 25.53 | 25.64 | 6.27 | 6.19 | 6.17 | 6.03 |
| H43 | 9 | 7.10 | 24.30 | 24.31 | 7.45 | 7.32 | 7.44 | 7.33 |
| H44 | 12 | 6.78 | 24.87 | 24.86 | 6.90 | 6.80 | 6.92 | 6.80 |
| H45 | 13 | 7.02 | 24.54 | 24.54 | 7.22 | 7.10 | 7.22 | 7.11 |
| H48 | 7′ | 6.73 | 24.83 | 24.67 | 6.94 | 6.84 | 7.10 | 6.99 |
| H49 | 10′ | 6.82 | 24.94 | 24.94 | 6.84 | 6.74 | 6.84 | 6.72 |
| H50 | 11′ | 6.61 | 25.08 | 24.88 | 6.70 | 6.61 | 6.89 | 6.77 |
| SS | RS | |
|---|---|---|
| sDP4+ (H data) | 99.87% | 0.13% |
| sDP4+ (C data) | 96.73% | 4.27% |
| sDP4+ (all data) | 100% | 0% |
| uDP4+ (H data) | 100% | 0% |
| uDP4+ (C data) | 79.89% | 20.11% |
| uDP4+ (all data) | 100% | 0% |
| DP4+ (H data) | 100% | 0% |
| DP4+ (C data) | 99.16% | 0.84% |
| DP4+ (all data) | 100% | 0% |
| Samples | IC50 (µM) | ||
|---|---|---|---|
| Colo 205 | Colo 320 | MRC-5 | |
| 1 | >100 | >100 | >100 |
| 6 | >100 | >100 | >100 |
| 7 | >100 | >100 | >100 |
| 8 | 11.65 ± 1.67 *** | 8.43 ± 1.1 | 7.92 ± 1.42 ** |
| Doxorubicin | 2.46 ± 0.26 | 7.44 ± 0.2 | > 20 |
| Samples | conc. (μM) | FSC | SSC | FL-1 | FAR |
|---|---|---|---|---|---|
| Tariquidar | 0.2 | 1945 | 837 | 64.100 | 5.533 |
| 1 | 20 | 2005 | 851 | 13.200 | 1.139 |
| 6 | 20 | 2074 | 861 | 11.900 | 1.027 |
| 7 | 20 | 2095 | 891 | 12.200 | 1.053 |
| 8 | 2 | 2099 | 857 | 10.100 | 0.872 |
| DMSO | 2.00% | 2073 | 848 | 9.590 | 0.828 |
| Colo 320 | - | 2052 | 841 | 8.870 | - |
| Compounds | DPPH EC50 (µg/mL) | ORAC Activity (mmol TE/g) |
|---|---|---|
| 2+3 | 0.72 ± 0.05 | 2.70 ± 0.03 |
| 4+5 | 0.25 ± 0.01 | 12.20 ± 0.92 |
| Ascorbic acid | 0.89 ± 0.02 | 6.94 ± 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béni, Z.; Dékány, M.; Sárközy, A.; Kincses, A.; Spengler, G.; Papp, V.; Hohmann, J.; Ványolós, A. Triterpenes and Phenolic Compounds from the Fungus Fuscoporia torulosa: Isolation, Structure Determination and Biological Activity. Molecules 2021, 26, 1657. https://doi.org/10.3390/molecules26061657
Béni Z, Dékány M, Sárközy A, Kincses A, Spengler G, Papp V, Hohmann J, Ványolós A. Triterpenes and Phenolic Compounds from the Fungus Fuscoporia torulosa: Isolation, Structure Determination and Biological Activity. Molecules. 2021; 26(6):1657. https://doi.org/10.3390/molecules26061657
Chicago/Turabian StyleBéni, Zoltán, Miklós Dékány, András Sárközy, Annamária Kincses, Gabriella Spengler, Viktor Papp, Judit Hohmann, and Attila Ványolós. 2021. "Triterpenes and Phenolic Compounds from the Fungus Fuscoporia torulosa: Isolation, Structure Determination and Biological Activity" Molecules 26, no. 6: 1657. https://doi.org/10.3390/molecules26061657
APA StyleBéni, Z., Dékány, M., Sárközy, A., Kincses, A., Spengler, G., Papp, V., Hohmann, J., & Ványolós, A. (2021). Triterpenes and Phenolic Compounds from the Fungus Fuscoporia torulosa: Isolation, Structure Determination and Biological Activity. Molecules, 26(6), 1657. https://doi.org/10.3390/molecules26061657







