Identification of Bioactive Compounds of Asparagus officinalis L.: Permutation Test Allows Differentiation among “Triguero” and Hybrid Green Varieties
Abstract
1. Introduction
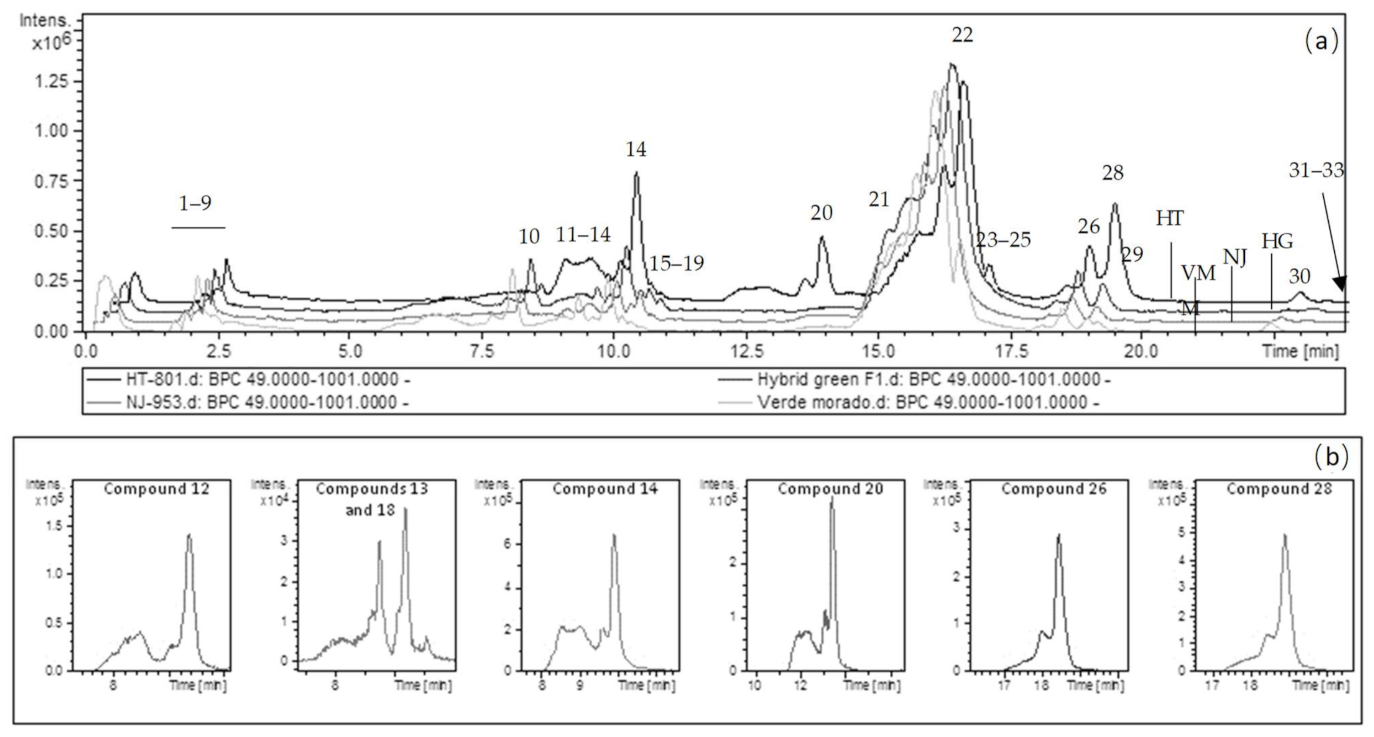
2. Results and Discussion
2.1. Analytical Characterization
2.1.1. Amino Acids
2.1.2. Carbohydrates
2.1.3. Organic Acids
2.1.4. Phenolic Compounds
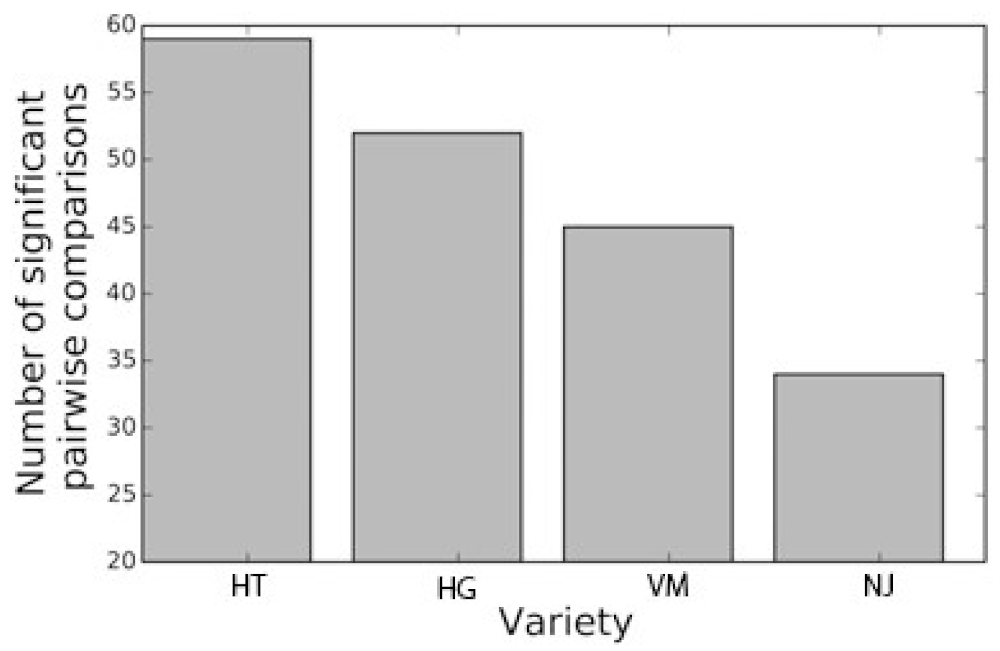
2.2. Permutation Test
3. Materials and Methods
3.1. Chemicals and Apparatus
3.2. Samples
- Commercial hybrid varieties: hybrid green F1 (HG), and NJ-953 (NJ).
- “Triguero” varieties: “verde-morado” (VM), and HT-801 (HT).
3.3. Extract Preparation
3.4. Analytical Characterization
3.5. Permutation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Moreno, R.; Espejo, J.; Cabrera, A.; Gil, J. Origin of tetraploid cultivated asparagus landraces inferred from nuclear ribosomal DNA internal transcribed spacers’ polymorphisms. Ann. Appl. Biol. 2008, 153, 233–241. [Google Scholar] [CrossRef]
- Riccardi, P.; Casali, P.E.; Mercati, F.; Falavigna, A.; Sunseri, F. Genetic characterization of asparagus doubled haploids collection and wild relatives. Sci. Hortic. 2011, 130, 691–700. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez, G.; Cermeño, P.; Jimenez-Araujo, A.; Guillén, R.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Identification of Flavonoid Diglycosides in Several Genotypes of Asparagus from the Huétor-Tájar Population Variety. J. Agric. Food Chem. 2007, 55, 10028–10035. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Jaramillo, S.; Rodrίguez-Gutiérrez, G.; Cermeño, P.; Espejo, J.A.; Jimenez-Araujo, A.; Guillén-Bejarano, R.; Fernández-Bolaños, J.; Arcos, R.R. Flavonoid Profile of Green Asparagus Genotypes. J. Agric. Food Chem. 2008, 56, 6977–6984. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Castilla, S.; De La Puerta, R.; Garcia-Gimenez, M.D.; Fernández-Arche, M.A.; Guillen-Bejarano, R. Bioactive Constituents from “Triguero” Asparagus Improve the Plasma Lipid Profile and Liver Antioxidant Status in Hypercholesterolemic Rats. Int. J. Mol. Sci. 2013, 14, 21227–21239. [Google Scholar] [CrossRef]
- Hahn, S.; Salmaso, L. A comparison of different synchronized permutation approaches to testing effects in two-level two-factor unbalanced ANOVA designs. Stat. Pap. 2017, 58, 123–146. [Google Scholar] [CrossRef]
- Westfall, P.H.; Young, S.S. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment; John Wiley & Sons Inc.: New York, NY, USA, 1993; 360p. [Google Scholar]
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Oliu, G.O.; Hertog, M.; Van De Poel, B.; Ampofo-Asiama, J.; Geeraerd, A.; Nicolaï, B. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol. 2011, 62, 7–16. [Google Scholar] [CrossRef]
- Ruhul Amin, M.; Tomita, Y.; Onodera, R. Rapid determination of phenylalanine and its related compounds in rumen fluid by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1995, 663, 201–207. [Google Scholar] [CrossRef]
- Taylor, W.R. The classification of amino acid conservation. J. Theor. Biol. 1986, 119, 205–218. [Google Scholar] [CrossRef]
- Lopez, G.; Ros, G.; Rincón, F.; Ortuño, J.; Periago, M.; Martínez, M. Amino acids and in vitro protein digestibility changes in green asparagus (Asparagus officinalis, L.) during growth and processing. Food Res. Int. 1996, 29, 617–625. [Google Scholar] [CrossRef]
- King, G.A.; Woollard, D.C.; Irving, D.E.; Borst, W.M. Physiological changes in asparagus spear tips after harvest. Physiol. Plantarum. 1990, 80, 393–400. [Google Scholar] [CrossRef]
- Hernández-Hernández, O.; Ruiz-Aceituno, L.; Sanz, M.L.; Martίnez-Castro, I. Determination of Free Inositols and Other Low Molecular Weight Carbohydrates in Vegetables. J. Agric. Food Chem. 2011, 59, 2451–2455. [Google Scholar] [CrossRef]
- Urakami, K.; Zangiacomi, V.; Yamaguchi, K.; Kusuhara, M. Quantitative metabolome profiling of Illicium anisatum by capillary electrophoresis time-of-flight mass spectrometry. Biomed. Res. 2010, 31, 161–163. [Google Scholar] [CrossRef]
- Barding, J.G.A.; Béni, S.; Fukao, T.; Bailey-Serres, J.; Larive, C.K. Comparison of GC-MS and NMR for Metabolite Profiling of Rice Subjected to Submergence Stress. J. Proteome Res. 2013, 12, 898–909. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Herppich, W. High CO2 effects on postharvest biochemical and textural properties of white asparagus (Asparagus officinalis L.) spears. Postharvest Biol. Technol. 2013, 75, 45–53. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jimenez-Araujo, A.; Guillén, R.; Heredia, A.; Fernández-Bolaños, J. Postharvest Changes in White Asparagus Cell Wall during Refrigerated Storage. J. Agric. Food Chem. 1999, 47, 3551–3557. [Google Scholar] [CrossRef]
- Ku, Y.-G.; Woolley, D. Effect of plant growth regulators and spear bud scales on growth of Asparagus officinalis spears. Sci. Hortic. 2006, 108, 238–242. [Google Scholar] [CrossRef]
- Jaramillo, S.; Rodríguez, R.; Jimenez-Araujo, A.; Guillén, R.; Heredia, A.; Fernández-Bolaños, J. Effects of storage conditions on the accumulation of ferulic acid derivatives in white asparagus cell walls. J. Sci. Food Agric. 2006, 87, 286–296. [Google Scholar] [CrossRef]
- Abad-García, B.; Berrueta, L.A.; Garmón-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar] [CrossRef]
- Abdennacer, B.; Karim, M.; Yassine, M.; Nesrine, R.; Mouna, D.; Mohamed, B. Determination of phytochemicals and antioxidant activity of methanol extracts obtained from the fruit and leaves of Tunisian Lycium intricatum Boiss. Food Chem. 2015, 174, 577–584. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Xiong, A.; Li, D.; Wang, Z. Authentication of Senecio scandens and S. vulgaris based on the comprehensive secondary metabolic patterns gained by UPLC–DAD/ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Jaramillo, S.; Guillén, R.; Jimenez-Araujo, A.; Fernández-Bolaños, J.; Heredia, A. Cell wall phenolics of white and green asparagus. J. Sci. Food Agric. 2005, 85, 971–978. [Google Scholar] [CrossRef]
- Jang, D.S.; Cuendet, M.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Constituents of Asparagus officinalis Evaluated for Inhibitory Activity against Cyclooxygenase-2. J. Agric. Food Chem. 2004, 52, 2218–2222. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Anesio, A.M.; Rassner, S.M.; Sattler, B.; Hubbard, B.; Perkins, W.T.; Young, M.; Griffith, G.W. Possible interactions between bacterial diversity, microbial activity and supraglacial hydrology of cryoconite holes in Svalbard. ISME J. 2010, 5, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Ludbrook, J.; Dudley, H. Why Permutation Tests Are Superior to t and F Tests in Biomedical Research. Am. Stat. 1998, 52, 127. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Manly, B. Randomization, Bootstrap and Monte Carlo Methods in Biology, 2nd ed.; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Jaramillo-Carmona, S.; Rodriguez-Arcos, R.; Jimenez-Araujo, A.; López, S.; Gil, J.; Moreno, R.; Guillén-Bejarano, R. Saponin Profile of Wild Asparagus Species. J. Food Sci. 2017, 82, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Tardío, J. Fatty acids profiles of some Spanish wild vegetables. Food Sci. Technol. Int. 2012, 18, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Bousserouel, S.; Le Grandois, J.; Gossé, F.; Werner, D.; Barth, S.W.; Marchioni, E.; Marescaux, J.; Raul, F. Methanolic extract of white asparagus shoots activates TRAIL apoptotic death pathway in human cancer cells and inhibits colon carcinogenesis in a preclinical model. Int. J. Oncol. 2013, 43, 394–404. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Zhao, J.; Wang, J.; Qu, W. Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. by-products in mice fed a high-fat diet. J. Sci. Food Agric. 2010, 90, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Sanae, M.; Yasuo, A. Green Asparagus (Asparagus officinalis) Prevented Hypertension by an Inhibitory Effect on Angiotensin-Converting Enzyme Activity in the Kidney of Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2013, 61, 5520–5525. [Google Scholar] [CrossRef] [PubMed]
- Hafizur, R.M.; Kabir, N.; Chishti, S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function in streptozotocin-induced type 2 diabetic rats. Br. J. Nutr. 2012, 108, 1586–1595. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Carretero, A.S.; Menendez, J.A.; Oliveras-Ferraros, C.; Cerretani, L.; Gutiérrez, A.F. Prediction of Extra Virgin Olive Oil Varieties through Their Phenolic Profile. Potential Cytotoxic Activity against Human Breast Cancer Cells. J. Agric. Food Chem. 2010, 58, 9942–9955. [Google Scholar] [CrossRef]
- Van Der Walt, S.; Colbert, S.C.; Varoquaux, G. The NumPy Array: A Structure for Efficient Numerical Computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Sánchez, C.; Pedregosa, F.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Identification of Bioactive Compounds of Asparagus officinalis L.: Permutation Test Allows Differentiation among “Triguero” and Hybrid Green Varieties. Molecules 2021, 26, 1640. https://doi.org/10.3390/molecules26061640
Jiménez-Sánchez C, Pedregosa F, Borrás-Linares I, Lozano-Sánchez J, Segura-Carretero A. Identification of Bioactive Compounds of Asparagus officinalis L.: Permutation Test Allows Differentiation among “Triguero” and Hybrid Green Varieties. Molecules. 2021; 26(6):1640. https://doi.org/10.3390/molecules26061640
Chicago/Turabian StyleJiménez-Sánchez, Cecilia, Fabián Pedregosa, Isabel Borrás-Linares, Jesús Lozano-Sánchez, and Antonio Segura-Carretero. 2021. "Identification of Bioactive Compounds of Asparagus officinalis L.: Permutation Test Allows Differentiation among “Triguero” and Hybrid Green Varieties" Molecules 26, no. 6: 1640. https://doi.org/10.3390/molecules26061640
APA StyleJiménez-Sánchez, C., Pedregosa, F., Borrás-Linares, I., Lozano-Sánchez, J., & Segura-Carretero, A. (2021). Identification of Bioactive Compounds of Asparagus officinalis L.: Permutation Test Allows Differentiation among “Triguero” and Hybrid Green Varieties. Molecules, 26(6), 1640. https://doi.org/10.3390/molecules26061640







