Tuning the Covering on Gold Surfaces by Grafting Amino-Aryl Films Functionalized with Fe(II) Phthalocyanine: Performance on the Electrocatalysis of Oxygen Reduction
Abstract
:1. Introduction
2. Results and Discussion
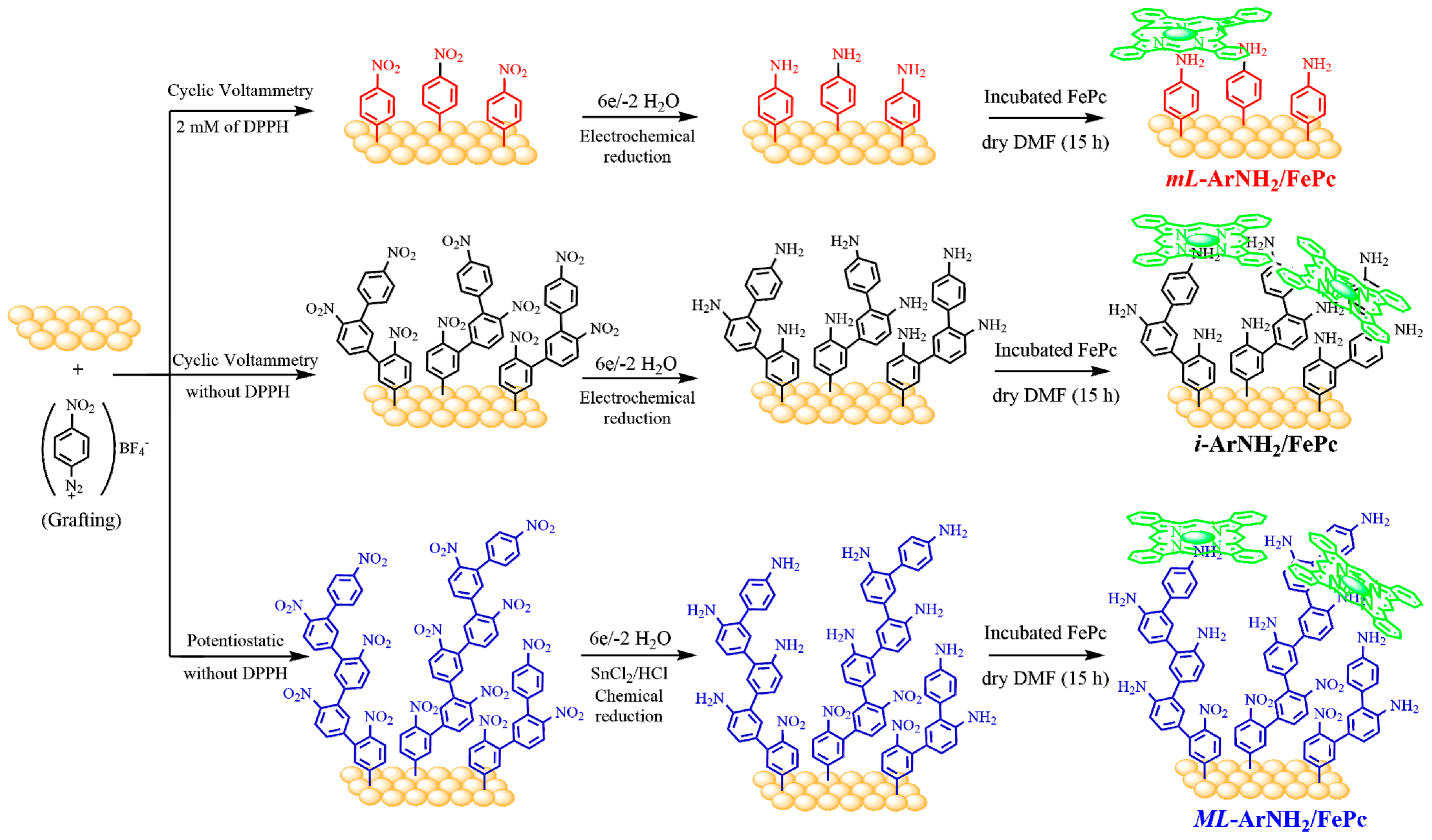
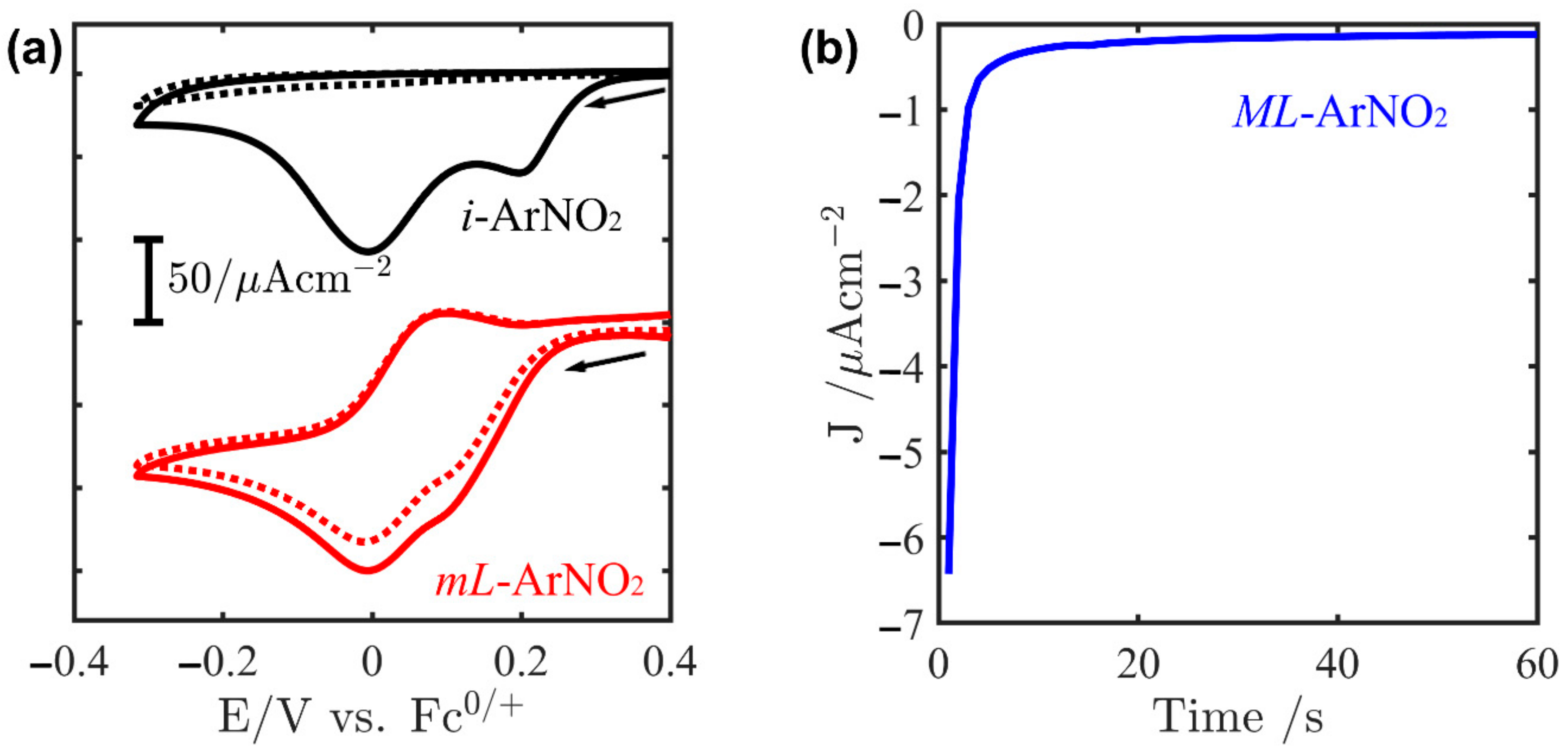
2.1. Electrografting of Gold Electrode whit 4-Nitrophenyl Diazonium
2.2. Electric Properties of Grafted Films; Electrochemical Impedance Spectroscopy
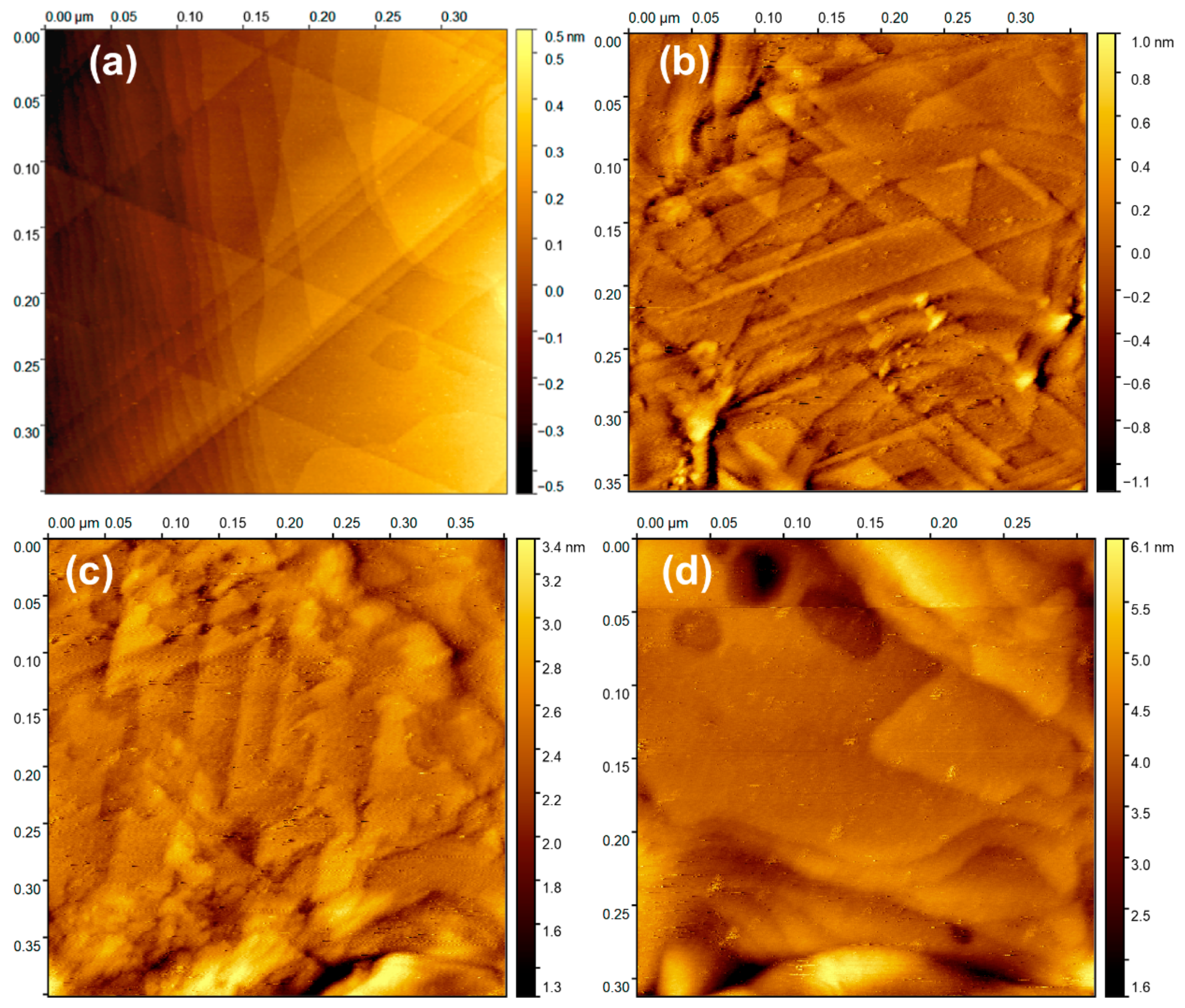
2.3. Surface Morphology of Amino-Aryl Grafting
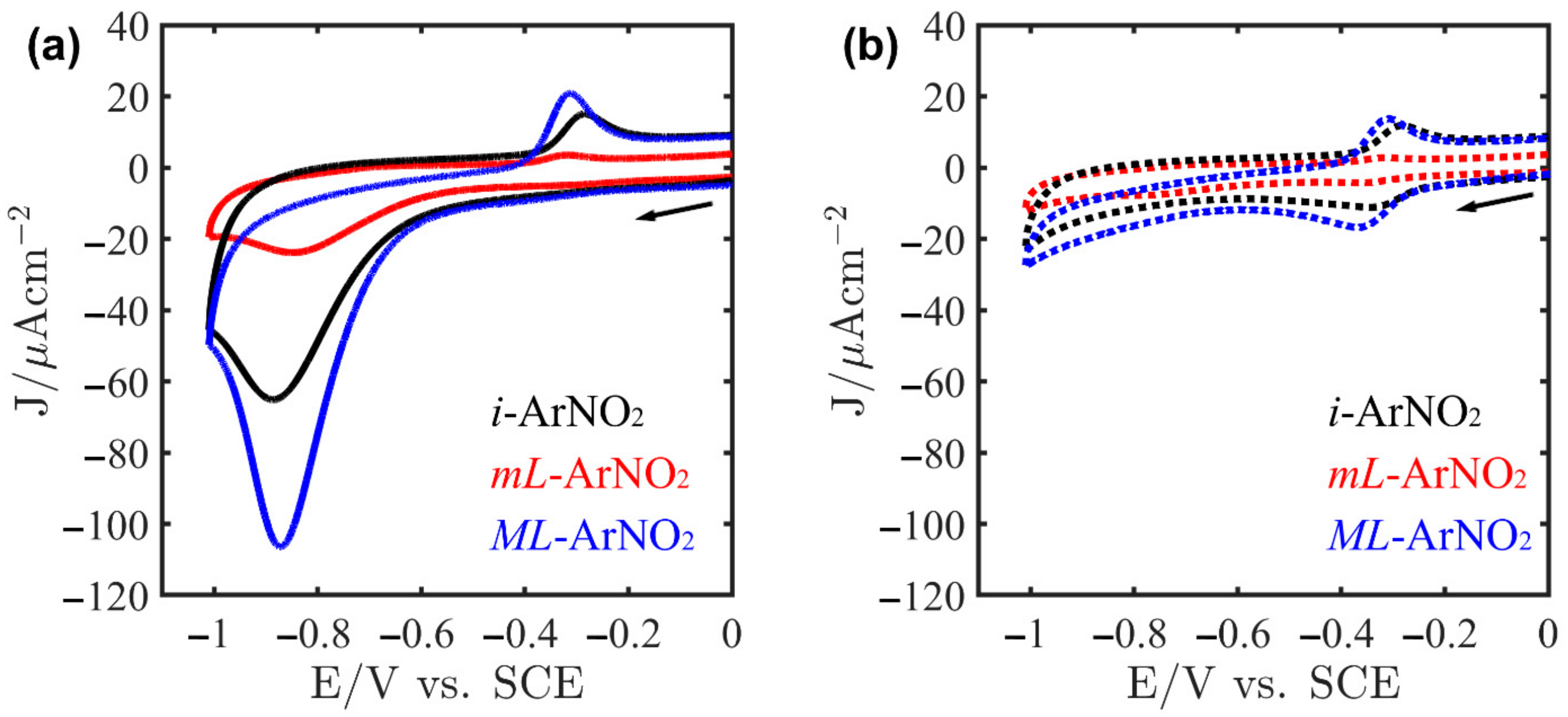
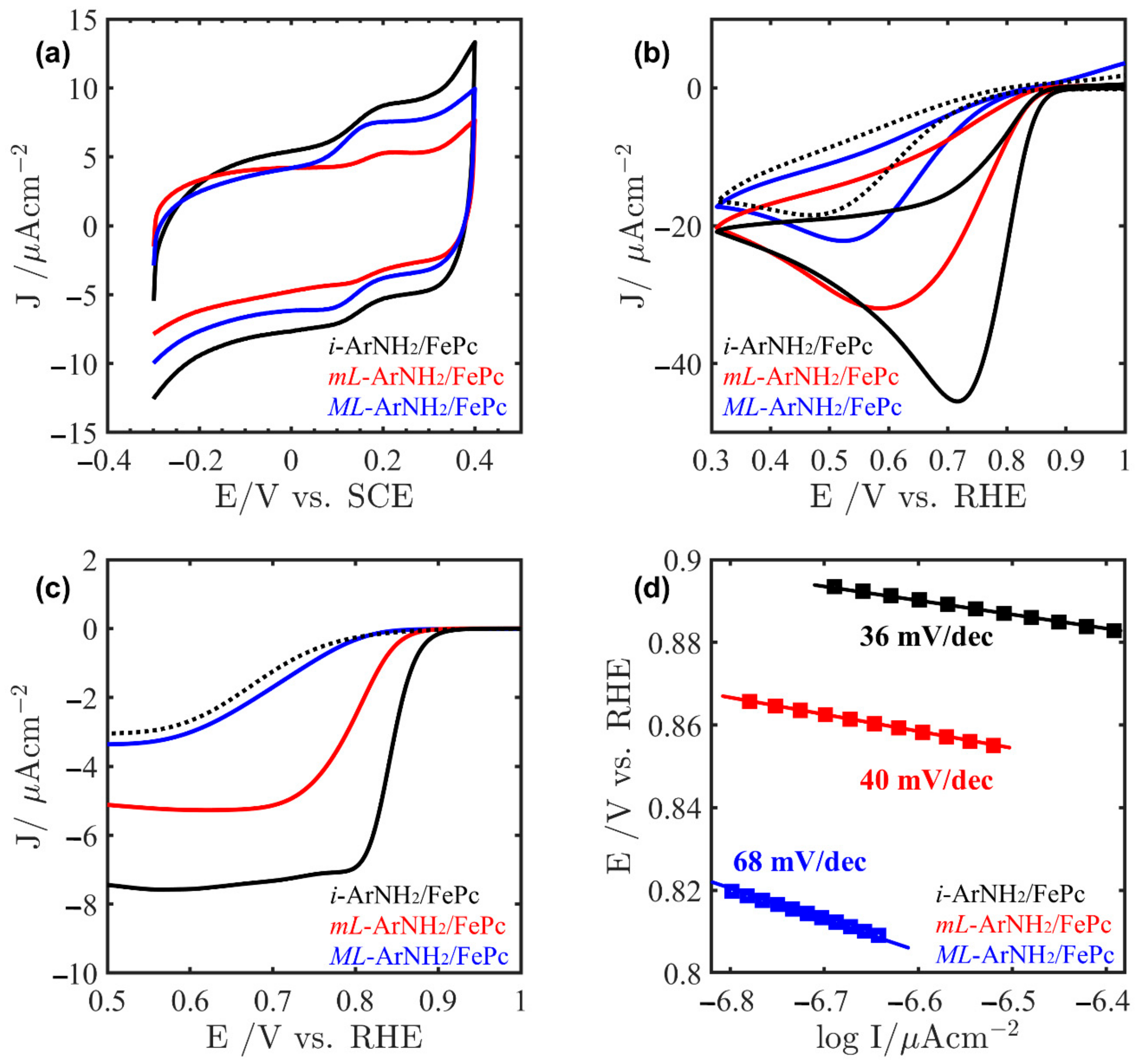
2.4. Electrocatalysis of ORR on Au/ArNH2/FePc Systems
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Adenier, A.; Bernard, M.-C.; Chehimi, M.M.; Cabet-Deliry, E.; Desbat, B.; Fagebaume, O.; Pinson, J.; Podvorica, F. Covalent Modification of Iron Surfaces by Electrochemical Reduction of Aryldiazonium Salts. J. Am. Chem. Soc. 2001, 123, 4541–4549. [Google Scholar] [CrossRef]
- Allongue, P.; Delamar, M.; Desbat, B.; Fagebaume, O.; Hitmi, R.; Pinson, J.; Savéant, J.-M. Covalent Modification of Carbon Surfaces by Aryl Radicals Generated from the Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc. 1997, 119, 201–207. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A Powerful Method for Surface Modification. Chem. Soc. Rev. 2011, 40, 3995. [Google Scholar] [CrossRef]
- Doppelt, P.; Hallais, G.; Pinson, J.; Podvorica, F.; Verneyre, S. Surface Modification of Conducting Substrates. Existence of Azo Bonds in the Structure of Organic Layers Obtained from Diazonium Salts. Chem. Mater. 2007, 19, 4570–4575. [Google Scholar] [CrossRef]
- Dyke, C.A.; Tour, J.M. Solvent-Free Functionalization of Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125, 1156–1157. [Google Scholar] [CrossRef] [PubMed]
- Pinson, J.; Podvorica, F. Attachment of Organic Layers to Conductive or Semiconductive Surfaces by Reduction of Diazonium Salts. Chem. Soc. Rev. 2005, 34, 429. [Google Scholar] [CrossRef]
- Anariba, F.; DuVall, S.H.; McCreery, R.L. Mono- and Multilayer Formation by Diazonium Reduction on Carbon Surfaces Monitored with Atomic Force Microscopy “Scratching”. Anal. Chem. 2003, 75, 3837–3844. [Google Scholar] [CrossRef]
- Kariuki, J.K.; McDermott, M.T. Formation of Multilayers on Glassy Carbon Electrodes via the Reduction of Diazonium Salts. Langmuir 2001, 17, 5947–5951. [Google Scholar] [CrossRef]
- Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Sterically Hindered Diazonium Salts for the Grafting of a Monolayer on Metals. J. Am. Chem. Soc. 2008, 130, 8576–8577. [Google Scholar] [CrossRef]
- Fontaine, O.; Ghilane, J.; Martin, P.; Lacroix, J.-C.; Randriamahazaka, H. Ionic Liquid Viscosity Effects on the Functionalization of Electrode Material through the Electroreduction of Diazonium. Langmuir 2010, 26, 18542–18549. [Google Scholar] [CrossRef]
- López, I.; Dabos-Seignon, S.; Breton, T. Use of Selective Redox Cross-Inhibitors for the Control of Organic Layer Formation Obtained via Diazonium Salt Reduction. Langmuir 2019, 35, 11048–11055. [Google Scholar] [CrossRef] [PubMed]
- Menanteau, T.; Levillain, E.; Breton, T. Electrografting via Diazonium Chemistry: From Multilayer to Monolayer Using Radical Scavenger. Chem. Mater. 2013, 25, 2905–2909. [Google Scholar] [CrossRef] [Green Version]
- Menanteau, T.; Levillain, E.; Downard, A.J.; Breton, T. Evidence of Monolayer Formation via Diazonium Grafting with a Radical Scavenger: Electrochemical, AFM and XPS Monitoring. Phys. Chem. Chem. Phys. 2015, 17, 13137–13142. [Google Scholar] [CrossRef] [PubMed]
- Menanteau, T.; Dias, M.; Levillain, E.; Downard, A.J.; Breton, T. Electrografting via Diazonium Chemistry: The Key Role of the Aryl Substituent in the Layer Growth Mechanism. J. Phys. Chem. C 2016, 120, 4423–4429. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef]
- Joshi, P.; Okada, T.; Miyabayashi, K.; Miyake, M. Evaluation of Alkylamine Modified Pt Nanoparticles as Oxygen Reduction Reaction Electrocatalyst for Fuel Cells via Electrochemical Impedance Spectroscopy. Anal. Chem. 2018, 90, 6116–6123. [Google Scholar] [CrossRef]
- Zagal, J.H. Metallophthalocyanines as Catalysts in Electrochemical Reactions. Coord. Chem. Rev. 1992, 119, 89–136. [Google Scholar] [CrossRef]
- Santander-Nelli, M.; Silva, C.P.; Espinoza-Vergara, J.; Silva, J.F.; Olguín, C.F.; Cortés-Arriagada, D.; Zagal, J.H.; Mendizabal, F.; Díez-Pérez, I.; Pavez, J. Tailoring Electroactive Surfaces by Non-Template Molecular Assembly. Towards Electrooxidation of L-Cysteine. Electrochim. Acta 2017, 254, 201–213. [Google Scholar] [CrossRef]
- Zagal, J.; Páez, M.; Tanaka, A.A.; dos Santos, J.R.; Linkous, C.A. Electrocatalytic Activity of Metal Phthalocyanines for Oxygen Reduction. J. Electroanal. Chem. 1992, 339, 13–30. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, S.; Kang, G.; Nguyen, D.; Schatz, G.C.; Van Duyne, R.P. Operando Characterization of Iron Phthalocyanine Deactivation during Oxygen Reduction Reaction Using Electrochemical Tip-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2019, 141, 15684–15692. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-Based Molecular Materials as Catalysts for Electrochemical Reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Zagal, J.H.; Koper, M.T.M. Reactivity Descriptors for the Activity of Molecular MN4 Catalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2016, 55, 14510–14521. [Google Scholar] [CrossRef]
- Li, B.; Zhao, C.; Chen, S.; Liu, J.; Chen, X.; Song, L.; Zhang, Q. Framework-Porphyrin-Derived Single-Atom Bifunctional Oxygen Electrocatalysts and Their Applications in Zn—Air Batteries. Adv. Mater. 2019, 31, 1900592. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Pavez, J.; Silva, C.P.; Zagal, J.H. Electrocatalytic Actvity of Modified Gold Electrodes Based on Self-Assembled Monolayers of 4-Mercaptopyridine and 4-Aminothiophenol on Au(111) Surfaces Chemically Functionalized with Substituted and Unsubstituted Iron Phthalocyanines. Electrochim. Acta 2013, 114, 7–13. [Google Scholar] [CrossRef]
- Kullapere, M.; Marandi, M.; Matisen, L.; Mirkhalaf, F.; Carvalho, A.E.; Maia, G.; Sammelselg, V.; Tammeveski, K. Blocking Properties of Gold Electrodes Modified with 4-Nitrophenyl and 4-Decylphenyl Groups. J. Solid State Electrochem. 2012, 16, 569–578. [Google Scholar] [CrossRef]
- Benedetto, A.; Balog, M.; Viel, P.; Le Derf, F.; Sallé, M.; Palacin, S. Electro-Reduction of Diazonium Salts on Gold: Why Do We Observe Multi-Peaks? Electrochim. Acta 2008, 53, 7117–7122. [Google Scholar] [CrossRef]
- Ricci, A.; Bonazzola, C.; Calvo, E.J. An FT-IRRAS Study of Nitrophenyl Mono- and Multilayers Electro-Deposited on Gold by Reduction of the Diazonium Salt. Phys. Chem. Chem. Phys. 2006, 8, 4297. [Google Scholar] [CrossRef]
- Brooksby, P.A.; Shields, J.D.; Farquhar, A.K.; Downard, A.J. Reduction of Nitrophenyl Films in Aqueous Solutions: How Many Electrons? ChemElectroChem 2016, 3, 2021–2026. [Google Scholar] [CrossRef]
- Breton, T.; Downard, A.J. Controlling Grafting from Aryldiazonium Salts: A Review of Methods for the Preparation of Monolayers. Aust. J. Chem. 2017, 70, 960. [Google Scholar] [CrossRef]
- Laforgue, A.; Addou, T.; Bélanger, D. Characterization of the Deposition of Organic Molecules at the Surface of Gold by the Electrochemical Reduction of Aryldiazonium Cations. Langmuir 2005, 21, 6855–6865. [Google Scholar] [CrossRef]
- Herrera, S.; Tasca, F.; Williams, F.J.; Calvo, E.J. Adsorption of 4,4′-Dithiodipyridine Axially Coordinated to Iron(II) Phthalocyanine on Au(111) as a New Strategy for Oxygen Reduction Electrocatalysis. ChemPhysChem 2018, 19, 1599–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.S.C.; Tan, E.S.Q.; Jane, R.T.; Downard, A.J. An Electrochemical and XPS Study of Reduction of Nitrophenyl Films Covalently Grafted to Planar Carbon Surfaces. Langmuir 2007, 23, 11074–11082. [Google Scholar] [CrossRef] [PubMed]
- González, M.C.R.; Orive, A.G.; Salvarezza, R.C.; Creus, A.H. Electrodeposition of Gold Nanoparticles on Aryl Diazonium Monolayer Functionalized HOPG Surfaces. Phys. Chem. Chem. Phys. 2016, 18, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Ponce, I.; Silva, J.F.; Oñate, R.; Rezende, M.C.; Paez, M.A.; Zagal, J.H.; Pavez, J.; Mendizabal, F.; Miranda-Rojas, S.; Muñoz-Castro, A.; et al. Enhancement of the Catalytic Activity of Fe Phthalocyanine for the Reduction of O2 Anchored to Au(111) via Conjugated Self-Assembled Monolayers of Aromatic Thiols As Compared to Cu Phthalocyanine. J. Phys. Chem. C 2012, 116, 15329–15341. [Google Scholar] [CrossRef]
- Zurilla, R.W.; Sen, R.K.; Yeager, E. The Kinetics of the Oxygen Reduction Reaction on Gold in Alkaline Solution. J. Electrochem. Soc. 1978, 125, 1103–1109. [Google Scholar] [CrossRef]
- Damjanovic, A.; Genshaw, M.A.; Bockris, J.O. Hydrogen Peroxide Formation in Oxygen Reduction at Gold Electrodes: II. Alkaline Solution. J. Electroanal. Chem. Interfacial Electrochem. 1967, 15, 173–180. [Google Scholar] [CrossRef]
- Baker, R.; Wilkinson, D.P.; Zhang, J. Facile Synthesis, Spectroscopy and Electrochemical Activity of Two Substituted Iron Phthalocyanines as Oxygen Reduction Catalysts in an Acidic Environment. Electrochim. Acta 2009, 54, 3098–3102. [Google Scholar] [CrossRef]
- Tsuda, M.; Diño, W.A.; Nakanishi, H.; Kasai, H. Orientation Dependence of O2 Dissociation from Heme—O2 Adduct. Chem. Phys. Lett. 2005, 402, 71–74. [Google Scholar] [CrossRef]
- Brooksby, P.A.; Downard, A.J. Electrochemical and Atomic Force Microscopy Study of Carbon Surface Modification via Diazonium Reduction in Aqueous and Acetonitrile Solutions. Langmuir 2004, 20, 5038–5045. [Google Scholar] [CrossRef] [PubMed]

| Systems | ArNO2 to ArNH2 Process 1 | ArNO2 to ArNHOH Process 1 | Total 1 |
|---|---|---|---|
| mL-ArNH2 | 1.78 × 10−10 | 1.37 × 10−11 | 1.91 × 10−10 |
| i-ArNH2 | 4.40 × 10−10 | 1.55 × 10−10 | 5.96 × 10−10 |
| ML-ArNH2 | 4.89 × 10−10 | 1.73 × 10−10 | 6.62 × 10−10 |
| Covering | Rct/kΩ | Cdl/μFcm−2 | ||
|---|---|---|---|---|
| ArNO2 | ArNH2 | ArNO2 | ArNH2 | |
| mL-ArNH2 | 4.70 | 4.04 | 0.60 | 2.82 |
| i-ArNH2 | 14.4 | 4.13 | 0.48 | 1.65 |
| ML-ArNH2 | 21.9 | 7.68 | 0.47 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín, C.F.; Agurto, N.; Silva, C.P.; Candia, C.P.; Santander-Nelli, M.; Oyarzo, J.; Gómez, A.; Silva, J.F.; Pavez, J. Tuning the Covering on Gold Surfaces by Grafting Amino-Aryl Films Functionalized with Fe(II) Phthalocyanine: Performance on the Electrocatalysis of Oxygen Reduction. Molecules 2021, 26, 1631. https://doi.org/10.3390/molecules26061631
Olguín CF, Agurto N, Silva CP, Candia CP, Santander-Nelli M, Oyarzo J, Gómez A, Silva JF, Pavez J. Tuning the Covering on Gold Surfaces by Grafting Amino-Aryl Films Functionalized with Fe(II) Phthalocyanine: Performance on the Electrocatalysis of Oxygen Reduction. Molecules. 2021; 26(6):1631. https://doi.org/10.3390/molecules26061631
Chicago/Turabian StyleOlguín, Camila F., Nicolás Agurto, Carlos P. Silva, Carolina P. Candia, Mireya Santander-Nelli, Juan Oyarzo, Alejandra Gómez, Juan F. Silva, and Jorge Pavez. 2021. "Tuning the Covering on Gold Surfaces by Grafting Amino-Aryl Films Functionalized with Fe(II) Phthalocyanine: Performance on the Electrocatalysis of Oxygen Reduction" Molecules 26, no. 6: 1631. https://doi.org/10.3390/molecules26061631
APA StyleOlguín, C. F., Agurto, N., Silva, C. P., Candia, C. P., Santander-Nelli, M., Oyarzo, J., Gómez, A., Silva, J. F., & Pavez, J. (2021). Tuning the Covering on Gold Surfaces by Grafting Amino-Aryl Films Functionalized with Fe(II) Phthalocyanine: Performance on the Electrocatalysis of Oxygen Reduction. Molecules, 26(6), 1631. https://doi.org/10.3390/molecules26061631