Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET
Abstract
1. Introduction
2. Historical Transitions of Modern Medical Imaging Techniques
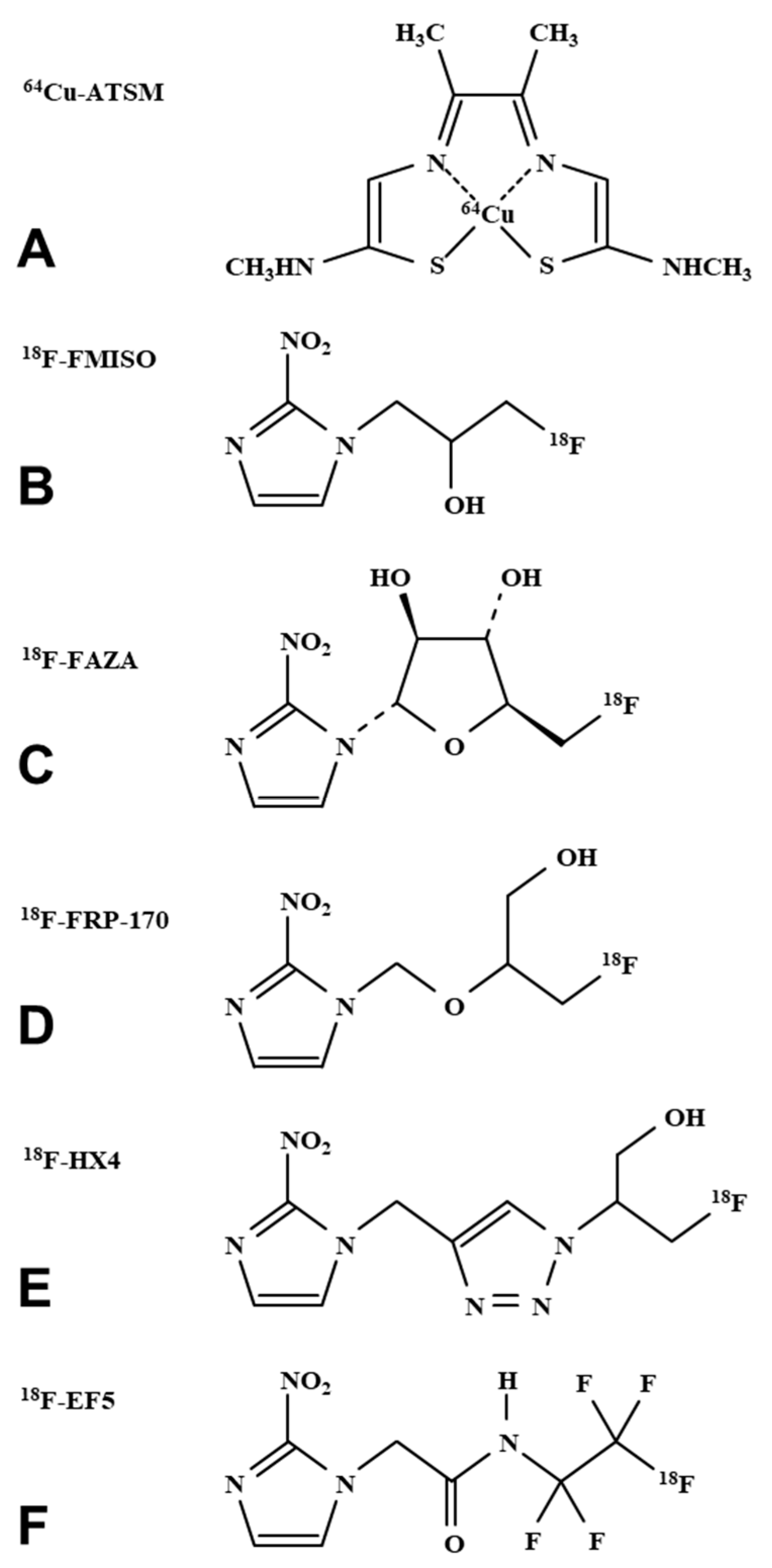
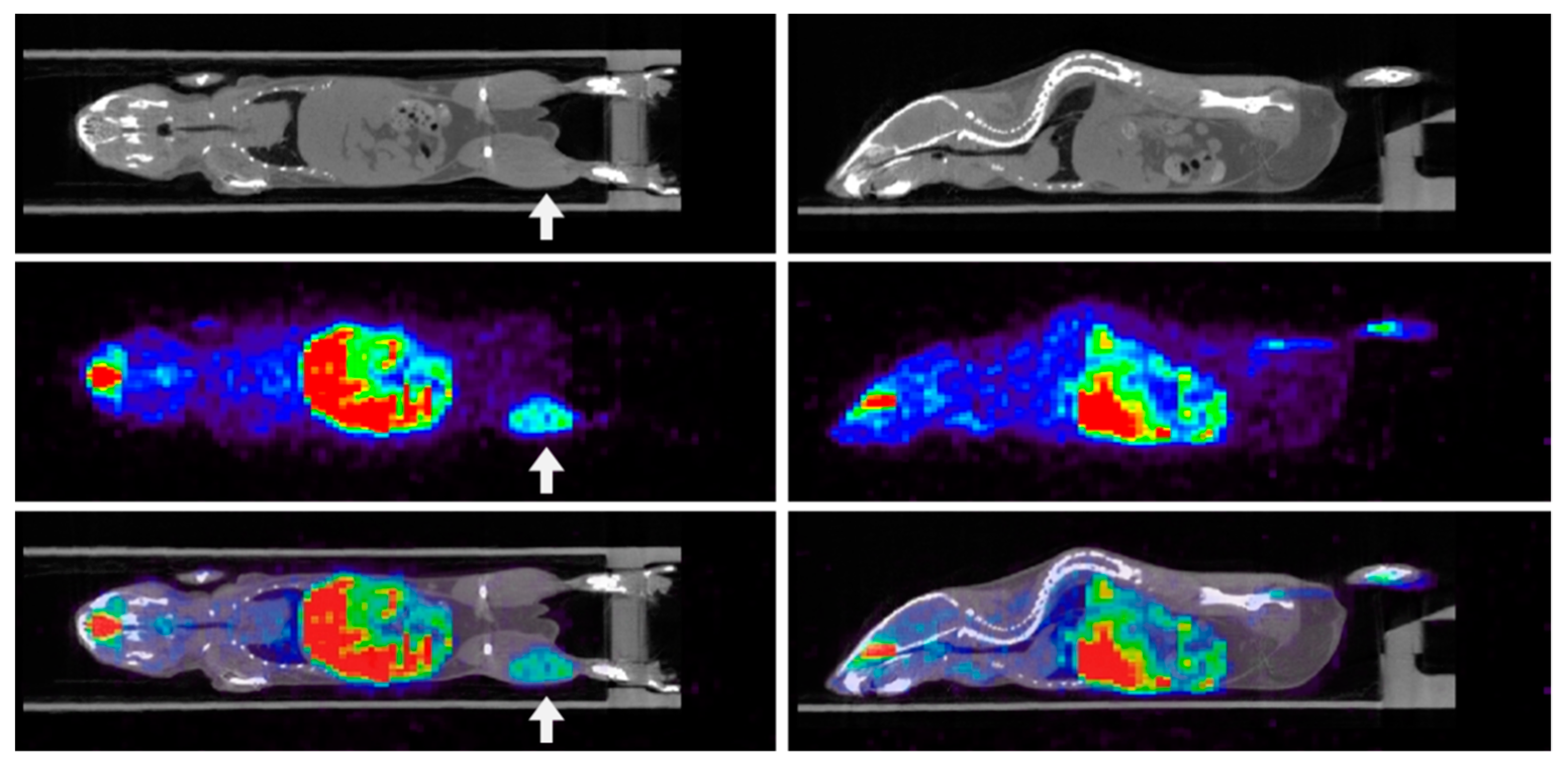
3. Imaging Hypoxia by PET
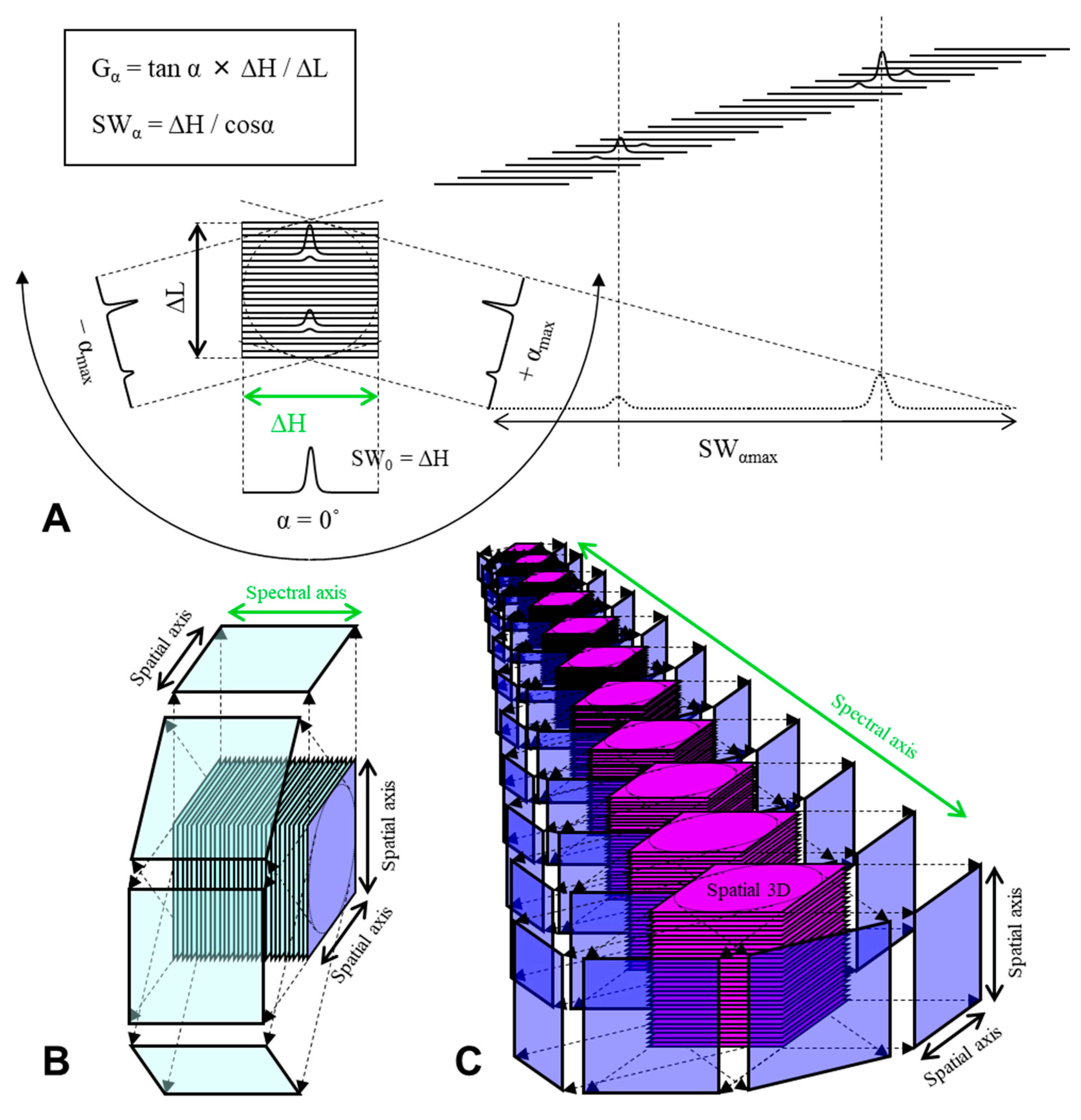
4. EPR Oxygen Mapping
5. MRI Based Oxygenation Imaging
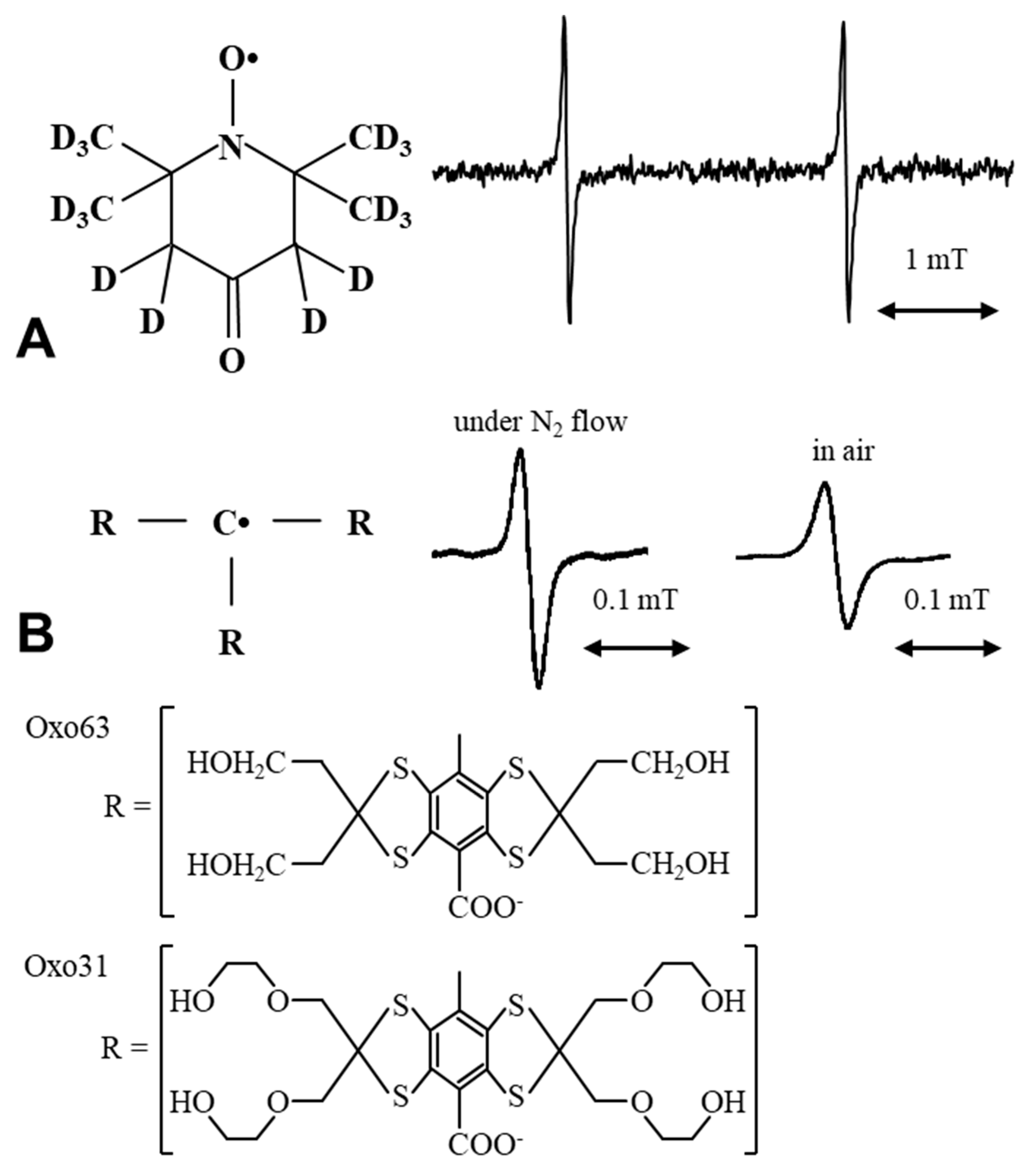
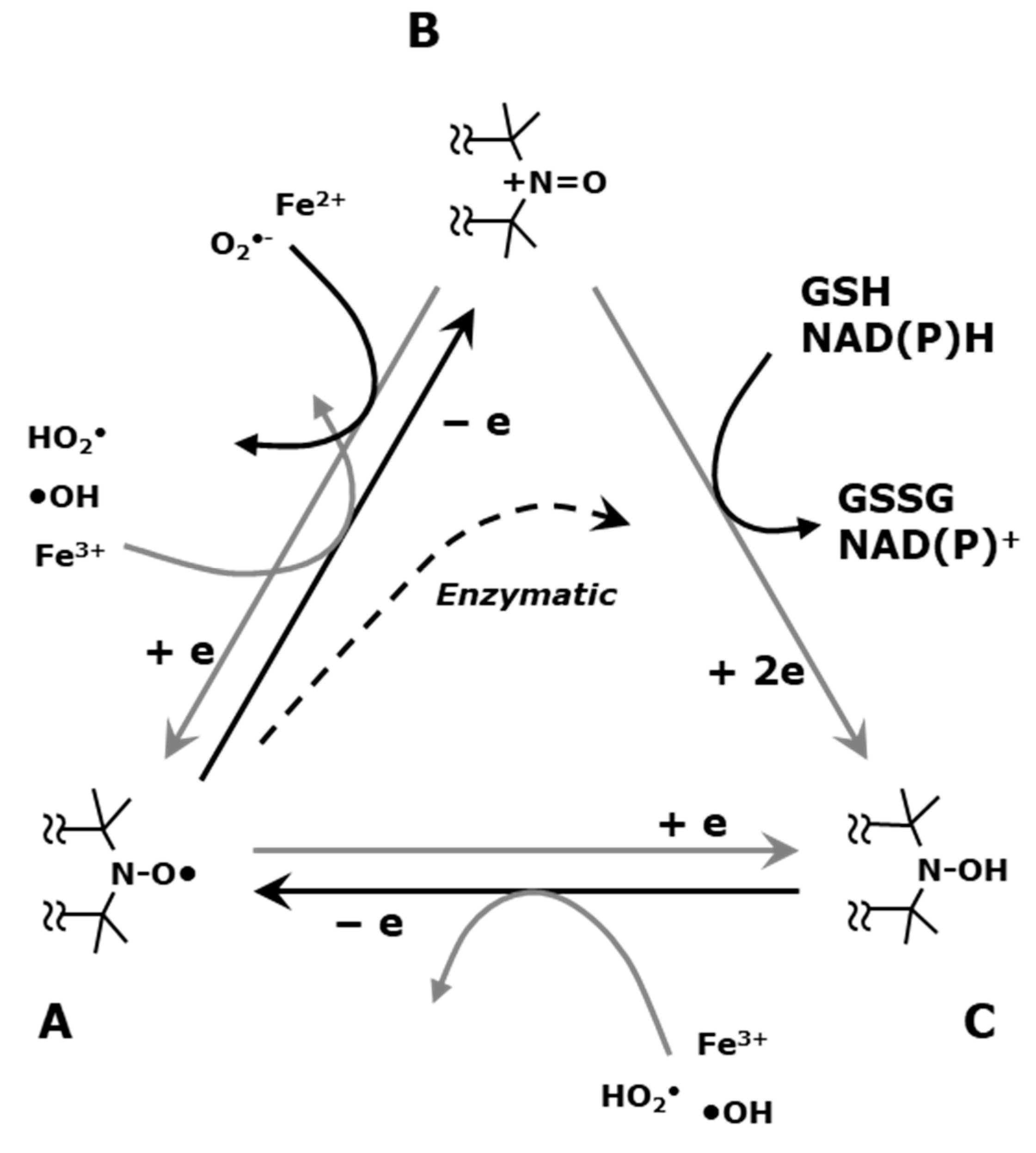
6. Imaging Tissue Redox Status (Redox Imaging) Using Redox Sensitive Nitroxyl Contrast Agents
7. Nitroxyl Radical as Radioprotector (Contrast Agents Having a Medicinal Effect)
8. Applications of Redox Sensitive Nitroxyl Contrast Agents and Multimodal Contrast Agent
9. Metabolic Imaging and Multimodal Comparison
10. Future Directions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spitz, D.R.; Buettner, G.R.; Petronek, M.S.; St-Aubin, J.J.; Flynn, R.T.; Waldron, T.J.; Limoli, C.L. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother. Oncol. 2019, 139, 23–27. [Google Scholar] [CrossRef]
- Kesarwala, A.H.; Krishna, M.C.; Mitchell, J.B. Oxidative stress in oral diseases. Oral Dis. 2016, 22, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Sanz, A.G.; García, G.; Sanche, L. Radiation damage to DNA: The indirect effect of low energy electrons. J. Phys. Chem. Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J. Scientific view of low-level radiation risks. Radiographics 1991, 11, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, R.; Ito, A.; Tomita, M.; Tsukada, T.; Yatagai, F.; Noguchi, M.; Matsumoto, Y.; Kase, Y.; Ando, K.; Okayasu, R.; et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat. Res. 2009, 171, 212–218. [Google Scholar] [CrossRef]
- Hirayama, R.; Matsumoto, Y.; Kase, Y.; Noguchi, M.; Ando, K.; Ito, A.; Okayasu, R.; Furusawa, Y. Radioprotection by DMSO in nitrogen-saturated mammalian cells exposed to helium ion beams. Radiat. Phys. Chem. 2009, 78, 1175–1178. [Google Scholar] [CrossRef]
- Hirayama, R.; Ito, A.; Noguchi, M.; Matsumoto, Y.; Uzawa, A.; Kobashi, G.; Okayasu, R.; Furusawa, Y. OH radicals from the indirect actions of X-rays induce cell lethality and mediate the majority of the oxygen enhancement effect. Radiat. Res. 2013, 180, 514–523. [Google Scholar] [CrossRef]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 2002, 16, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Zhao, D.; Pacheco-Torres, J.; Cui, W.; Kodibagkar, V.D.; Gulaka, P.K.; Hao, G.; Thorpe, P.; Hahn, E.W.; Peschke, P. Multimodality imaging of hypoxia in preclinical settings. Q. J. Nucl. Med. Mol. Imaging 2010, 54, 259–280. [Google Scholar]
- Busk, M.; Overgaard, J.; Horsman, M.R. Imaging of tumor hypoxia for radiotherapy: Current status and future directions. Semin. Nucl. Med. 2020, 50, 562–583. [Google Scholar] [CrossRef]
- Lee, C.T.; Boss, M.K.; Dewhirst, M.W. Imaging tumor hypoxia to advance radiation oncology. Antioxid. Redox Signal. 2014, 21, 313–337. [Google Scholar] [CrossRef]
- Krishna, M.C.; Matsumoto, S.; Yasui, H.; Saito, K.; Devasahayam, N.; Subramanian, S.; Mitchell, J.B. Electron paramagnetic resonance imaging of tumor pO2. Radiat. Res. 2012, 177, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Anemone, A.; Consolino, L.; Arena, F.; Capozza, M.; Longo, D.L. Imaging tumor acidosis: A survey of the available techniques for mapping in vivo tumor pH. Cancer Metastasis Rev. 2019, 38, 25–49. [Google Scholar] [CrossRef]
- Hashim, A.I.; Zhang, X.; Wojtkowiak, J.W.; Martinez, G.V.; Gillies, R.J. Imaging pH and metastasis. NMR Biomed. 2011, 24, 582–591. [Google Scholar] [CrossRef]
- Tang, H.; Li, C.; Zhang, Y.; Zheng, H.; Cheng, Y.; Zhu, J.; Chen, X.; Zhu, Z.; Piao, J.G.; Li, F. Targeted Manganese doped silica nano GSH-cleaner for treatment of liver cancer by destroying the intracellular redox homeostasis. Theranostics 2020, 10, 9865–9887. [Google Scholar] [CrossRef]
- Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. A highly sensitive, cell-membrane-permeable fluorescent probe for glutathione. Bioorg. Med. Chem. Lett. 2014, 24, 4363–4366. [Google Scholar] [CrossRef]
- Hadavand, M.A.; Mayer, D.; Chen, W.; Wnorowski, A.; Siddiqui, M.M. Role of metabolic imaging in diagnosis of primary, metastatic, and recurrent prostate cancer. Curr. Opin. Oncol. 2020, 32, 223–231. [Google Scholar] [CrossRef]
- von Morze, C.; Merritt, M.E. Cancer in the crosshairs: Targeting cancer metabolism with hyperpolarized carbon-13 MRI technology. NMR Biomed. 2019, 32, e3937. [Google Scholar] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Brender, J.R.; Chandramouli, G.V.R.; Saida, Y.; Yamamoto, K.; Mitchell, J.B.; Krishna, M.C. Hypoxia-activated prodrug evofosfamide treatment in pancreatic ductal adenocarcinoma xenografts alters the tumor redox status to potentiate radiotherapy. Antioxid. Redox Signal. 2020; Online ahead of print. [Google Scholar]
- Ahn, K.H.; Scott, G.; Stang, P.; Conolly, S.; Hristov, D. Multiparametric imaging of tumor oxygenation, redox status, and anatomical structure using Overhauser-enhanced MRI-prepolarized MRI system. Magn. Reson. Med. 2011, 65, 1416–1422. [Google Scholar] [CrossRef]
- Golman, K.; Zandt, R.I.; Lerche, M.; Pehrson, R.; Ardenkjaer-Larsen, J.H. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006, 66, 10855–10860. [Google Scholar] [CrossRef]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography). 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef]
- Ambrose, J. Computerized transverse axial scanning (tomography). 2. Clinical application. Br. J. Radiol. 1973, 46, 1023–1047. [Google Scholar] [CrossRef] [PubMed]
- Lauterbur, P.C. Image formation by iInduced local interactions: Examples employing nuclear magnetic resonance. Nature 1973, 242, 190–191. [Google Scholar] [CrossRef]
- Kumar, A.; Welti, D.; Ernst, R.R. NMR Fourier zeugmatography. J. Magn. Reson. 1975, 18, 69–83. [Google Scholar] [CrossRef]
- Reivich, M. Application of the deoxyglucose method to human cerebral dysfunction. The use of [2-18F] fluoro-2-deoxy-D-glucose in man. Neurosci. Res. Program. Bull. 1976, 14, 502–504. [Google Scholar]
- Yamada, K.; Kuppusamy, P.; English, S.; Yoo, J.; Irie, A.; Subramanian, S.; Mitchell, J.B.; Krishna, M.C. Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol. 2002, 43, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Itoh, O.; Ohya-Nishiguchi, H.; Kamada, H. Reducing ability of the striatum and cerebral cortex in rats following acute administration of risperidone or haloperidol: An estimation by in vivo electron paramagnetic resonance imaging. Neurochem. Res. 2002, 27, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Wang, P.; Zweier, J.L.; Krishna, M.C.; Mitchell, J.B.; Ma, L.; Trimble, C.E.; Hsia, C.J. Electron paramagnetic resonance imaging of rat heart with nitroxide and polynitroxyl-albumin. Biochemistry 1996, 35, 7051–7057. [Google Scholar] [CrossRef] [PubMed]
- Panagiotelis, I.; Nicholson, I.; Foster, M.A.; Hutchison, J.M. T*1e and T*2e maps derived in vivo from the rat using longitudinally detected electron spin resonance phase imaging: Application to abdominal oxygen mapping. Magn. Reson. Med. 2001, 46, 1223–1232. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Chzhan, M.; Vij, K.; Shteynbuk, M.; Lefer, D.J.; Giannella, E.; Zweier, J.L. Three-dimensional spectral-spatial EPR imaging of free radicals in the heart: A technique for imaging tissue metabolism and oxygenation. Proc. Natl. Acad. Sci. USA 1994, 91, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Chzhan, M.; Samouilov, A.; Wang, P.; Zweier, J.L. Mapping the spin-density and lineshape distribution of free radicals using 4D spectral-spatial EPR imaging. J. Magn. Reson. B 1995, 107, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.L.; Lauterbur, P.C. Rapid medium- resolution 3-D NMR zeugmatographic imaging of the head. Eur. J. Radiol. 1983, 3 (Suppl. 1), 257–263. [Google Scholar]
- Maltempo, M.M. Differenciation of spectral and spatial components in EPR imaging using 2-D image reconstruction algorithms. J. Magn. Reson. 1986, 69, 156–161. [Google Scholar]
- Subramanian, S.; Devasahayam, N.; Murugesan, R.; Yamada, K.; Cook, J.; Taube, A.; Mitchell, J.B.; Lohman, J.A.; Krishna, M.C. Single-point (constant-time) imaging in radiofrequency Fourier transform electron paramagnetic resonance. Magn. Reson. Med. 2002, 48, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Nayak, A.S.; Glynn, P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990, 14, 68–78. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M. Magnetic resonance imaging of blood vessels at high fields: In vivo and in vitro measurements and image simulation. Magn. Reson. Med. 1990, 16, 9–18. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Ikehira, H.; Girard, F.; Obata, T.; Ito, H.; Yoshitomi, H.; Miyazaki, M.; Nakajima, N.; Kamei, H.; Kanazawa, Y.; Takano, H.; et al. A preliminary study for clinical pharmacokinetics of oral fluorine anticancer medicines using the commercial MRI system 19F-MRS. Br. J. Radiol. 1999, 72, 584–589. [Google Scholar] [CrossRef]
- Arias-Mendoza, F.; Brown, T.R. In vivo measurement of phosphorous markers of disease. Dis. Markers 2004, 19, 49–68. [Google Scholar] [CrossRef][Green Version]
- Narazaki, M.; Kanazawa, Y.; Koike, S.; Ando, K.; Ikehira, H. Quantitative 17O imaging towards oxygen consumption study in tumor bearing mice at 7 T. Magn. Reson. Imaging 2013, 31, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Pak, R.W.; Hadjiabadi, D.H.; Senarathna, J.; Agarwal, S.; Thakor, N.V.; Pillai, J.J.; Pathak, A.P. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. J. Cereb. Blood Flow Metab. 2017, 37, 3475–3487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Lee, V.S. Renal perfusion imaging by MRI. J. Magn. Reson. Imaging 2020, 52, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Gallez, B.; Neveu, M.A.; Danhier, P.; Jordan, B.F. Manipulation of tumor oxygenation and radiosensitivity through modification of cell respiration. A critical review of approaches and imaging biomarkers for therapeutic guidance. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 700–711. [Google Scholar] [CrossRef]
- Doi, Y.; Shimmura, T.; Kuribayashi, H.; Tanaka, Y.; Kanazawa, Y. Quantitative 19F imaging of nmol-level F-nucleotides/-sides from 5-FU with T2 mapping in mice at 9.4T. Magn. Reson. Med. 2009, 62, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, Z.; Bakalova, R.; Aoki, I.; Matsumoto, K.; Gadjeva, V.; Anzai, K.; Kanno, I. Nitroxyl radicals as low toxic spin-labels for non-invasive magnetic resonance imaging of blood-brain barrier permeability for conventional therapeutics. Chem. Commun. 2009, 2009, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Raufman, J.P.; Xu, S.; Cheng, K.; Khurana, S.; Johnson, D.; Shao, C.; Kane, M.A.; Shi, D.; Gullapalli, R.; Polli, J. In vivo magnetic resonance imaging to detect biliary excretion of 19F-labeled drug in mice. Drug Metab. Dispos. 2011, 39, 736–739. [Google Scholar] [CrossRef]
- Lopes, S.I.L.; Ferreira, S.; Caetano, M. PET/CT in the evaluation of hypoxia for radiotherapy planning in head and neck tumors: Systematic literature review. J. Nucl. Med. Technol. 2020; Online ahead of print. [Google Scholar]
- Lewis, J.S.; McCarthy, D.W.; McCarthy, T.J.; Fujibayashi, Y.; Welch, M.J. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J. Nucl. Med. 1999, 40, 177–183. [Google Scholar]
- Matsumoto, K.; Szajek, L.; Krishna, M.C.; Cook, J.A.; Seidel, J.; Grimes, K.; Carson, J.; Sowers, A.L.; English, S.; Green, M.V.; et al. The influence of tumor oxygenation on hypoxia imaging in murine squamous cell carcinoma using [64Cu]Cu-ATSM or [18F]Fluoromisonidazole positron emission tomography. Int. J. Oncol. 2007, 30, 873–881. [Google Scholar] [CrossRef]
- Piert, M.; Machulla, H.J.; Picchio, M.; Reischl, G.; Ziegler, S.; Kumar, P.; Wester, H.J.; Beck, R.; McEwan, A.J.; Wiebe, L.I.; et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J. Nucl. Med. 2005, 46, 106–113. [Google Scholar]
- Kaneta, T.; Takai, Y.; Kagaya, Y.; Yamane, Y.; Wada, H.; Yuki, M.; Iwata, R.; Tsujitani, M.; Takahashi, S.; Yamada, S. Imaging of ischemic but viable myocardium using a new 18F-labeled 2-nitroimidazole analog, 18F-FRP170. J. Nucl. Med. 2002, 43, 109–116. [Google Scholar]
- Dolbier, W.R., Jr.; Li, A.R.; Koch, C.J.; Shiue, C.Y.; Kachur, A.V. [18F]-EF5, a marker for PET detection of hypoxia: Synthesis of precursor and a new fluorination procedure. Appl. Radiat. Isot. 2001, 54, 73–80. [Google Scholar] [CrossRef]
- van Loon, J.; Janssen, M.H.; Ollers, M.; Aerts, H.J.; Dubois, L.; Hochstenbag, M.; Dingemans, A.M.; Lalisang, R.; Brans, B.; Windhorst, B.; et al. PET imaging of hypoxia using [18F]HX4: A phase I trial. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Saito, H.; Nishikido, F.; Takahashi, M.; Yamaya, T. Oxygen sensing ability of positronium atom for tumor hypoxia imaging. Commun. Phys. 2020, 3, 173. [Google Scholar] [CrossRef]
- Yoshida, E.; Tashima, H.; Nagatsu, K.; Tsuji, A.B.; Kamada, K.; Prrodi, K.; Yamaya, T. Whole gamma imaging: A new concept of PET combined with Compton imaging. Phys. Med. Biol. 2020, 65, 125013. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; English, S.; Yoo, J.; Yamada, K.; Devasahayam, N.; Cook, J.A.; Mitchell, J.B.; Subramanian, S.; Krishna, M.C. Pharmacokinetics of a triarylmethyl-type paramagnetic spin probe used in EPR oximetry. Magn. Reson. Med. 2004, 52, 885–892. [Google Scholar] [CrossRef]
- Subramanian, S.; Matsumoto, K.; Mitchell, J.B.; Krishna, M.C. Radio frequency continuous-wave and time-domain EPR imaging and Overhauser-enhanced magnetic resonance imaging of small animals: Instrumental developments and comparison of relative merits for functional imaging. NMR Biomed. 2004, 17, 263–294. [Google Scholar] [CrossRef] [PubMed]
- Elas, M.; Williams, B.B.; Parasca, A.; Mailer, C.; Pelizzari, C.A.; Lewis, M.A.; River, J.N.; Karczmar, G.S.; Barth, E.D.; Halpern, H.J. Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): Methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magn. Reson. Med. 2003, 49, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Chandrika, B.; Lohman, J.A.B.; Mitchell, J.B.; Krishna, M.C.; Subramanian, S. Application of continuous-wave EPR spectral-spatial image reconstruction techniques for in vivo oxymetry: Comparison of projection reconstruction and constant-time modalities. Magn. Reson. Med. 2003, 50, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Subramanian, S.; Devasahayam, N.; Aravalluvan, T.; Murugesan, R.; Cook, J.A.; Mitchell, J.B.; Krishna, M.C. Electron paramagnetic resonance imaging of tumor hypoxia: Enhanced spatial and temporal resolution for in vivo pO2 determination. Magn. Reson. Med. 2006, 55, 1157–1163. [Google Scholar] [CrossRef]
- Mailer, C.; Sundramoorthy, S.V.; Pelizzari, C.A.; Halpern, H.J. Spin echo spectroscopic electron paramagnetic resonance imaging. Magn. Reson. Med. 2006, 55, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kishimoto, S.; Devasahayam, N.; Chandramouli, G.V.R.; Ogawa, Y.; Matsumoto, S.; Krishna, M.C.; Subramanian, S. EPR-based oximetric imaging: A combination of single point-based spatial encoding and T1 weighting. Magn. Reson. Med. 2018, 80, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.; Sundramoorthy, S.V.; Barth, E.D.; Mailer, C.; Halpern, H.J. Comparison of 250 MHz electron spin echo and continuous wave oxygen EPR imaging methods for in vivo applications. Med. Phys. 2011, 38, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Matsumoto, K.; Murugesan, R.; Subramanian, S.; Devasahayam, N.; Koscielniak, J.W.; Hyodo, F.; Cook, J.A.; Mitchell, J.B.; Krishna, M.C. Continuous wave EPR oximetric imaging at 300 MHz using radiofrequency power saturation effects. Antioxid. Redox Signal. 2007, 9, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; English, S.; Yamada, K.; Yoo, J.; Murugesan, R.; Devasahayam, N.; Cook, J.A.; Golman, K.; Ardenkjaer-Larsen, J.H.; Subramanian, S.; et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: Coregistration of tumor anatomy and tissue oxygen concentration. Proc. Natl. Acad. Sci. USA 2002, 99, 2216–2221. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; He, G.; Kuppusamy, P.; Lurie, D.J.; Zweier, J.L. Proton electron double resonance imaging of the in vivo distribution and clearance of a triaryl methyl radical in mice. Magn. Reson. Med. 2002, 48, 530–534. [Google Scholar] [CrossRef]
- Grucker, D.; Chambron, J. Oxygen imaging in perfused hearts by dynamic nuclear polarization. Magn. Reson. Imaging 1993, 11, 691–696. [Google Scholar] [CrossRef]
- Chubarov, A.; Spitsyna, A.; Krumkacheva, O.; Mitin, D.; Suvorov, D.; Tormyshev, V.; Fedin, M.; Bowman, M.K.; Bagryanskaya, E. Reversible dimerization of human serum albumin. Molecules 2020, 26, 108. [Google Scholar] [CrossRef] [PubMed]
- Tormyshev, V.M.; Chubarov, A.S.; Krumkacheva, O.A.; Trukhin, D.V.; Rogozhnikova, O.Y.; Spitsyna, A.S.; Kuzhelev, A.A.; Koval, V.V.; Fedin, M.V.; Godovikova, T.S.; et al. Methanethiosulfonate derivative of OX063 trityl: A promising and efficient reagent for side-directed spin labeling of proteins. Chemistry 2020, 26, 2705–2712. [Google Scholar] [CrossRef]
- Ketter, S.; Gopinath, A.; Rogozhnikova, O.; Trukhin, D.; Tormyshev, V.M.; Bagryanskaya, E.G.; Joseph, B. In situ labeling and distance measurements of membrane proteins in E. coli using finland and OX063 trityl labels. Chemistry 2021, 27, 2299–2304. [Google Scholar] [CrossRef]
- Kishimoto, S.; Matsumoto, K.; Saito, K.; Enomoto, A.; Matsumoto, S.; Mitchell, J.B.; Devasahayam, N.; Krishna, M.C. Pulsed electron paramagnetic resonance imaging: Applications in the studies of tumor physiology. Antioxid. Redox Signal. 2018, 28, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Hyodo, F.; Subramanian, S.; Devasahayam, N.; Munasinghe, J.; Hyodo, E.; Gadisetti, C.; Cook, J.A.; Mitchell, J.B.; Krishna, M.C. Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J. Clin. Investig. 2008, 118, 1965–1973. [Google Scholar] [CrossRef]
- Yasui, H.; Matsumoto, S.; Devasahayam, N.; Munasinghe, J.P.; Choudhuri, R.; Saito, K.; Subramanian, S.; Mitchell, J.B.; Krishna, M.C. Low-field magnetic resonance imaging to visualize chronic and cycling hypoxia in tumor-bearing mice. Cancer Res. 2010, 70, 6427–6436. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Krishna, M.C.; Khramtsov, V.V.; Utsumi, H.; Lurie, D.J. In vivo application of proton-electron double-resonance imaging. Antioxid. Redox Signal. 2018, 28, 1345–1364. [Google Scholar] [CrossRef]
- Matsumoto, K.; Subramanian, S.; Murugesan, R.; Mitchell, J.B.; Krishna, M.C. Spatially resolved biologic information from in vivo EPRI, OMRI, and MRI. Antioxid. Redox Signal. 2007, 9, 1125–1141. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Bernardo, M.; Subramanian, S.; Choyke, P.; Mitchell, J.B.; Krishna, M.C.; Lizak, M.J. MR assessment of changes of tumor in response to hyperbaric oxygen treatment. Magn. Reson. Med. 2006, 56, 240–246. [Google Scholar] [CrossRef]
- Englund, E.K.; Langham, M.C. Quantitative and dynamic MRI measures of peripheral vascular function. Front. Physiol. 2020, 11, 120. [Google Scholar] [CrossRef]
- Niendorf, T.; Seeliger, E.; Cantow, K.; Flemming, B.; Waiczies, S.; Pohlmann, A. Probing renal blood volume with magnetic resonance imaging. Acta Physiol. 2020, 228, e13435. [Google Scholar] [CrossRef]
- Chaudhry, A.A.; Naim, S.; Gul, M.; Chaudhry, A.; Chen, M.; Jandial, R.; Badie, B. Utility of preoperative blood-oxygen-level-dependent functional MR imaging in patients with a central nervous system neoplasm. Radiol. Clin. N. Am. 2019, 57, 1189–1198. [Google Scholar] [CrossRef]
- Koopmans, P.J.; Yacoub, E. Strategies and prospects for cortical depth dependent T2 and T2* weighted BOLD fMRI studies. Neuroimage 2019, 197, 668–676. [Google Scholar] [CrossRef]
- Gore, J.C.; Li, M.; Gao, Y.; Wu, T.L.; Schilling, K.G.; Huang, Y.; Mishra, A.; Newton, A.T.; Rogers, B.P.; Chen, L.M.; et al. Functional MRI and resting state connectivity in white matter—A mini-review. Magn. Reson. Imaging 2019, 63, 1–11. [Google Scholar] [CrossRef]
- Breuer, K.; Weick, S.; Ströhle, S.P.; Breuer, F.A.; Kleine, P.; Veldhoen, S.; Richter, A.; Lapa, C.; Flentje, M.; Polat, B. Feasibility of 4D T2* quantification in the lung with oxygen gas challenge in patients with non-small cell lung cancer. Phys. Med. 2020, 72, 46–51. [Google Scholar] [CrossRef]
- Ando, K.; Nagao, M.; Watanabe, E.; Sakai, A.; Suzuki, A.; Nakao, R.; Ishizaki, U.; Sakai, S.; Hagiwara, N. Association between myocardial hypoxia and fibrosis in hypertrophic cardiomyopathy: Analysis by T2* BOLD and T1 mapping MRI. Eur. Radiol. 2020, 30, 4327–4336. [Google Scholar] [CrossRef]
- Periquito, J.S.; Starke, L.; Santos, C.M.; Freitas, A.C.; Loução, N.; Polo, P.G.; Nunes, R.G.; Niendorf, T.; Pohlmann, A. Analysis protocols for MRI mapping of the blood oxygenation-sensitive parameters T2* and T2 in the kidney. Methods Mol. Biol. 2021, 2216, 591–610. [Google Scholar]
- Virani, N.; Kwon, J.; Zhou, H.; Mason, R.; Berbeco, R.; Protti, A. In vivo hypoxia characterization using blood oxygen level dependent magnetic resonance imaging in a preclinical glioblastoma mouse model. Magn. Reson. Imaging 2021, 76, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bane, O.; Besa, C.; Wagner, M.; Oesingmann, N.; Zhu, H.; Fiel, M.I.; Taouli, B. Feasibility and reproducibility of BOLD and TOLD measurements in the liver with oxygen and carbogen gas challenge in healthy volunteers and patients with hepatocellular carcinoma. J. Magn. Reson. Imaging 2016, 43, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P. Oxygen breathing challenge- the simplest theranostic. Theranostics 2017, 7, 3873–3875. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mitchell, J.B.; Krishna, M.C. Effects of oxygen challenging to tissue redox and pO2 status. Free Radic. Biol. Med. 2019, 130, 343–347. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. Therapeutic and clinical applications of nitroxide compounds. Antioxid. Redox Signal. 2007, 9, 1731–1743. [Google Scholar] [CrossRef]
- Davis, R.M.; Mitchell, J.B.; Krishna, M.C. Nitroxides as cancer imaging agents. Anticancer Agents Med. Chem. 2011, 11, 347–358. [Google Scholar] [CrossRef]
- Bačić, G.; Pavićević, A.; Peyrot, F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol. 2016, 8, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Nyui, M.; Nakanishi, I.; Anzai, K.; Ozawa, T.; Matsumoto, K. Reactivity of redox sensitive paramagnetic nitroxyl contrast agents with reactive oxygen species. J. Clin. Biochem. Nutr. 2019, 64, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Soule, B.P.; Matsumoto, K.; Matusmoto, S.; Cook, J.A.; Hyodo, E.; Sowers, A.L.; Krishna, M.C.; Mitchell, J.B. Assessment of tissue redox status using metabolic responsive contrast agents and magnetic resonance imaging. J. Pharm. Pharmacol. 2008, 60, 1049–1060. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hyodo, F.; Anzai, K.; Utsumi, H.; Mitchell, J.B.; Krishna, M.C. Brain redox imaging. Methods Mol. Biol. 2011, 711, 397–419. [Google Scholar] [PubMed]
- Kuppusamy, P.; Afeworki, M.; Shankar, R.A.; Coffin, D.; Krishna, M.C.; Hahn, S.M.; Mitchell, J.B.; Zweier, J.L. In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res. 1998, 58, 1562–1568. [Google Scholar] [PubMed]
- Brasch, R.C.; London, D.A.; Wesbey, G.E.; Tozer, T.N.; Nitecki, D.E.; Williams, R.D.; Doemeny, J.; Tuck, L.D.; Lallemand, D.P. Work in progress: Nuclear magnetic resonance study of paramagnetic nitroxide contrast agent for enhancement of renal structures in experimental animals. Radiology 1983, 147, 773–779. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hyodo, F.; Matsumoto, A.; Koretsky, A.P.; Sowers, A.L.; Mitchell, J.B.; Krishna, M.C. High resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin. Cacer Res. 2006, 12, 2455–2462. [Google Scholar] [CrossRef]
- Hyodo, F.; Matsumoto, K.; Matsumoto, A.; Mitchell, J.B.; Krishna, M.C. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006, 66, 9921–9928. [Google Scholar] [CrossRef]
- Zhelev, Z.; Aoki, I.; Gadjeva, V.; Nikolova, B.; Bakalova, R.; Saga, T. Tissue redox activity as a sensing platform for imaging of cancer based on nitroxide redox cycle. Eur. J. Cancer 2013, 49, 1467–1478. [Google Scholar] [CrossRef]
- Lazarova, D.; Shibata, S.; Ishii, I.; Zlateva, G.; Zhelev, Z.; Aoki, I.; Higashi, T.; Bakalova, R. Nitroxide-enhanced magnetic resonance imaging of kidney dysfunction in vivo based on redox-imbalance and oxidative stress. Gen. Physiol. Biophys. 2019, 38, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, Z.; Georgieva, E.; Lazarova, D.; Semkova, S.; Aoki, I.; Gulubova, M.; Higashi, T.; Bakalova, R. “Redox imaging” to distinguish cells with different proliferative indexes: Superoxide, hydroperoxides, and their ratio as potential biomarkers. Oxid. Med. Cell. Longev. 2019, 2019, 6373685. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; DeGraff, W.G.; Kaufman, D.; Krishna, M.C.; Samuni, A.; Finkelstein, E.; Ahn, M.S.; Hahn, S.M.; Gamson, J.; Russo, A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, Tempol. Arch. Biochem. Biophy.s 1991, 289, 62–70. [Google Scholar] [CrossRef]
- Hahn, S.M.; Krishna, M.C.; DeLuca, A.M.; Coffin, D.; Mitchell, J.B. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic. Biol. Med. 2000, 28, 953–958. [Google Scholar] [CrossRef]
- Hahn, S.M.; Tochner, Z.; Krishna, C.M.; Glass, J.; Wilson, L.; Samuni, A.; Sprague, M.; Venzon, D.; Glatstein, E.; Mitchell, J.B.; et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992, 52, 1750–1753. [Google Scholar]
- Hahn, S.M.; Sullivan, F.J.; DeLuca, A.M.; Krishna, M.C.; Wersto, N.; Venzon, D.; Russon, A.; Mitchell, J.B. Evaluation of TEMPOL radioprotection in a murine tumor model. Free Radic. Biol. Med. 1997, 22, 1211–1216. [Google Scholar] [CrossRef]
- Cotrim, A.P.; Hyodo, F.; Matsumoto, K.; Sowers, A.L.; Cook, J.A.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin. Cancer Res. 2007, 13, 4928–4933. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002, 62, 307–312. [Google Scholar]
- Hyodo, F.; Davis, R.M.; Hyodo, E.; Masumoto, S.; Krishna, M.C.; Mitchell, J.B. The relationship between tissue oxygenation and redox status using magnetic resonance imaging. Int. J. Oncol. 2012, 41, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Chuang, K.H.; Goloshevsky, A.G.; Sulima, A.; Griffiths, G.L.; Mitchell, J.B.; Koretsky, A.P.; Krishna, M.C. Brain redox imaging using blood-brain barrier-permeable nitroxide MRI contrast agent. J. Cereb. Blood Flow Metab. 2008, 28, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.M.; Sowers, A.L.; DeGraff, W.; Bernardo, M.; Thetford, A.; Krishna, M.C.; Mitchell, J.B. A novel nitroxide is an effective brain redox imaging contrast agent and in vivo radioprotector. Free Radic. Biol. Med. 2011, 51, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamasaki, T.; Nakamura, M.; Ishikawa, J.; Ueno, M.; Nakanishi, I.; Sekita, A.; Ozawa, Y.; Kamada, T.; Aoki, I.; et al. Brain contrasting ability of blood-brain-barrier-permeable nitroxyl contrast agents for magnetic resonance redox imaging. Magn. Reson. Med. 2016, 76, 935–945. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamasaki, T.; Ueno, M.; Shibata, S.; Ozawa, Y.; Kamada, T.; Nakanishi, I.; Yamada, K.; Aoki, I.; Matsumoto, K. Radiation-induced redox alteration in the mouse brain. Free Radic. Biol. Med. 2019, 143, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, A.; Emoto, M.C.; Suzuki, S.; Iwahara, N.; Hisahara, S.; Kawamata, J.; Suzuki, H.; Yamauchi, A.; Sato-Akaba, H.; Fujii, H.G.; et al. Evaluation of oxidative stress in the brain of a transgenic mouse model of Alzheimer disease by in vivo electron paramagnetic resonance imaging. Free Radic. Biol. Med. 2015, 85, 165–173. [Google Scholar] [CrossRef]
- Emoto, M.C.; Yamato, M.; Sato-Akaba, H.; Yamada, K.; Fujii, H.G. Brain redox imaging in the pentylenetetrazole (PTZ)-induced kindling model of epilepsy by using in vivo electron paramagnetic resonance and a nitroxide imaging probe. Neurosci. Lett. 2015, 608, 40–44. [Google Scholar] [CrossRef]
- Emoto, M.C.; Sato-Akaba, H.; Matsuoka, Y.; Yamada, K.; Fujii, H.G. Non-invasive mapping of glutathione levels in mouse brains by in vivo electron paramagnetic resonance (EPR) imaging: Applied to a kindling mouse model. Neurosci. Lett. 2019, 690, 6–10. [Google Scholar] [CrossRef]
- Zhelev, Z.; Matsumoto, K.; Gadjeva, V.; Bakalova, R.; Aoki, I.; Zheleva, A.; Anzai, K. EPR signal reduction kinetic of several nitroxyl derivatives in blood in vitro and in vivo. Gen. Physiol. Biophys. 2009, 28, 356–362. [Google Scholar] [CrossRef]
- Emoto, M.C.; Sato, S.; Fujii, H.G. Development of nitroxide-based theranostic compounds that act both as anti-inflammatory drugs and brain redox imaging probes in MRI. Magn. Reson. Chem. 2016, 54, 705–711. [Google Scholar] [CrossRef]
- Emoto, M.C.; Sasaki, K.; Maeda, K.; Fujii, H.G.; Sato, S. Synthesis and evaluation as a blood-brain barrier-permeable probe of 7-N-(PROXYL-3-yl-methyl)theophylline. Chem. Pharm. Bull 2018, 66, 887–891. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Khramtsov, V.V. In Vivo EPR Assessment of pH, pO2, Redox Status, and Concentrations of Phosphate and Glutathione in the Tumor Microenvironment. J. Vis. Exp. 2018, 133, e56624. [Google Scholar]
- Sowers, M.A.; McCombs, J.R.; Wang, Y.; Paletta, J.T.; Morton, S.W.; Dreaden, E.C.; Boska, M.D.; Ottaviani, M.F.; Hammond, P.T.; Rajca, A.; et al. Redox-responsive branched-bottlebrush polymers for in vivo MRI and fluorescence imaging. Nat. Commun. 2014, 5, 5460. [Google Scholar] [CrossRef]
- Dharmarwardana, M.; Martins, A.F.; Chen, Z.; Palacios, P.M.; Nowak, C.M.; Welch, R.P.; Li, S.; Luzuriaga, M.A.; Bleris, L.; Pierce, B.S.; et al. Nitroxyl modified tobacco mosaic virus as a metal-free high-relaxivity MRI and EPR active superoxide Sensor. Mol. Pharm. 2018, 15, 2973–2983. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, C.; Li, M.; Wang, K.; Wang, Y.; Xu, P.; Hu, Y. Nitroxide-radicals-modified gold nanorods for in vivo CT/MRI-guided photothermal cancer therapy. Int. J. Nanomed. 2018, 13, 7123–7134. [Google Scholar] [CrossRef] [PubMed]
- Rajca, A.; Wang, Y.; Boska, M.; Paletta, J.T.; Olankitwanit, A.; Swanson, M.A.; Mitchell, D.G.; Eaton, S.S.; Eaton, G.R.; Rajca, S. Organic radical contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 2012, 134, 15724–15727. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.-T.; Chen, Q.; Paletta, J.T.; Harvey, P.; Jiang, Y.; Zhang, H.; Boska, M.D.; Ottaviani, M.F.; Jasanoff, A.; Rajca, A.; et al. Nitroxide-based macromolecular contrast agents with unprecedented transverse relaxivity and stability for magnetic resonance imaging of Tumors. ACS Cent. Sci. 2017, 3, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.-T.; Detappe, A.; Harvey, P.; Gallagher, N.; Mathieu, C.; Agius, M.P.; Zavidij, O.; Wang, W.; Jiang, Y.; Rajca, A.; et al. Pro-organic radical contrast agents (“pro-ORCAs”) for real-time MRI of pro-drug activation in biological systems. Polym. Chem. 2020, 11, 4768–4779. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Detappe, A.; Gallagher, N.M.; Zhang, H.; Harvey, P.; Yan, C.; Mathieu, C.; Golder, M.R.; Jiang, Y.; Ottaviani, M.F.; et al. Triply loaded nitroxide brush-arm star polymers enable metal-free millimetric tumor detection by magnetic resonance imaging. ACS Nano 2018, 12, 11343–11354. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, S.; Kutseikin, S.; Morozov, D.; Krumkacheva, O.; Spitsyna, A.; Gatilov, Y.; Silnikov, V.; Angelovski, G.; Bowman, M.K.; Kirilyuk, I.; et al. Human serum albumin labelled with sterically-hindered nitroxides as potential MRI contrast agents. Molecules 2020, 25, 1709. [Google Scholar] [CrossRef] [PubMed]
- Soikkeli, M.; Horkka, K.; Moilanen, J.O.; Timonen, M.; Kavakka, J.; Heikkinen, S. Synthesis, Stability and relaxivity of TEEPO-Met: An organic radical as a potential tumour targeting contrast agent for magnetic resonance imaging. Molecules 2018, 23, 1034. [Google Scholar] [CrossRef] [PubMed]
- Koonjoo, N.; Parzy, E.; Massot, P.; Lepetit-Coiffé, M.; Marque, S.R.; Franconi, J.M.; Thiaudiere, E.; Mellet, P. In vivo Overhauser-enhanced MRI of proteolytic activity. Contrast Media Mol. Imaging 2014, 9, 363–371. [Google Scholar] [CrossRef]
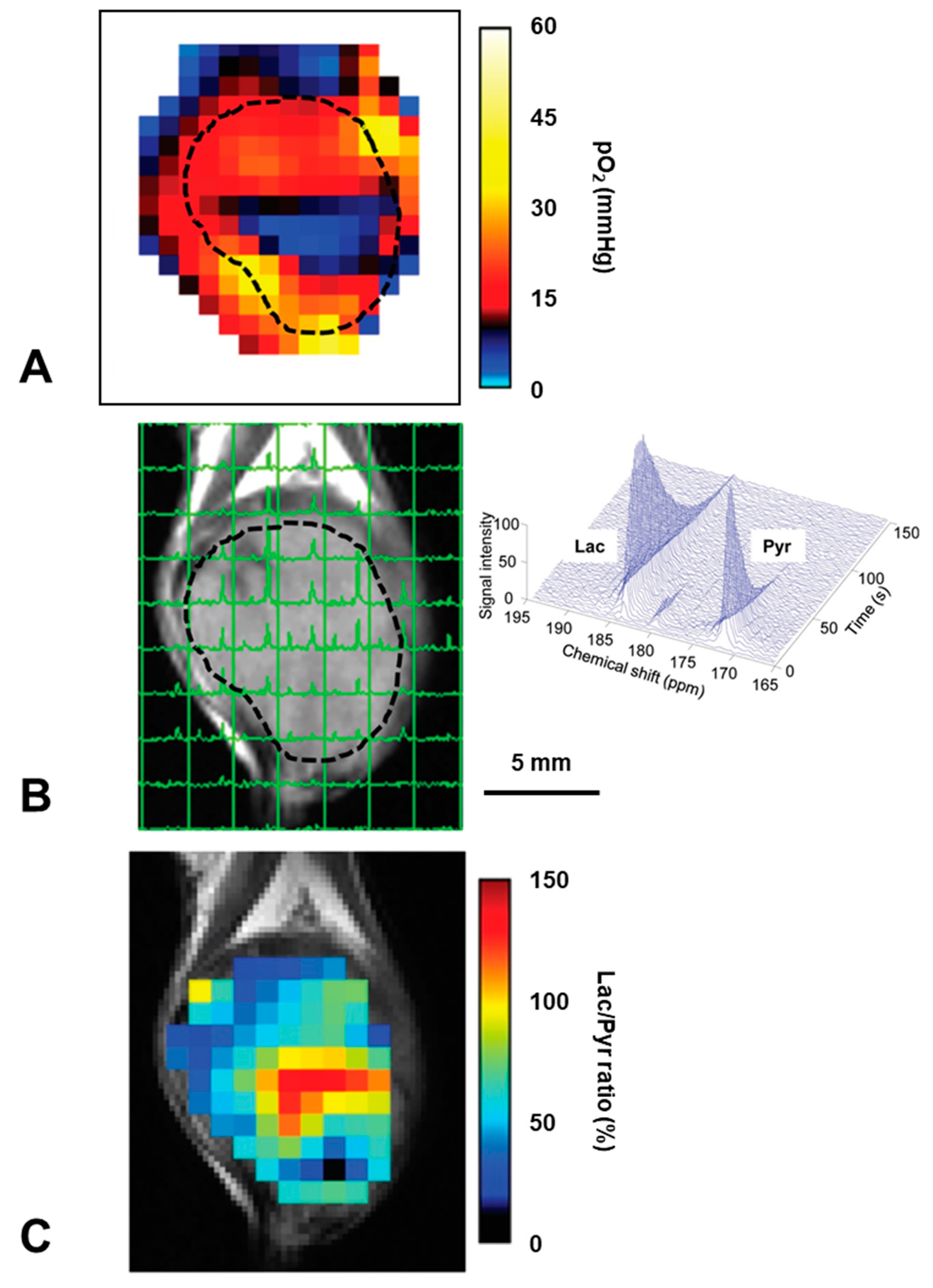
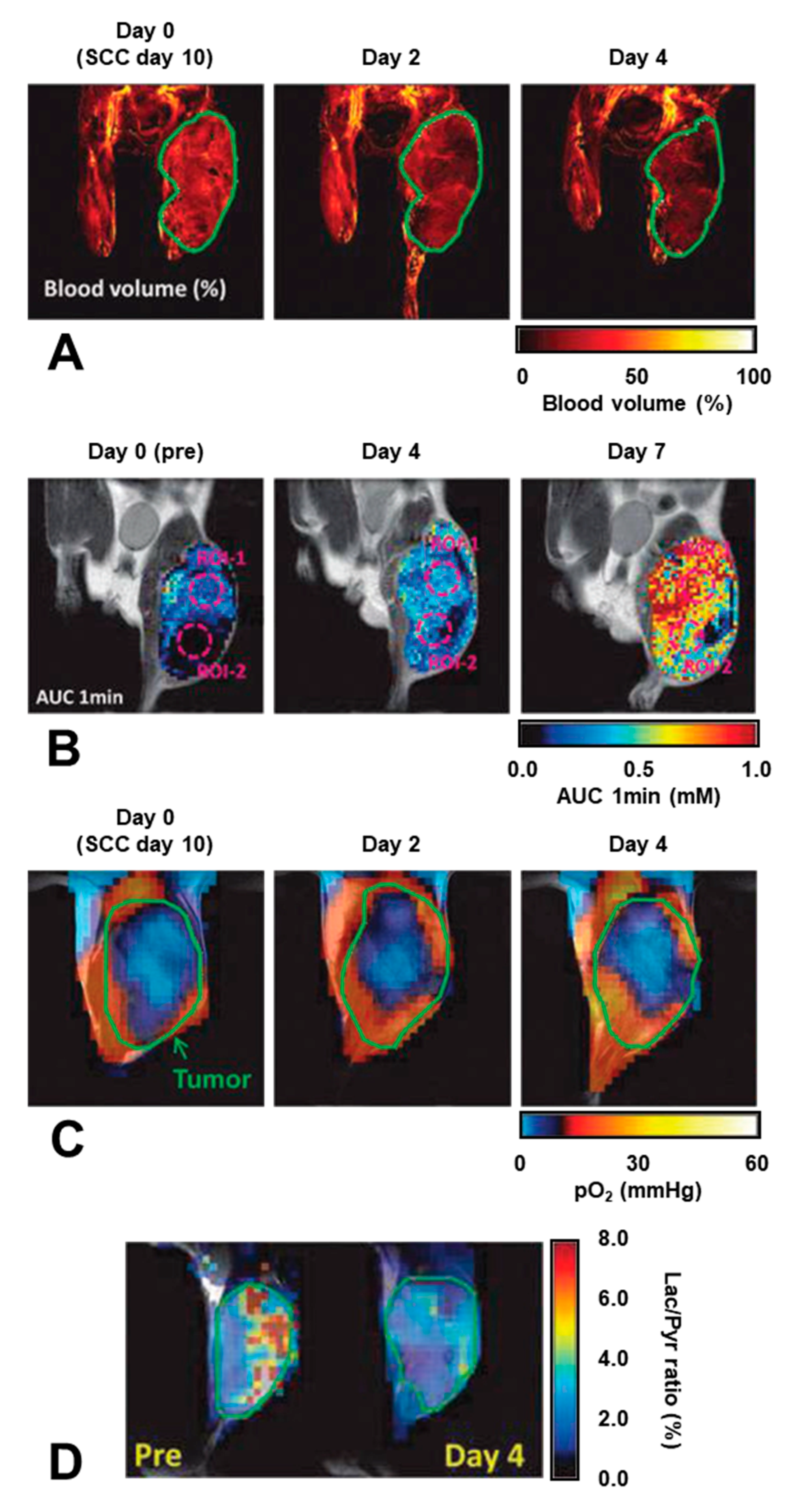
- Matsuo, M.; Kawai, T.; Kishimoto, S.; Saito, K.; Munasinghe, J.; Devasahayam, N.; Mitchell, J.B.; Krishna, M.C. Co-imaging of the tumor oxygenation and metabolism using electron paramagnetic resonance imaging and 13-C hyperpolarized magnetic resonance imaging before and after irradiation. Oncotarget 2018, 9, 25089–25100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, S.; Saito, K.; Takakusagi, Y.; Matsuo, M.; Munasinghe, J.P.; Morris, H.D.; Lizak, M.J.; Merkle, H.; Yasukawa, K.; Devasahayam, N.; et al. In vivo imaging of tumor physiological, metabolic, and redox changes in response to the anti-angiogenic agent sunitinib: Longitudinal assessment to identify transient vascular renormalization. Antioxid. Redox Signal. 2014, 21, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
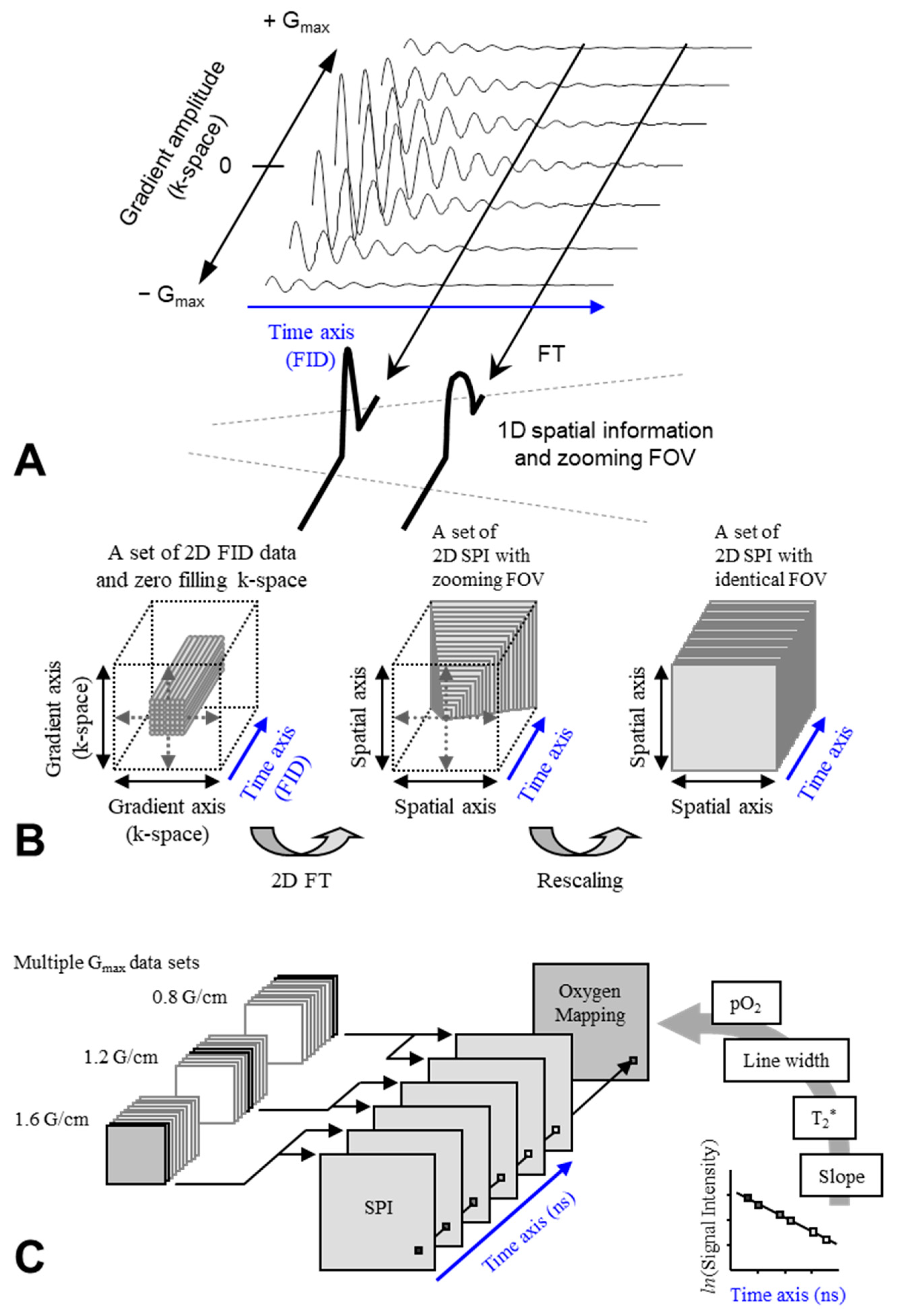
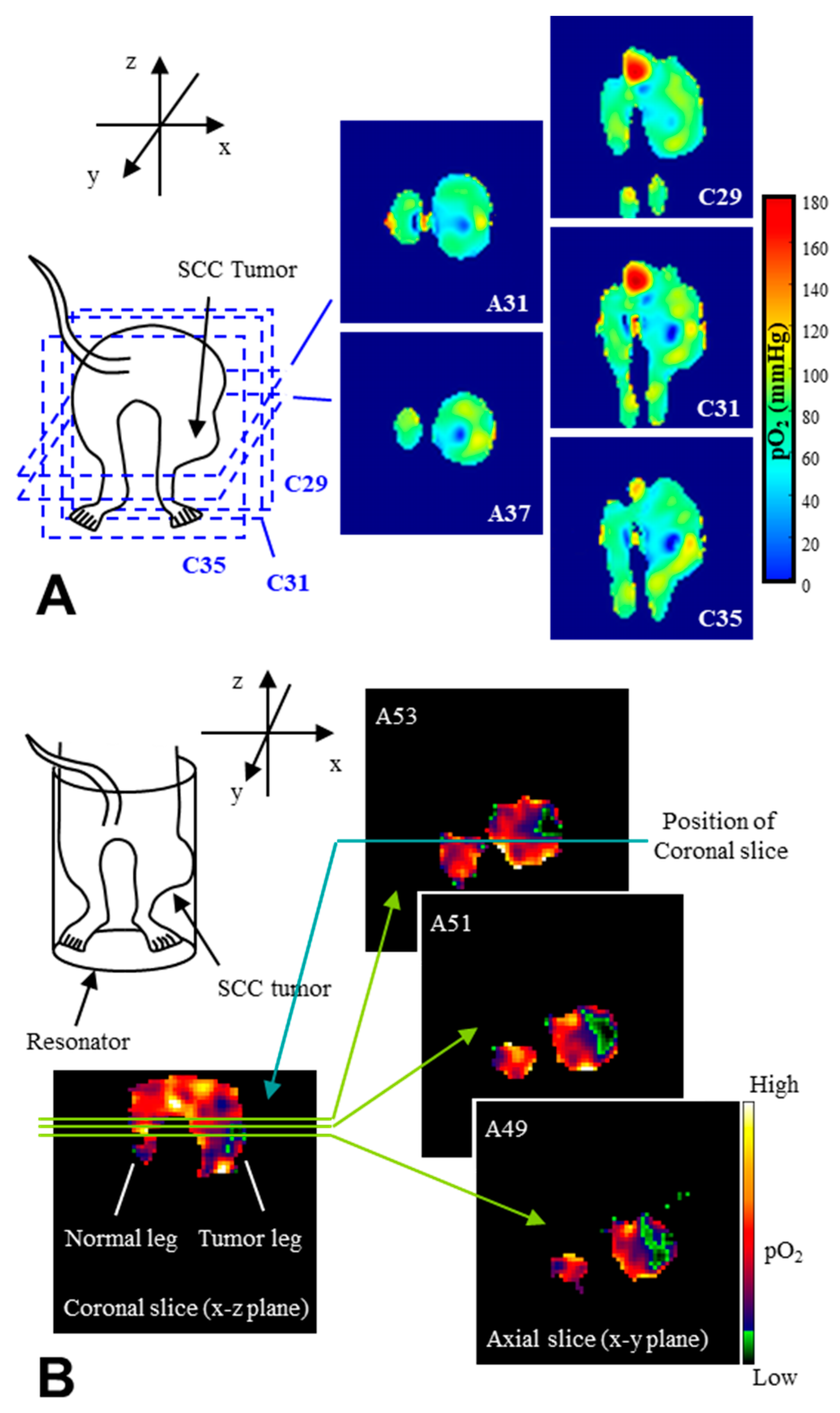
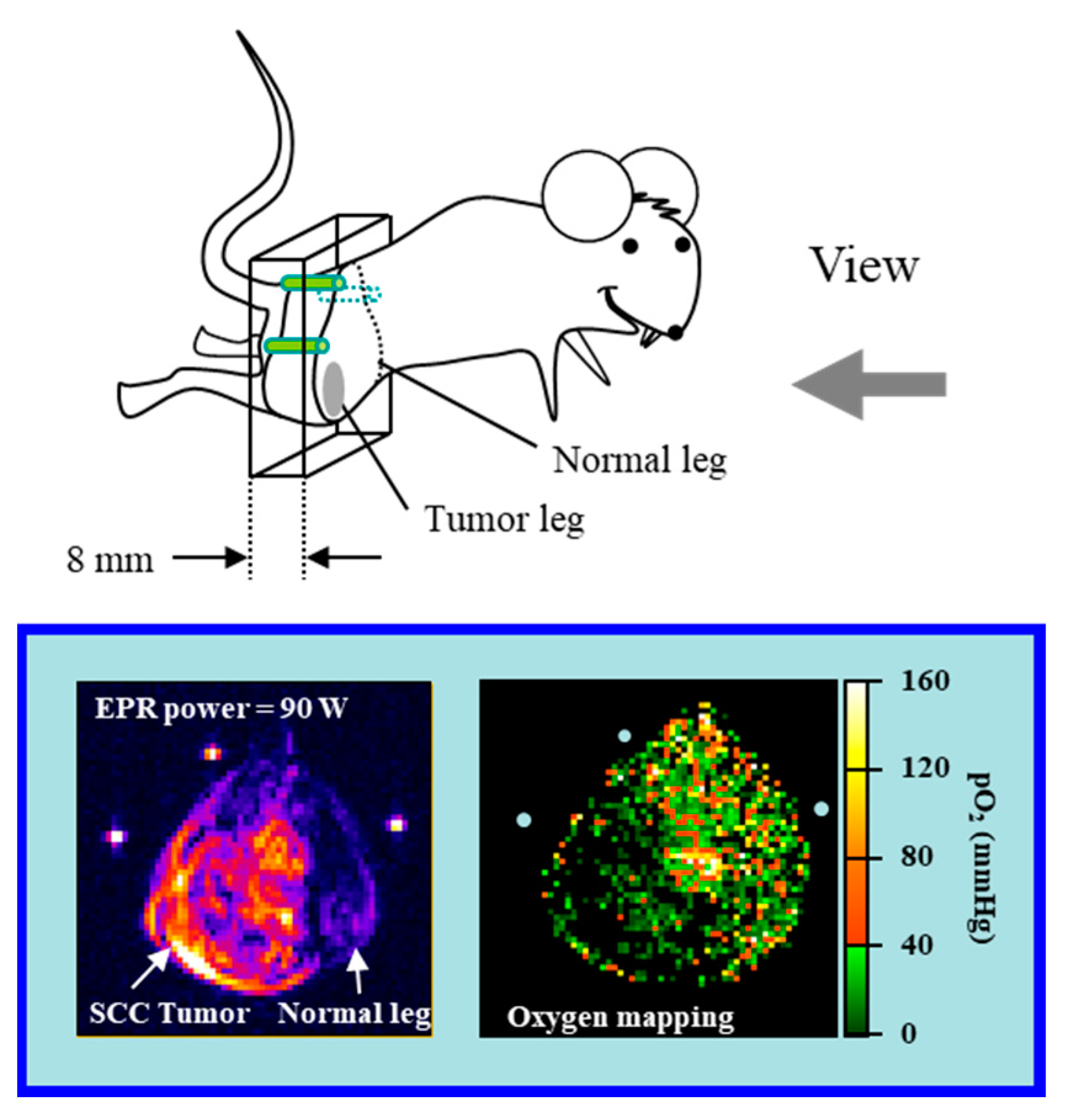
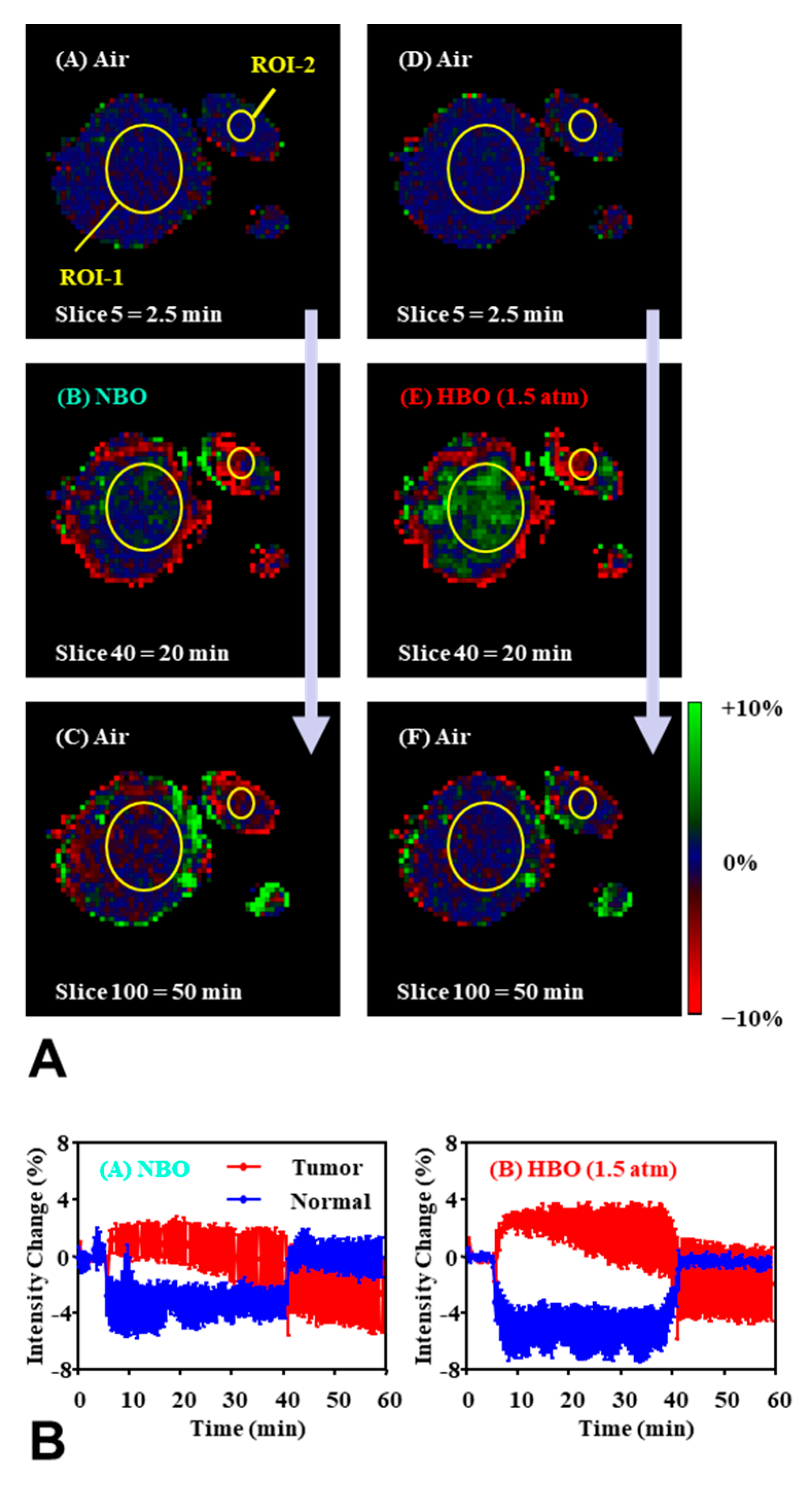
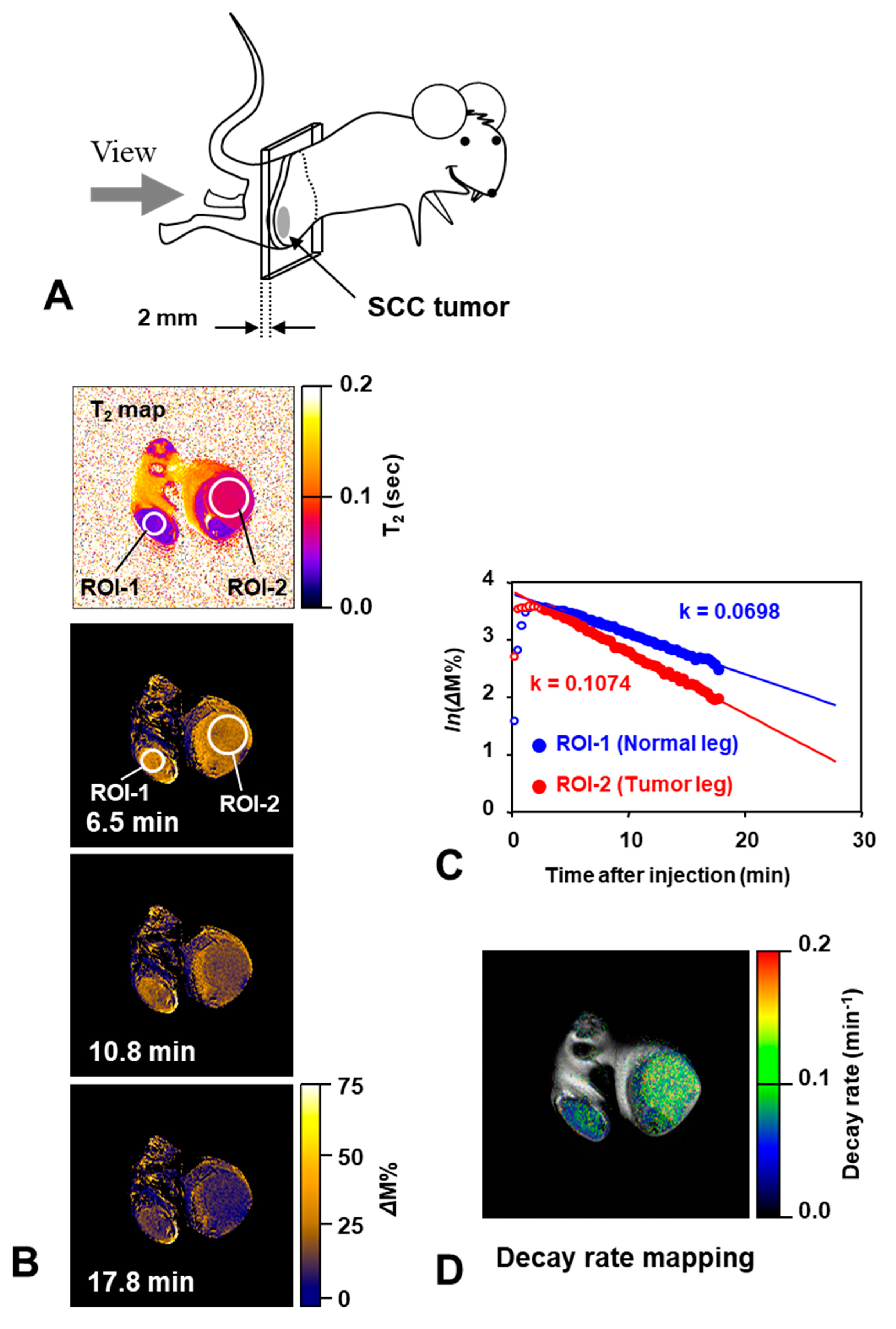
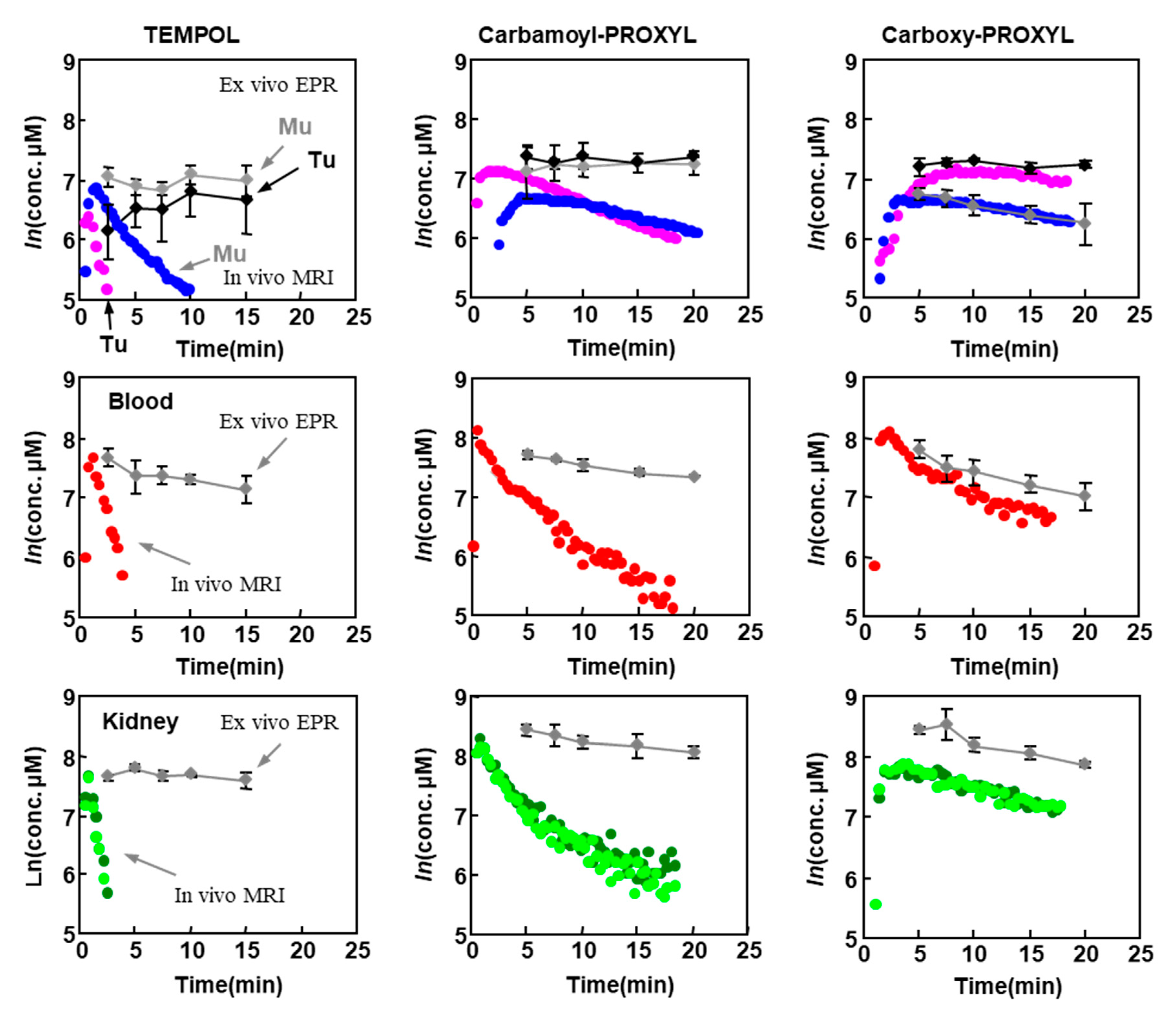
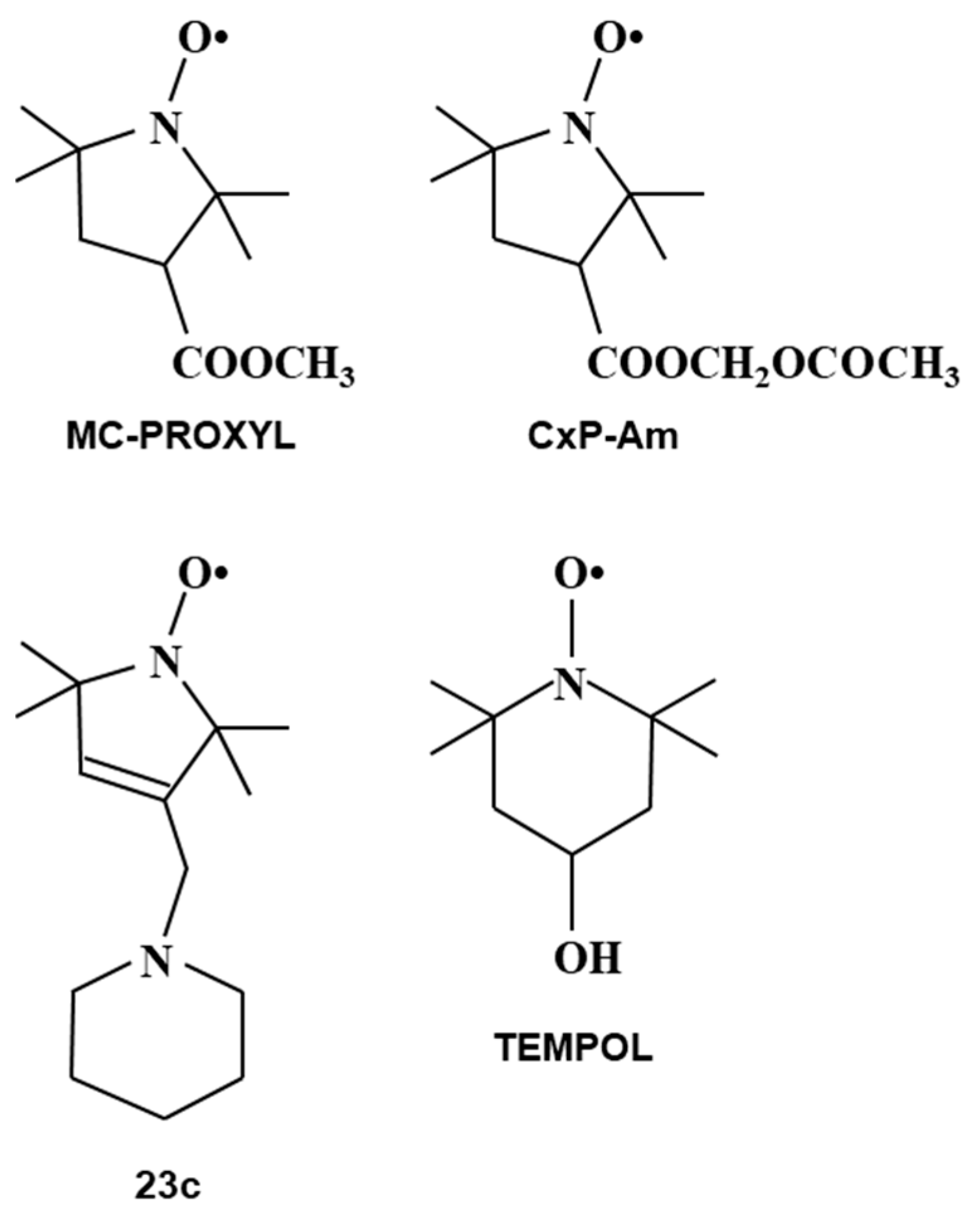
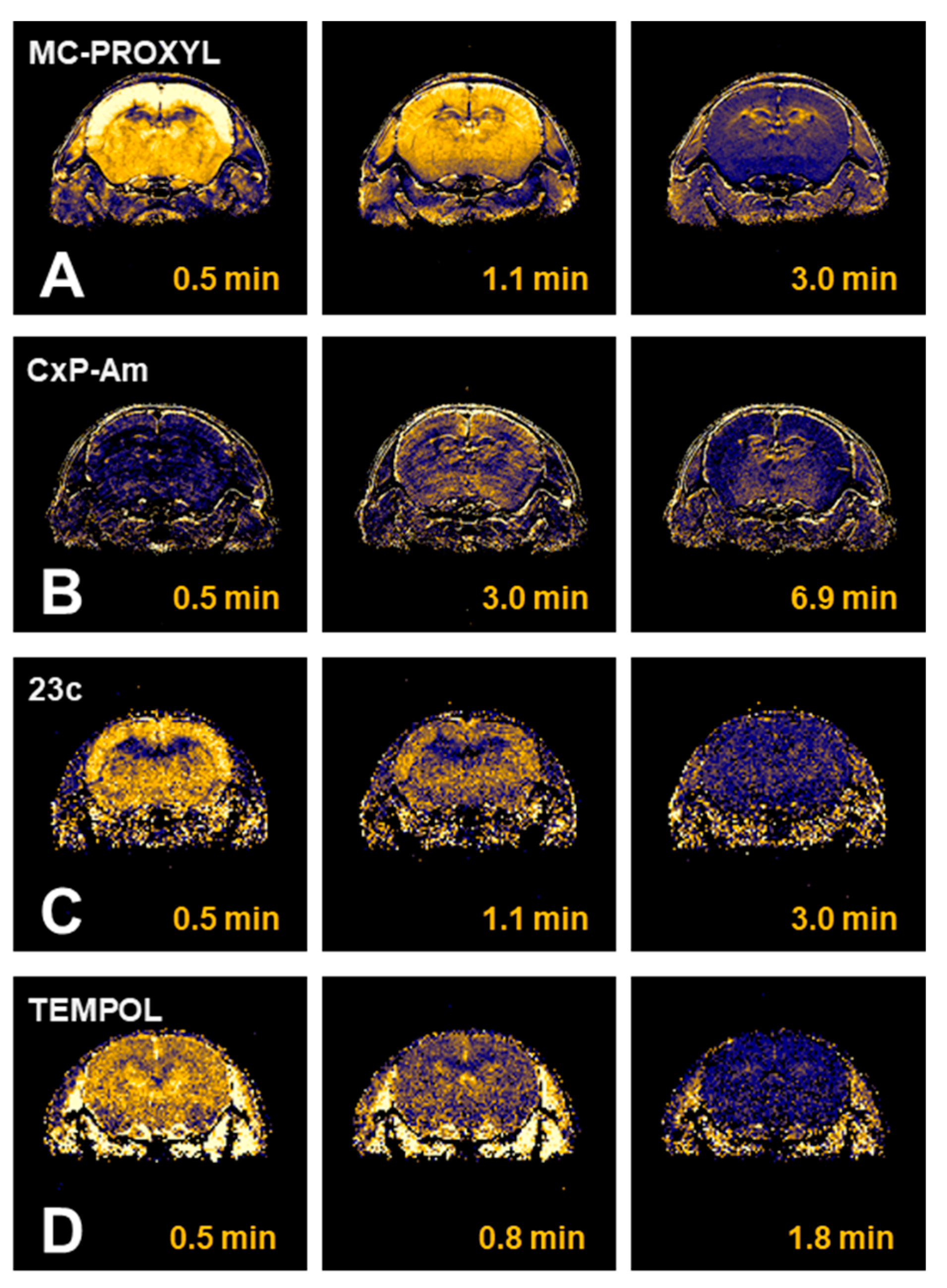
| Tissues | TEMPOL Decay Rate (min−1) | Carbamoyl-PROXYL Decay Rate (min−1) | Carboxy-PROXYL Decay Rate (min−1) |
|---|---|---|---|
| Normal muscle | 0.319 ± 0.025 | 0.056 ± 0.013 | 0.029 ± 0.014 |
| Tumor tissue | 1.095 ± 0.203 ** | 0.107 ± 0.20 * | 0.020 ± 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, K.-i.; Mitchell, J.B.; Krishna, M.C. Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET. Molecules 2021, 26, 1614. https://doi.org/10.3390/molecules26061614
Matsumoto K-i, Mitchell JB, Krishna MC. Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET. Molecules. 2021; 26(6):1614. https://doi.org/10.3390/molecules26061614
Chicago/Turabian StyleMatsumoto, Ken-ichiro, James B. Mitchell, and Murali C. Krishna. 2021. "Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET" Molecules 26, no. 6: 1614. https://doi.org/10.3390/molecules26061614
APA StyleMatsumoto, K.-i., Mitchell, J. B., & Krishna, M. C. (2021). Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET. Molecules, 26(6), 1614. https://doi.org/10.3390/molecules26061614







