EGCG Inhibits Adipose-Derived Mesenchymal Stem Cells Differentiation into Adipocytes and Prevents a STAT3-Mediated Paracrine Oncogenic Control of Triple-Negative Breast Cancer Cell Invasive Phenotype
Abstract
1. Introduction
2. Results
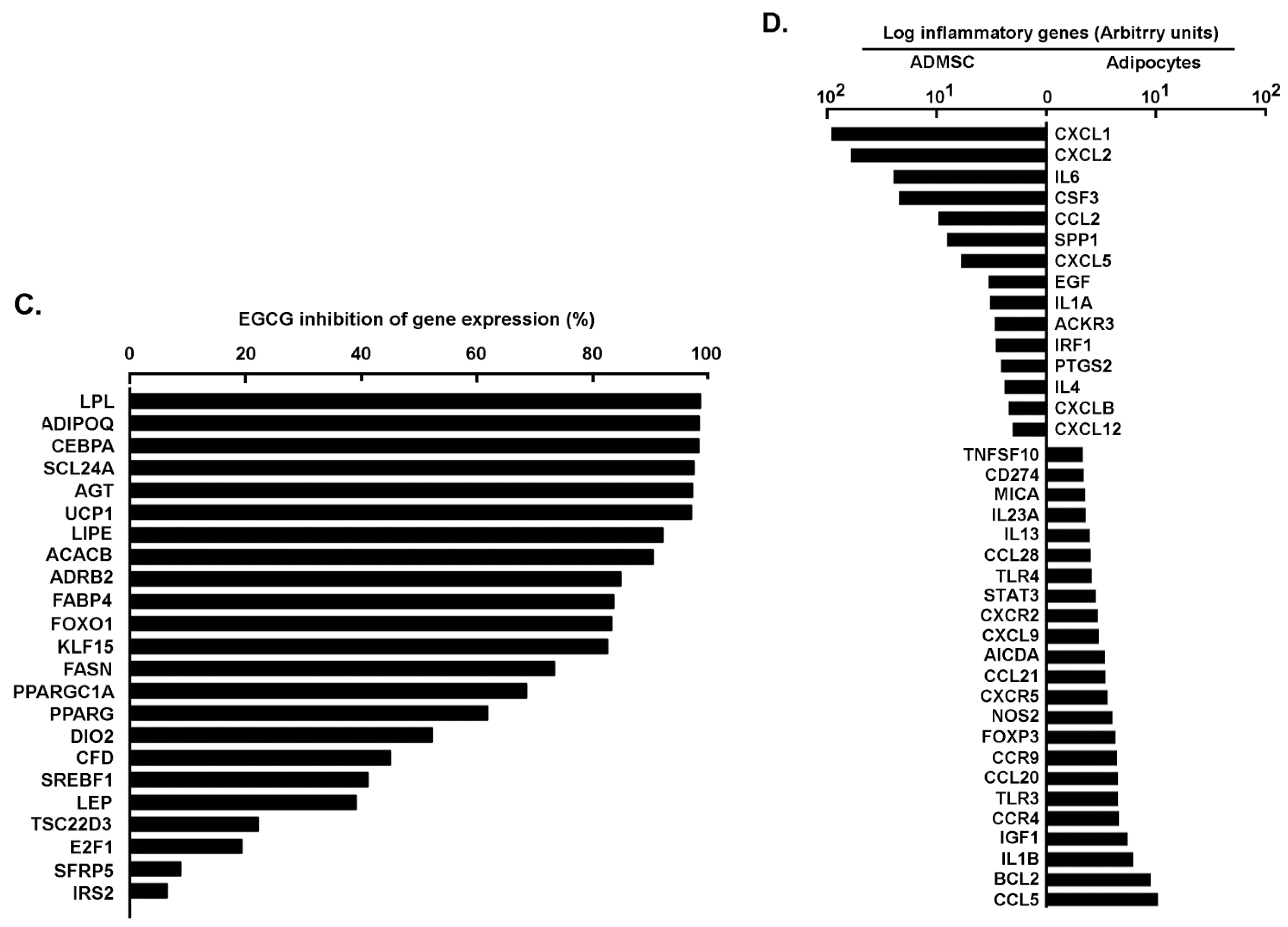
2.1. Phenotypical and Transcriptional Assessment of Adipose-Derived Mesenchymal Stem Cells Differentiation and Inhibition of Adipogenesis by Green Tea-Derived EGCG
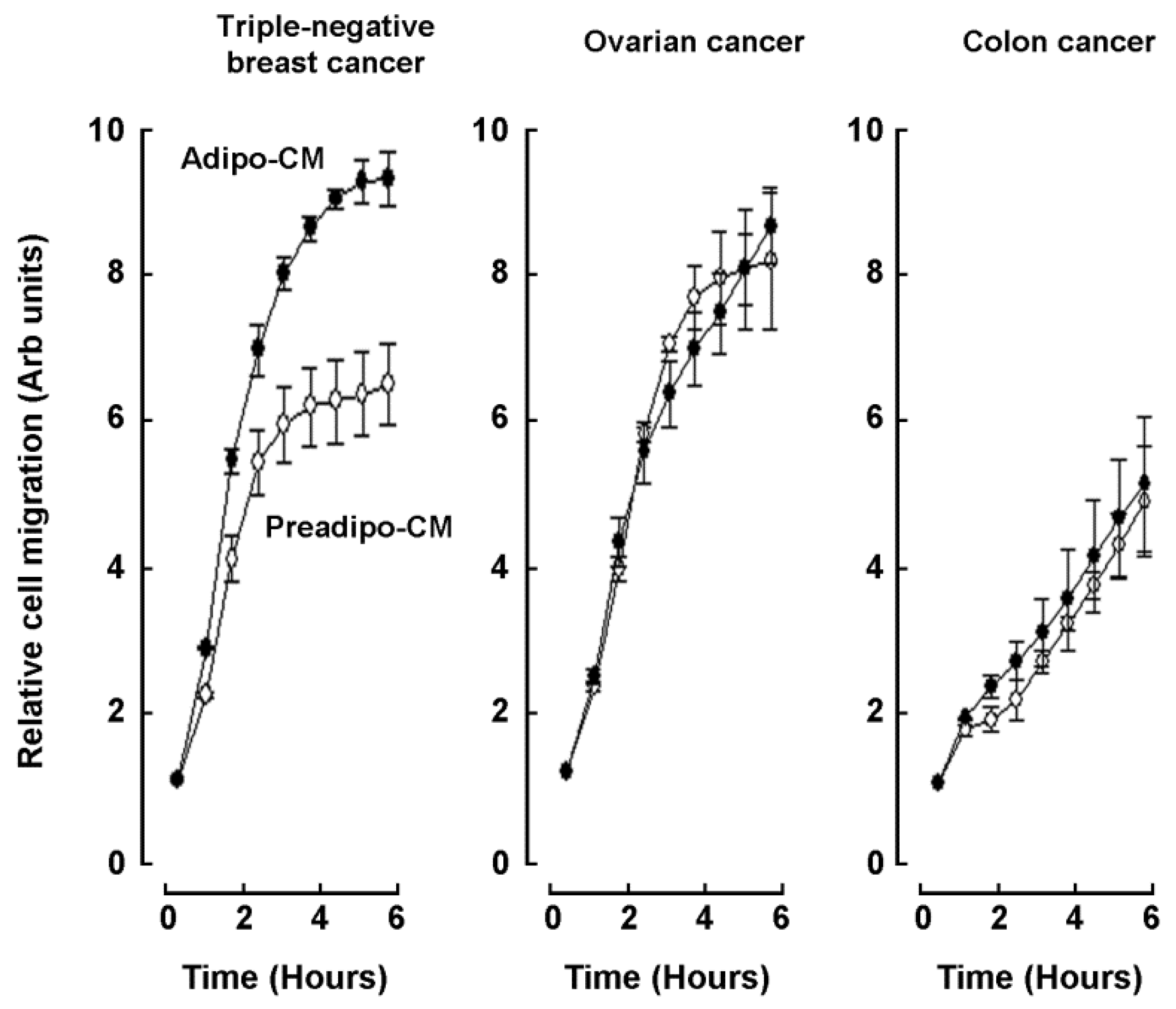
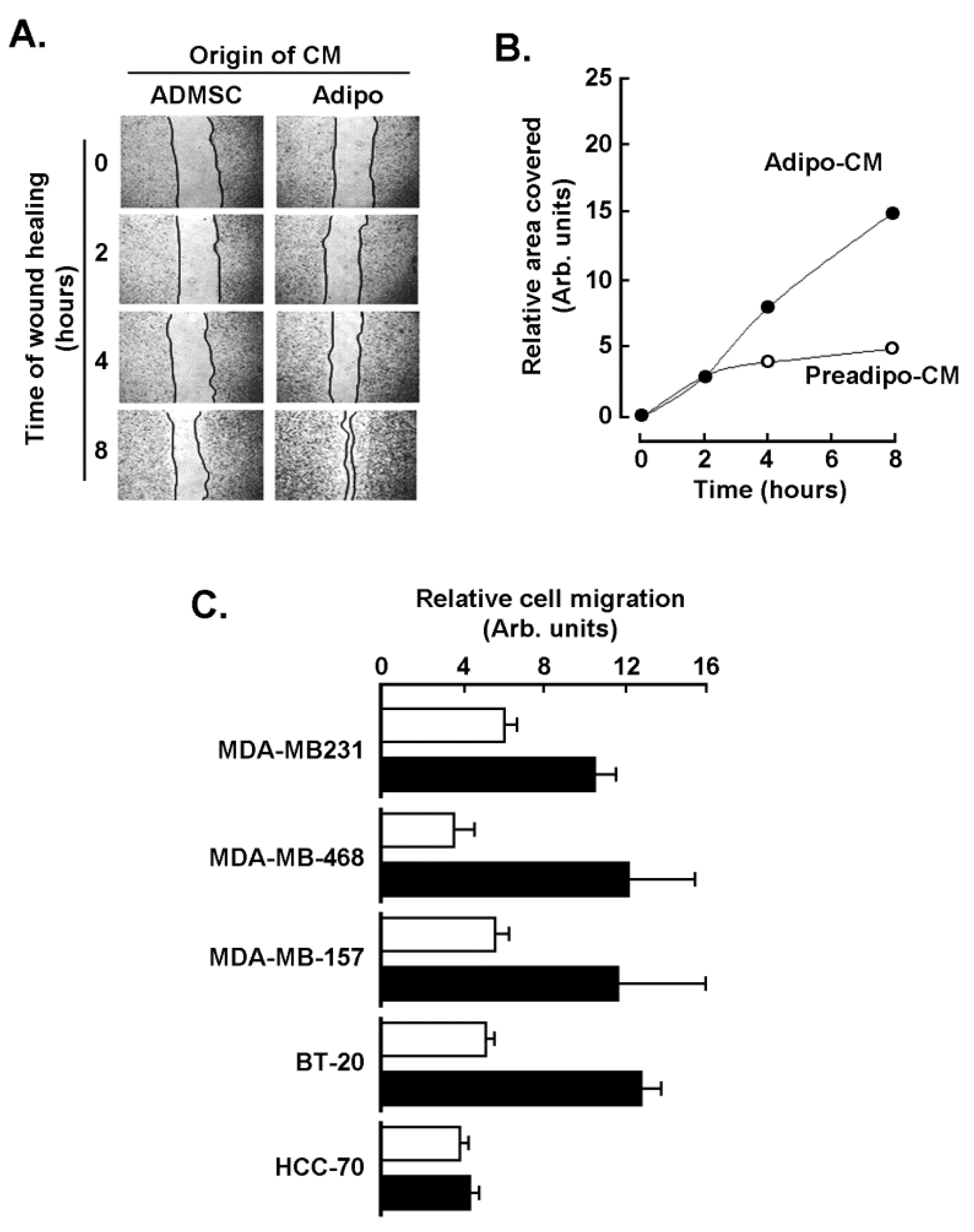
2.2. Adipocytes Secretome, but Not that of Adipose-Derived Mesenchymal Stem Cells, Triggers Increased TNBC-Derived Cell Migration
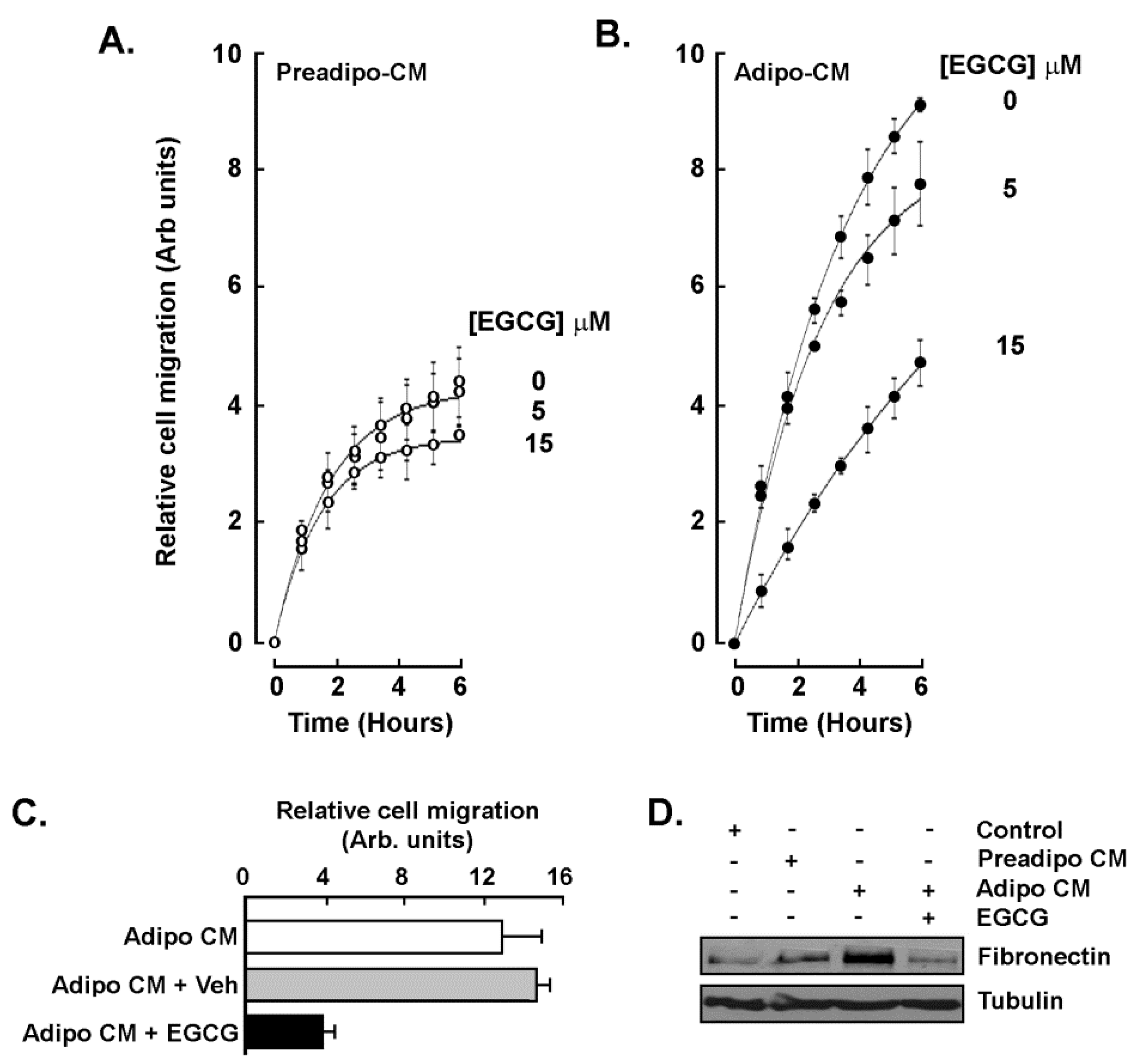
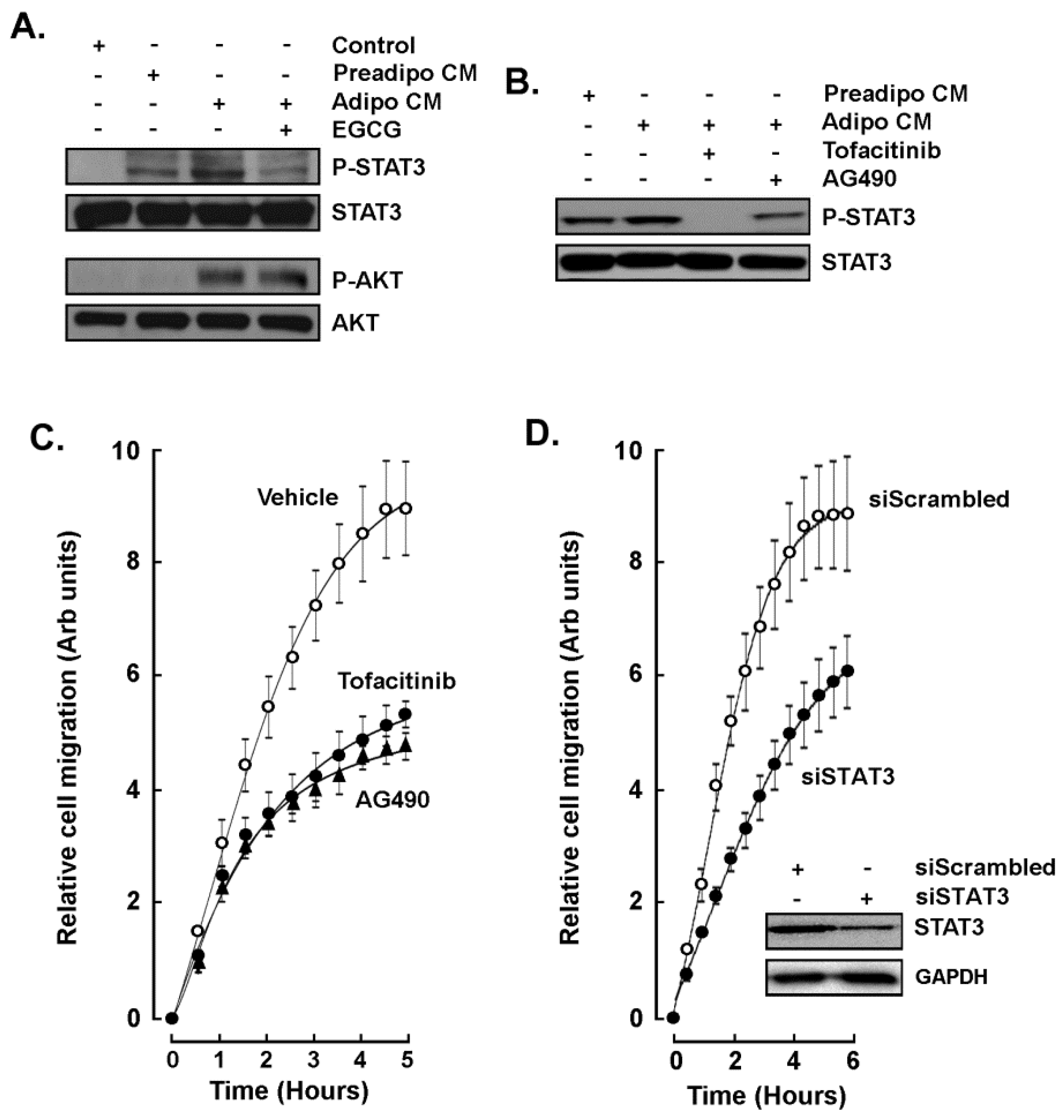
2.3. EGCG Inhibits Both the Acute Response and the Paracrine Regulation of TNBC Cell Chemotaxis Response to Mature Adipocytes Secretome
2.4. STAT3 Is Involved in the Paracrine Chemotaxis Response to Adipocytes Secretome
2.5. Adipocytes Secretome Triggers In Vitro 3D-Capillary-Like Structure Maturation, and STAT3 Inhibition Prevents such Maturation
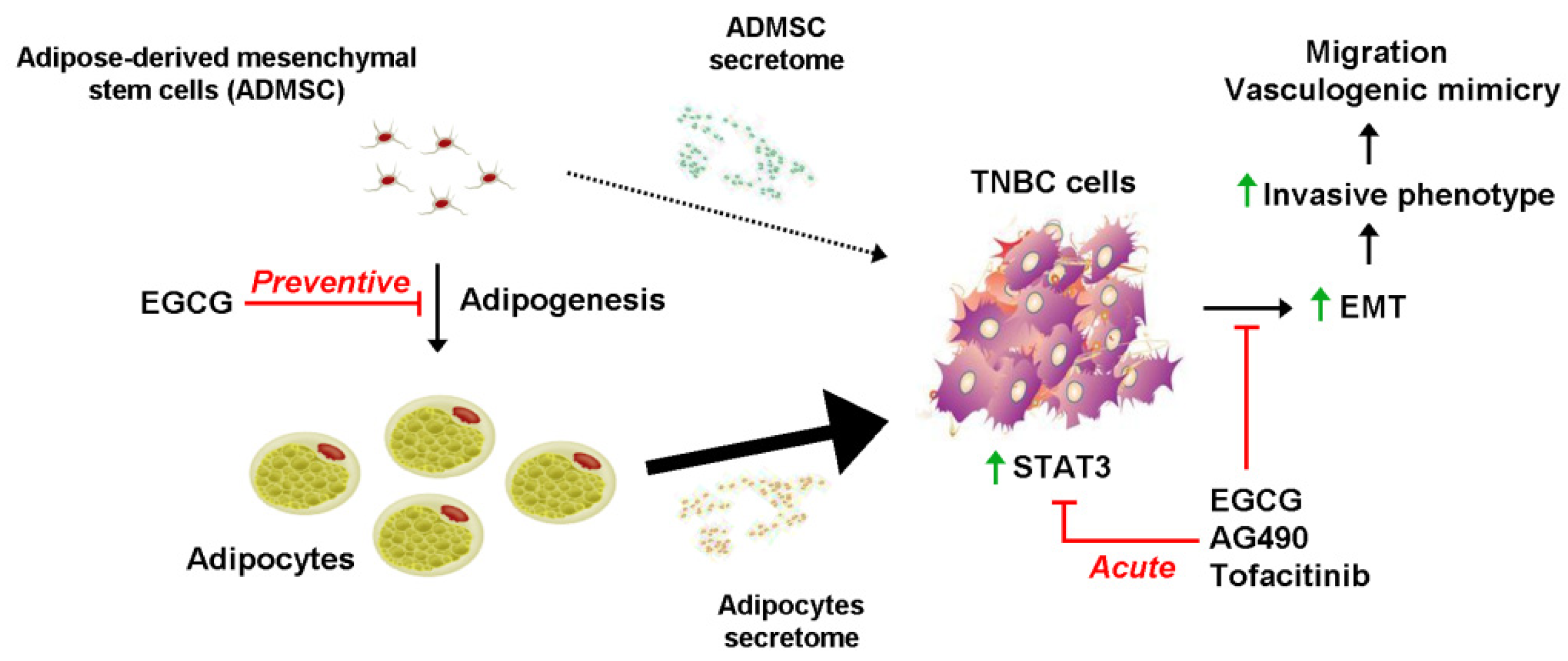
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Adipose-Derived Mesenchymal Stem Cell Differentiation
4.4. Oil Red O Staining
4.5. Total RNA Isolation, cDNA Synthesis and Real-Time Quantitative PCR
4.6. Human Adipogenesis and Inflammation PCR Arrays
4.7. Western Blotting
4.8. Chemotactic Cell Migration Assay
4.9. Wound-Healing Assay
4.10. In Vitro Vasculogenic Mimicry Assay
4.11. Statistical Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ADMSC | Adipose-derived mesenchymal stem cells |
| AKT | Protein kinase B |
| C/EBPα | CCAAT/enhancer-binding protein alpha |
| CM | Conditioned media |
| CSC | Cancer stem cells |
| EGCG | Epigallocatechin-3-gallate |
| EMT | Epithelial-mesenchymal transition |
| FABP4 | Fatty acid binding protein 4 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNBC | Triple negative breast cancer |
| VM | Vasculogenic mimicry |
References
- Dagpo, T.D.; Nolan, C.J.; Delghingaro-Augusto, V. Exploring therapeutic targets to reverse or prevent the transition from metabolically healthy to unhealthy obesity. Cells 2020, 9, 1596. [Google Scholar] [CrossRef]
- Heyn, G.S.; Corrêa, L.H.; Magalhães, K.G. The impact of adipose tissue-derived miRNAs in metabolic syndrome, obesity, and cancer. Front. Endocrinol. 2020, 11, 563816. [Google Scholar] [CrossRef]
- Himbert, C.; Delphan, M.; Scherer, D.; Bowers, L.W.; Hursting, S.; Ulrich, C.M. Signals from the adipose microenvironment and the obesity—Cancer link—A systematic review. Cancer Prev. Res. 2017, 10, 494–506. [Google Scholar] [CrossRef]
- Onzi, G.R.; Faccioni, J.L.; Pereira, L.C.; Thomé, M.P.; Bertoni, A.P.S.; Buss, J.H.; Fazolo, T.; Filippi-Chiela, E.; Wink, M.R.; Lenz, G. Adipose-derived stromal cell secretome disrupts autophagy in glioblastoma. J. Mol. Med. 2019, 97, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Sacca, P.A.; Mazza, O.N.; Scorticati, C.; Vitagliano, G.; Casas, G.; Calvo, J.C. Human periprostatic adipose tissue: Secretome from patients with prostate cancer or benign prostate hyperplasia. Cancer Genom. Proteom. 2019, 16, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Kokocha, A.; Bajek, A.; Drewa, T. The interplay between adipose-derived stem cells and bladder cancer cells. Sci. Rep. 2018, 8, 15118. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Nguyen, T.T.; Thanh, V.V.; Quang, T.L.; Minh, L.B.; Pham, V.H.; Ngoc, V.T.N.; et al. The effects of adipocytes on the regulation of breast cancer in the tumor microenvironment: An update. Cells 2019, 8, 857. [Google Scholar] [CrossRef]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef]
- Zimta, A.A.; Tigu, A.B.; Muntean, M.; Cenariu, D.; Slaby, O.; Berindan-Neagoe, I. Molecular links between central obesity and breast cancer. Int. J. Mol. Sci. 2019, 20, 5364. [Google Scholar] [CrossRef]
- Jasinski-Bergner, S.; Kielstein, H. Adipokines regulate the expression of tumor-relevant microRNAs. Obes. Facts 2019, 12, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Miran, I.; Scherer, D.; Ostyn, P.; Mazouni, C.; Drusch, F.; Bernard, M.; Louvet, E.; Adam, J.; Mathieu, M.C.; Haffa, M.; et al. Adipose tissue properties in tumor-bearing breasts. Front. Oncol. 2020, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Nawaz, A.; Evans, M. Drug therapy in obesity: A review of current and emerging treatments. Diabetes Ther. 2020, 11, 1199–1216. [Google Scholar] [CrossRef]
- Li, H.; Qi, J.; Li, L. Phytochemicals as potential candidates to combat obesity via adipose non-shivering thermogenesis. Pharmacol. Res. 2019, 147, 104393. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Lu, J.; Peng, C.; Lin, L. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Doseff, A.I. The targeted impact of flavones on obesity-induced inflammation and the potential synergistic role in cancer and the gut microbiota. Molecules 2020, 25, 2477. [Google Scholar] [CrossRef]
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective (The Third Expert Report). 2007. Available online: https://www.wcrf.org/dietandcancer (accessed on 13 February 2021).
- Andersen, C.; Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and adipogenesis. Biofactors 2010, 36, 415–422. [Google Scholar] [CrossRef]
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: From molecular to clinical studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and epigenomic regulation of adipogenesis. Mol. Cell Biol. 2019, 39, e00601–e00618. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Zeng, J.; Sauter, E.R.; Li, B. FABP4: A new player in obesity-associated breast cancer. Trends Mol. Med. 2020, 26, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Balanis, N.; Wendt, M.K.; Schiemann, B.J.; Wang, Z.; Schiemann, W.P.; Carlin, C.R. Epithelial to mesenchymal transition promotes breast cancer progression via a fibronectin-dependent STAT3 signaling pathway. J. Biol. Chem. 2013, 288, 17954–17967. [Google Scholar] [CrossRef]
- Balanis, N.; Carlin, C.R. Stress-induced EGF receptor signaling through STAT3 and tumor progression in triple-negative breast cancer. Mol. Cell. Endocrinol. 2017, 451, 24–30. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Kasembeli, M.M.; Robinson, P.; Tweardy, D.J. Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: Rationale, progress, and caution. Pharmacol. Rev. 2020, 72, 486–526, Erratum in 2020, 72, 605. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Liu, J.; Li, G.W.; Shen, J.X.; Huang, Y.T. The transcriptional STAT3 is a potential target, whereas transcriptional STAT5A/5B/6 are new biomarkers for prognosis in human breast carcinoma. Oncotarget 2017, 8, 36279–36288. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. 2019, 38, 195. [Google Scholar] [CrossRef]
- Lyons, T.G. Targeted therapies for triple-negative breast cancer. Curr. Treat. Options Oncol. 2019, 20, 82. [Google Scholar] [CrossRef]
- Jabbarzadeh Kaboli, P.; Salimian, F.; Aghapour, S.; Xiang, S.; Zhao, Q.; Li, M.; Wu, X.; Du, F.; Zhao, Y.; Shen, J.; et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—A comprehensive review from chemotherapy to immunotherapy. Pharmacol. Res. 2000, 156, 104806. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Tweardy, D.J. Targeting Stat3 in cancer therapy. Anticancer Drugs 2005, 16, 601–607. [Google Scholar] [CrossRef]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Javadian, M.; Babaloo, Z.; Baradaran, B. Janus kinase inhibitors: A therapeutic strategy for cancer and autoimmune diseases. J. Cell. Physiol. 2020, 235, 5903–5924. [Google Scholar] [CrossRef] [PubMed]
- Jitariu, A.A.; Cîmpean, A.M.; Ribatti, D.; Raica, M. Triple negative breast cancer: The kiss of death. Oncotarget 2017, 8, 46652–46662. [Google Scholar] [CrossRef] [PubMed]
- Avram, M.M.; Avram, A.S.; James, W.D. Subcutaneous fat in normal and diseased states 3. Adipogenesis: From stem cell to fat cell. J. Am. Acad. Dermatol. 2007, 56, 472–492. [Google Scholar] [CrossRef]
- Farmer, S.R. Regulation of PPARgamma activity during adipogenesis. Int. J. Obes. 2005, 29, S13–S16. [Google Scholar] [CrossRef] [PubMed]
- Harkins, J.M.; Moustaid-Moussa, N.; Chung, Y.J.; Penner, K.M.; Pestka, J.J.; North, C.M.; Claycombe, K.J. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J. Nutr. 2004, 134, 2673–2677. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef]
- Klöting, N.; Koch, L.; Wunderlich, T.; Kern, M.; Ruschke, K.; Krone, W.; Brüning, J.C.; Blüher, M. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 2008, 57, 2074–2082. [Google Scholar] [CrossRef]
- Lowenstein, C.J.; Padalko, E. iNOS (NOS2) at a glance. J. Cell Sci. 2004, 117, 2865–2867. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. 2001, 226, 997–1002. [Google Scholar] [CrossRef]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: Functions and therapeutic implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K.B. Targeting oncogenic transcription factors by polyphenols: A novel approach for cancer therapy. Pharmacol. Res. 2018, 130, 273–291. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, X.; Deng, C.; Yang, L.; Yan, E.; Guo, T.; Li, Y.; Xu, M.X. Mechanism of the inhibition of the STAT3 signaling pathway by EGCG. Oncol. Rep. 2013, 30, 2691–2696. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, K.; Xu, Y.; Peng, H.; Wang, Z.; Liu, S.; Zhang, G. (-)-Epigallocatechin-3-gallate induces cell apoptosis in chronic myeloid leukaemia by regulating Bcr/Abl-mediated p38-MAPK/JNK and JAK2/STAT3/AKT signalling pathways. Clin. Exp. Pharmacol. Physiol. 2019, 46, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Pinheiro, M.; Granja, A.; Farabegoli, F.; Reis, S.; Attar, R.; Sabitaliyevich, U.Y.; Xu, B.; Ahmad, A. EGCG mediated targeting of deregulated signaling pathways and non-coding RNAs in different cancers: Focus on JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL mediated signaling pathways. Cancers 2020, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Sharma, N.; Ghosh, M.; Park, Y.H.; Jeong, D.K. Emerging importance of dietary phytochemicals in fight against cancer: Role in targeting cancer stem cells. Crit. Rev. Food Sci. Nutr. 2017, 57, 3449–3463. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; McKee, D.L. The “Big Five” phytochemicals targeting cancer stem cells: Curcumin, EGCG, sulforaphane, resveratrol and genistein. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Verma, S.S.; Aggarwal, S.; Gupta, S.C. Drug repurposing for breast cancer therapy: Old weapon for new battle. Semin. Cancer Biol. 2019, 68, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ávalos-Moreno, M.; López-Tejada, A.; Blaya-Cánovas, J.L.; Cara-Lupiañez, F.E.; González-González, A.; Lorente, J.A.; Sánchez-Rovira, P.; Granados-Principal, S. Drug repurposing for triple-negative breast cancer. J. Pers. Med. 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Stewart, T.A.; Thompson, E.W.; Monteith, G.R. Targeting EMT in cancer: Opportunities for pharmacological intervention. Trends Pharmacol. Sci. 2014, 35, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin-gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef]
- Sicard, A.A.; Dao, T.; Suarez, N.G.; Annabi, B. Diet-derived gallated catechins prevent TGF-β-mediated epithelial-mesenchymal transition, cell migration and vasculogenic mimicry in chemosensitive ES-2 ovarian cancer cells. Nutr. Cancer 2021, 73, 169–180. [Google Scholar] [CrossRef]
- D’Esposito, V.; Ambrosio, M.R.; Giuliano, M.; Cabaro, S.; Miele, C.; Beguinot, F.; Formisano, P. Mammary adipose tissue control of breast cancer progression: Impact of obesity and diabetes. Front. Oncol. 2020, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef]
- Santander, A.M.; Lopez-Ocejo, O.; Casas, O.; Agostini, T.; Sanchez, L.; Lamas-Basulto, E.; Carrio, R.; Cleary, M.P.; Gonzlez-Perez, R.R.; Torroella-Kouri, M. Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: The role of obesity and inflammation in breast adipose tissue. Cancers 2015, 7, 143–178. [Google Scholar] [CrossRef]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a matter of fat: The crosstalk between adipose tissue and tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Yan, X.; Yan, F.; Sun, Y.; Zeng, J.; Waigel, S.; Yin, Y.; Fraig, M.M.; Egilmez, N.K.; et al. Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metab. 2018, 28, 689–705.e5. [Google Scholar] [CrossRef]
- Guaita-Esteruelas, S.; Saavedra-García, P.; Bosquet, A.; Borràs, J.; Girona, J.; Amiliano, K.; Rodríguez-Balada, M.; Heras, M.; Masana, L.; Gumà, J. Adipose-derived fatty acid-binding proteins plasma concentrations are increased in breast cancer patients. Oncologist 2017, 22, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Francisco Fernandez, M.; Charfi, C.; Piloto-Ferrer, J.; Lidia González, M.; Lamy, S.; Annabi, B. Targeting ovarian cancer cell cytotoxic drug resistance phenotype with Xanthium strumarium L. extract. Evid. Based Complement. Altern. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Fernández-González, R.; Muñoz-Barrutia, A.; Ortiz-De-Solorzano, C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010, 73, 1019–1029. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Suarez, N.; Rodriguez Torres, S.; Ouanouki, A.; El Cheikh-Hussein, L.; Annabi, B. EGCG Inhibits Adipose-Derived Mesenchymal Stem Cells Differentiation into Adipocytes and Prevents a STAT3-Mediated Paracrine Oncogenic Control of Triple-Negative Breast Cancer Cell Invasive Phenotype. Molecules 2021, 26, 1506. https://doi.org/10.3390/molecules26061506
Gonzalez Suarez N, Rodriguez Torres S, Ouanouki A, El Cheikh-Hussein L, Annabi B. EGCG Inhibits Adipose-Derived Mesenchymal Stem Cells Differentiation into Adipocytes and Prevents a STAT3-Mediated Paracrine Oncogenic Control of Triple-Negative Breast Cancer Cell Invasive Phenotype. Molecules. 2021; 26(6):1506. https://doi.org/10.3390/molecules26061506
Chicago/Turabian StyleGonzalez Suarez, Narjara, Sahily Rodriguez Torres, Amira Ouanouki, Layal El Cheikh-Hussein, and Borhane Annabi. 2021. "EGCG Inhibits Adipose-Derived Mesenchymal Stem Cells Differentiation into Adipocytes and Prevents a STAT3-Mediated Paracrine Oncogenic Control of Triple-Negative Breast Cancer Cell Invasive Phenotype" Molecules 26, no. 6: 1506. https://doi.org/10.3390/molecules26061506
APA StyleGonzalez Suarez, N., Rodriguez Torres, S., Ouanouki, A., El Cheikh-Hussein, L., & Annabi, B. (2021). EGCG Inhibits Adipose-Derived Mesenchymal Stem Cells Differentiation into Adipocytes and Prevents a STAT3-Mediated Paracrine Oncogenic Control of Triple-Negative Breast Cancer Cell Invasive Phenotype. Molecules, 26(6), 1506. https://doi.org/10.3390/molecules26061506





