On the Development of Selective Chelators for Cadmium: Synthesis, Structure and Chelating Properties of 3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)benzo[d]isothiazole 1,1-dioxide, a Novel Thiadiazolyl Saccharinate
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure Analysis
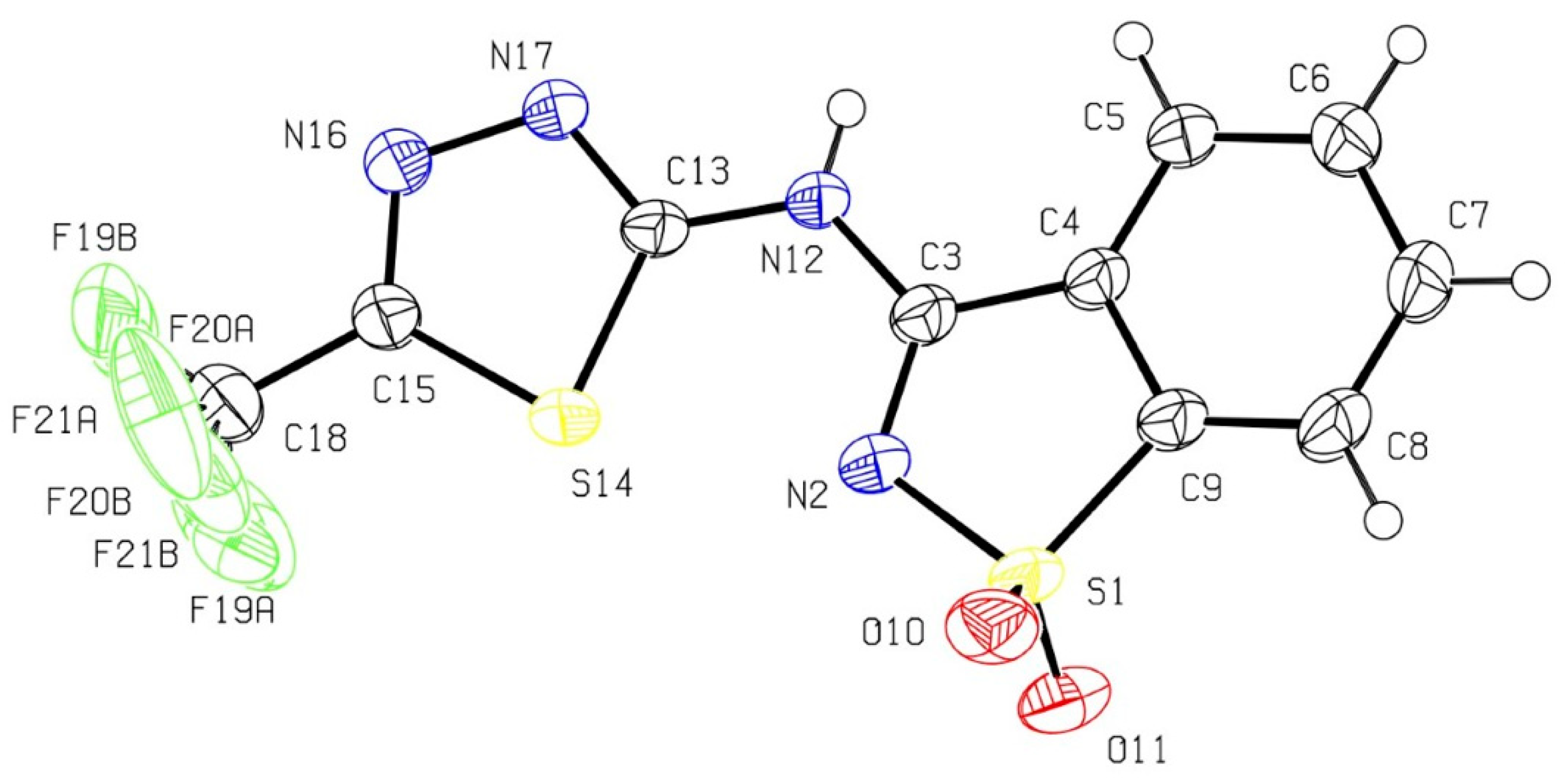
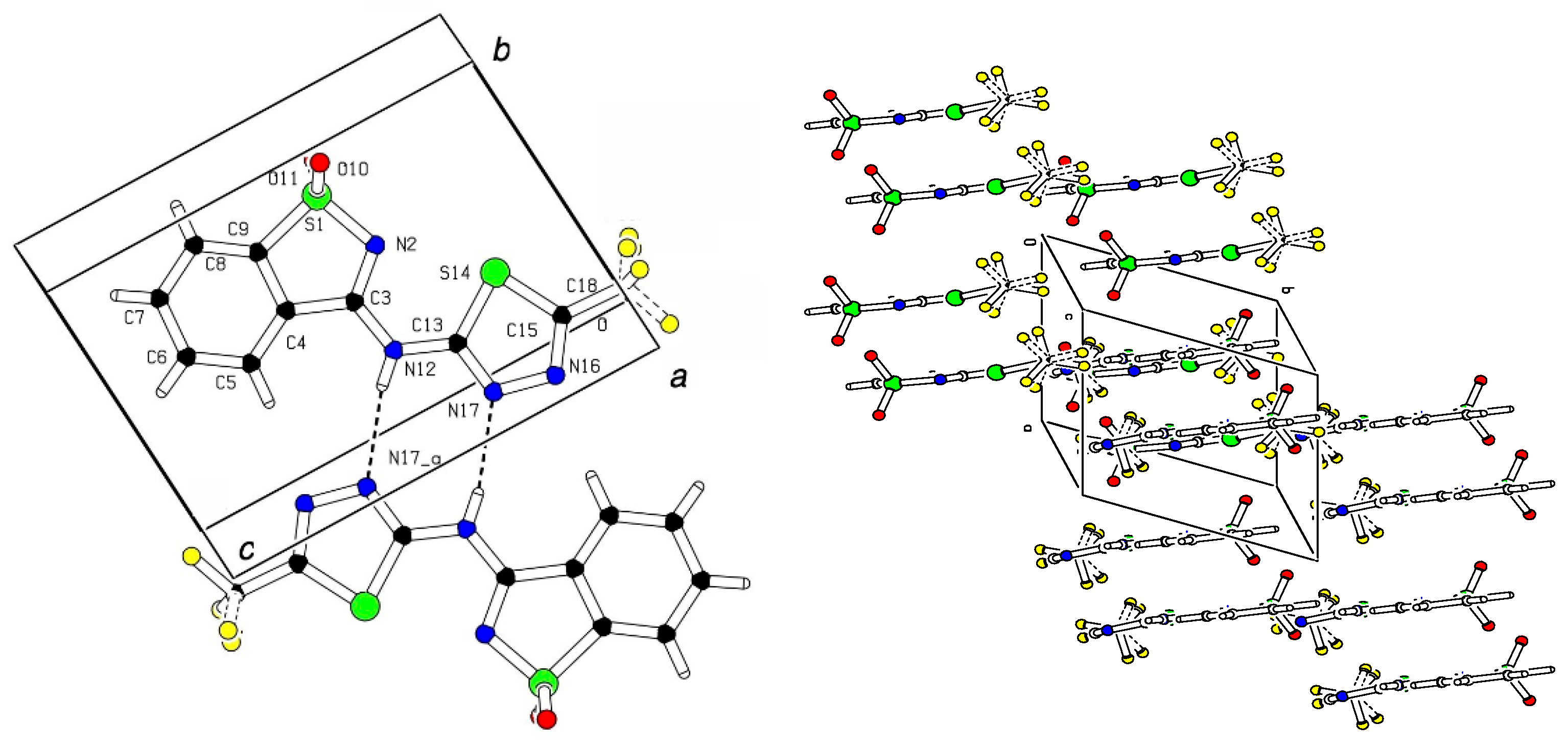
2.2. X-ray Crystal Analysis
2.3. Metal Chelating Activities
2.4. UV-vis Titrations
3. Materials and Methods
3.1. Chemicals
3.2. Equipment
3.3. Synthesis Procedure
3.4. X-ray Crystallography
3.5. Metal Chelating Activity
3.6. Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, X.; Liu, L.; Wang, Y.; Luo, G.; Chen, X.; Yang, X.; Gao, B.; He, X. Integrated Assessment of Heavy Metal Contamination in Sediments from a Coastal Industrial Basin, NE China. PLoS ONE 2012, 7, e39690. [Google Scholar] [CrossRef] [PubMed]
- Cotté-Krief, M.H.; Guieu, C.; Thomas, A.J.; Martin, J.M. Sources of Cd, Cu, Ni and Zn in Portuguese Coastal Waters. Mar. Chem. 2000, 71, 199–214. [Google Scholar] [CrossRef]
- Micheline, G.; Rachida, C.; Céline, M.; Gaby, K.; Rachid, A.; Petru, J. Levels of Pb, Cd, Hg and As in Fishery Products from the Eastern Mediterranean and Human Health Risk Assessment Due to Their Consumption. Int. J. Environ. Res. 2019, 13, 443–455. [Google Scholar] [CrossRef]
- Diop, M.; Howsam, M.; Diop, C.; Goossens, J.F.; Diouf, A.; Amara, R. Assessment of Trace Element Contamination and Bioaccumulation in Algae (Ulva Lactuca), Mussels (Perna Perna), Shrimp (Penaeus Kerathurus), and Fish (Mugil Cephalus, Saratherondon Melanotheron) along the Senegalese Coast. Mar. Pollut. Bull. 2016, 103, 339–343. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Caeiro, S.; Vaz-Fernandes, P.; Martinho, A.P.; Costa, P.M.; Silva, M.J.; Lavinha, J.; Matias-Dias, C.; Machado, A.; Castanheira, I.; Costa, M.H. Environmental Risk Assessment in a Contaminated Estuary: An Integrated Weight of Evidence Approach as a Decision Support Tool. Ocean Coast. Manag. 2017, 143, 51–62. [Google Scholar] [CrossRef]
- Caeiro, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Medeiros, G.; Painho, M. Assessing Heavy Metal Contamination in Sado Estuary Sediment: An Index Analysis Approach. Ecol. Indic. 2005, 5, 151–169. [Google Scholar] [CrossRef]
- Moberg, C.; Muhammed, M.; Svensson, G.; Weber, M. Novel Quinaldic Acids for Selective Chelation of Cadmium(II). X-Ray Crystal Structure of [Cd(C18H12NO4)2](Me2SO)·2H2O. J. Chem. Soc. Chem. Commun. 1988, 810–812. [Google Scholar] [CrossRef]
- Xiao, L.; Wildgoose, G.G.; Crossley, A.; Knight, R.; Jones, J.H.; Compton, R.G. Removal of Toxic Metal-Ion Pollutants from Water by Using Chemically Modified Carbon Powders. Chem. Asian J. 2006, 1, 614–622. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, B.; Pan, B.; Zhang, W.; Jia, K.; Zhang, Q. Selective Sorption of Lead, Cadmium and Zinc Ions by a Polymeric Cation Exchanger Containing Nano-Zr(HPO3S)2. Environ. Sci. Technol. 2008, 42, 4140–4145. [Google Scholar] [CrossRef] [PubMed]
- Merrikhpour, H.; Jalali, M. Comparative and Competitive Adsorption of Cadmium, Copper, Nickel, and Lead Ions by Iranian Natural Zeolite. Clean Technol. Environ. Policy 2013, 15, 303–316. [Google Scholar] [CrossRef]
- Choudhary, S.; Sar, P. Characterization of a Metal Resistant Pseudomonas Sp. Isolated from Uranium Mine for Its Potential in Heavy Metal (Ni2+, Co2+, Cu2+, and Cd2+) Sequestration. Bioresour. Technol. 2009, 100, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, S.; Gianguzza, A.; Pettignano, A.; Piazzese, D.; Sammartano, S. Complex Formation of Copper(II) and Cadmium(II) with Pectin and Polygalacturonic Acid in Aqueous Solution. An ISE-H+ and ISE-Me2+ Electrochemical Study. Int. J. Electrochem. Sci. 2012, 7, 6722–6737. [Google Scholar]
- Huang, W.; Liu, Z.M. Biosorption of Cd(II)/Pb(II) from Aqueous Solution by Biosurfactant-Producing Bacteria: Isotherm Kinetic Characteristic and Mechanism Studies. Colloids Surf. B 2013, 105, 113–119. [Google Scholar] [CrossRef]
- Isaac, P.J.; Amaravadi, S.; Kamil, M.S.M.; Cheralathan, K.K.; Lakshmipathy, R. Synthesis of Zeolite/Activated Carbon Composite Material for the Removal of Lead (II) and Cadmium (II) Ions. Environ. Prog. Sustain. Energy 2019, 38, e13246. [Google Scholar] [CrossRef]
- Wan, S.; Qiu, L.; Tang, G.; Chen, W.; Li, Y.; Gao, B.; He, F. Ultrafast Sequestration of Cadmium and Lead from Water by Manganese Oxide Supported on a Macro-Mesoporous Biochar. Chem. Eng. J. 2020, 387, 124095. [Google Scholar] [CrossRef]
- Ismael, A.; Henriques, M.S.C.; Marques, C.; Rodrigues, M.; Barreira, L.; Paixao, J.A.; Fausto, R.; Cristiano, M.L.S. Exploring Saccharinate-Tetrazoles as Selective Cu(II) Ligands: Structure, Magnetic Properties and Cytotoxicity of Copper(II) Complexes Based on 5-(3-Aminosaccharyl)-Tetrazoles. RSC Adv. 2016, 6, 71628–71637. [Google Scholar] [CrossRef]
- Ismael, A.; Borba, A.; Henriques, M.S.C.; Paixão, J.A.; Fausto, R.; Cristiano, M.L.S. Structure and Photochemistry of a Saccharyl Thiotetrazole. J. Org. Chem. 2015, 80, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.I.L.; Brás, E.M.; Henriques, M.S.C.; Marques, C.; Frija, L.M.T.; Barreira, L.; Paixão, J.A.; Fausto, R.; Cristiano, M.L.S. Synthesis, Structure, and Cytotoxicity of a New Sulphanyl-Bridged Thiadiazolyl-Saccharinate Conjugate: The Relevance of S⋅⋅⋅N Interaction. Chem. Eur. J. 2018, 24, 3251–3262. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Fausto, R.; Loureiro, R.M.S.; Cristiano, M.L.S. Synthesis and Structure of Novel Benzisothiazole-Tetrazolyl Derivatives for Potential Application as Nitrogen Ligands. J. Mol. Catal. A Chem. 2009, 305, 142–146. [Google Scholar] [CrossRef]
- Ismael, A.; Abe, M.; Fausto, R.; Cristiano, M.L.S. Insights into the Photochemistry of 5-Aminotetrazole Derivatives with Applications in Coordination Chemistry. Effect of the Saccharyl Moiety on the Photostability. Pure Appl. Chem. 2020, 92, 49–62. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Cristiano, M.L.S.; Gómez-Zavaglia, A.; Reva, I.; Fausto, R. Genesis of Rare Molecules Using Light-Induced Reactions of Matrix-Isolated Tetrazoles. J. Photochem. Photobiol. C 2014, 18, 71–90. [Google Scholar] [CrossRef]
- Zhang, Q.; Teng, J.; Zhang, Z.; Nie, G.; Zhao, H.; Peng, Q.; Jiao, T. Unique and Outstanding Cadmium Sequestration by Polystyrene-Supported Nanosized Zirconium Hydroxides: A Case Study. RSC Adv. 2015, 5, 55445–55452. [Google Scholar] [CrossRef]
- Mu, G.N.; Tang, L.B. Adsorption of Cd(II) Ion and Its Complex Compounds from Solution on the Surface of Charcoal Treated with an Oxidation-Negative Ionizing Method. J. Colloid Interface Sci. 2002, 247, 504–506. [Google Scholar] [CrossRef]
- Llamas, N.E.; di Nezio, M.S.; Palomeque, M.E.; Fernández Band, B.S. Direct Determination of Saccharin and Acesulfame-K in Sweeteners and Fruit Juices Powders. Food Anal. Methods 2008, 1, 43–48. [Google Scholar] [CrossRef]
- Fernandes, V.N.O.; Fernandes, L.B.; Vasconcellos, J.P.; Jager, A.V.; Tonin, F.G.; Leal De Oliveira, M.A. Simultaneous Analysis of Aspartame, Cyclamate, Saccharin and Acesulfame-K by CZE under UV Detection. Anal. Methods 2013, 5, 1524–1532. [Google Scholar] [CrossRef]
- Leal, J.F.; Esteves, V.I.; Santos, E.B.H. Solar Photodegradation of Oxytetracycline in Brackish Aquaculture Water: New Insights about Effects of Ca2+ and Mg2+. J. Photochem. Photobiol. A Chem. 2019, 372, 218–225. [Google Scholar] [CrossRef]
- Ismael, A.; Borba, A.; Duarte, L.; Giuliano, B.M.; Gómez-Zavaglia, A.; Cristiano, M.L.S. Structure and Photochemistry of a Novel Tetrazole-Saccharyl Conjugate Isolated in Solid Argon. J. Mol. Struct. 2012, 1025, 105–116. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Megías, C.; Pastor-Cavada, E.; Torres-Fuentes, C.; Girón-Calle, J.; Alaiz, M.; Juan, R.; Pastor, J.; Vioque, J. Chelating, Antioxidant and Antiproliferative Activity of Vicia Sativa Polyphenol Extracts. Eur. Food Res. Technol. 2009, 230, 353–359. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Chowdhury, M.T.I. A Simple Spectrophotometric Method for the Determination of Cadmium in Industrial, Environmental, Biological and Soil Samples Using 5,7-Dibromo-8-Hydroxyquinoline. Anal. Sci. 2004, 20, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX3; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Bruker. SAINT V8.38A; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]

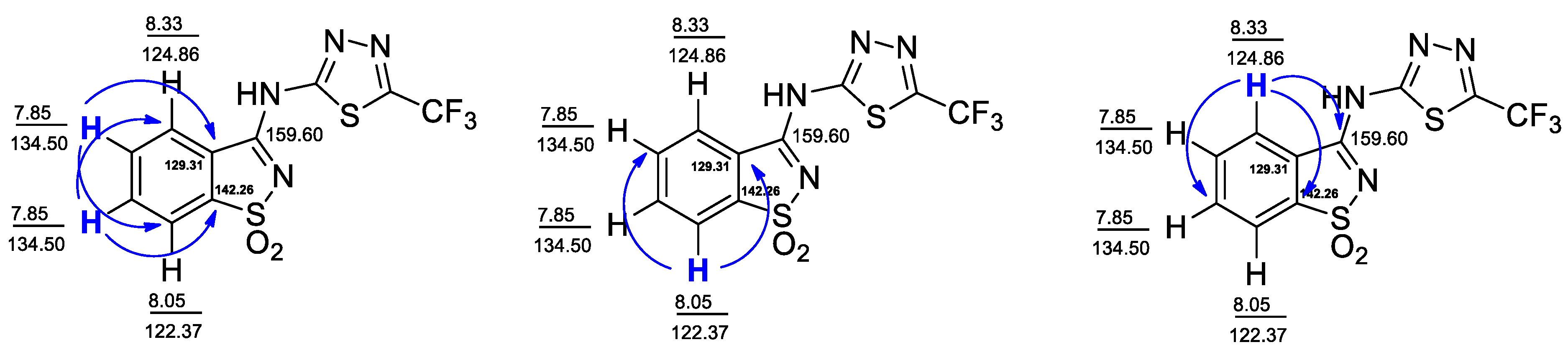


| 1H, δ (ppm) | 1H, δ (ppm) TOCSY | HSQC | 13C, δ (ppm) HMBC |
|---|---|---|---|
| 7.86 | 8.05; 8.33 | 134.50 | 122.23; 124.92; 129.31; 134.44; 142.26 |
| 8.05 | 7.86; 8.33 | 122.37 | 129.31; 134.20; 142.26 |
| 8.33 | 7.86; 8.05 | 124.86 | 129.07; 134.69; 142,26; 159.60 |
| Concentration (mM). | Cd(II) | Cu(II) | Fe(II) |
|---|---|---|---|
| 1 | 14.7 ± 6.7 | NA | NA |
| 5 | 39.8 ± 3.1 | 17.7 ± 6.2 | NA |
| 10 | 58.7 ± 4.2 | 29.2 ± 5.8 | NA |
| 15 | 64.0 ± 4.2 | - | - |
| 20 | 69.0 ± 3.3 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, J.F.; Guerreiro, B.; Amado, P.S.M.; Fernandes, A.L.; Barreira, L.; Paixão, J.A.; Cristiano, M.L.S. On the Development of Selective Chelators for Cadmium: Synthesis, Structure and Chelating Properties of 3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)benzo[d]isothiazole 1,1-dioxide, a Novel Thiadiazolyl Saccharinate. Molecules 2021, 26, 1501. https://doi.org/10.3390/molecules26061501
Leal JF, Guerreiro B, Amado PSM, Fernandes AL, Barreira L, Paixão JA, Cristiano MLS. On the Development of Selective Chelators for Cadmium: Synthesis, Structure and Chelating Properties of 3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)benzo[d]isothiazole 1,1-dioxide, a Novel Thiadiazolyl Saccharinate. Molecules. 2021; 26(6):1501. https://doi.org/10.3390/molecules26061501
Chicago/Turabian StyleLeal, Joana F., Bruno Guerreiro, Patrícia S. M. Amado, André L. Fernandes, Luísa Barreira, José A. Paixão, and Maria L. S. Cristiano. 2021. "On the Development of Selective Chelators for Cadmium: Synthesis, Structure and Chelating Properties of 3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)benzo[d]isothiazole 1,1-dioxide, a Novel Thiadiazolyl Saccharinate" Molecules 26, no. 6: 1501. https://doi.org/10.3390/molecules26061501
APA StyleLeal, J. F., Guerreiro, B., Amado, P. S. M., Fernandes, A. L., Barreira, L., Paixão, J. A., & Cristiano, M. L. S. (2021). On the Development of Selective Chelators for Cadmium: Synthesis, Structure and Chelating Properties of 3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)benzo[d]isothiazole 1,1-dioxide, a Novel Thiadiazolyl Saccharinate. Molecules, 26(6), 1501. https://doi.org/10.3390/molecules26061501









