Abstract
The leaves of Carica papaya (CP) are rich in natural antioxidants. Carica papaya has traditionally been used to treat various ailments, including skin diseases. This study aims to decipher the antioxidant effects and phytochemical content of different CP leaf extracts (CPEs) obtained using supercritical carbon dioxide (scCO2) and conventional extraction methods. The antioxidant activities of CPEs were evaluated by cell-free (1,1-diphenyl-2-picryl-hydrazyl (DPPH) and ferric-reduced antioxidative power (FRAP)) and cell-based (H2O2) assay. Both C. papaya leaf scCO2 extract with 5% ethanol (CPSCE) and C. papaya leaf scCO2 extract (CPSC) exhibited stronger DPPH radical scavenging activity than conventional extracts. In the FRAP assay, two hydrophilic extracts (C. papaya leaf ethanol extract (CPEE) and C. papaya freeze-dried leaf juice (CPFD)) showed relatively stronger reducing power compared to lipophilic extracts. Cell-based assays showed that CPFD significantly protected skin fibroblasts from H2O2-induced oxidative stress in both pre-and post-treatment. CPEE protected skin fibroblasts from oxidative stress in a dose-dependent manner while CPSCE significantly triggered the fibroblast recovery after treatment with H2O2. GC-MS analysis indicated that CPSCE had the highest α-tocopherol and squalene contents. By contrast, both CP hydrophilic extracts (CPEE and CPFD) had a higher total phenolic content (TPC) and rutin content than the lipophilic extracts. Overall, CPEs extracted using green and conventional extraction methods showed antioxidative potential in both cell-based and cell-free assays due to their lipophilic and hydrophilic antioxidants, respectively.
1. Introduction
The skin is the largest organ in the body and comprises the epidermis and dermis, which are formed by keratinocytes and fibroblasts [1]. The primary function of skin is to provide a protective barrier to prevent hazardous materials and pathogens from entering the body [2]. As the outermost layer of the body, the skin is directly exposed to environmental hazards and pollutants which are themselves oxidants or able to catalyze the production of reactive oxygen species (ROS) [3]. Reactive oxygen species (ROS) are a group of molecules which consist of free radicals (superoxide anion (O2•−), hydroxyl radical (OH−), and non-radical oxidants (hydrogen peroxide (H2O2)) [4]. Their sources can be varied and include environmental pollutants, UV radiation, and byproducts from cellular metabolism. ROS can be produced physiologically through the leaking of the electron from the mitochondria during oxidative respiration and as a byproduct of numerous enzymatic reactions such as those of NADPH oxidases [4]. At low levels, ROS act as a redox signaling messenger, regulating the physiological functions of the cells through activation of several antioxidant enzymes such as catalase, glutathione peroxidase, and superoxide dismutase [5]. However, imbalances in the production and removal of ROS cause oxidative stress, which is deleterious to the cells. ROS cause damage to the cell membrane through lipid peroxidation, mitochondrial dysfunction, and alterations in DNA and RNA structures, leading to apoptosis and cellular death [6]. Oxidative stress is a hallmark of many phenomena such as skin ageing, neurodegenerative diseases, and diabetic kidney [7,8,9]. Thus, maintaining the homeostasis between the oxidation and reduction of ROS is important in preventing diseases.
Carica papaya (CP) belongs to the family Caricaseae and is a perennial tree which is planted domestically for fruit production. Besides the fruit (which is used as a delicacy worldwide), the leaves have traditionally been used in treating parasitic worms, gastric digestion problems, fever, and burns, and for the relief of asthma [10,11,12]. Pharmacological studies have revealed that the leaves display antioxidant, anti-inflammatory, antiviral, antitumor, and antibacterial activities [13]. Our previous study showed that C. papaya leaf supercritical carbon dioxide (scCO2) extract (CPSC) and its constitutive phytosterols were cytotoxic towards squamous carcinoma cells (SCC25), a widely used model for skin cancer [14]. However, the effects of CPSC on non-cancerous skin cells are yet to be explored. In contrast, a study on human skin fibroblasts (HSF1184) revealed the wound-healing properties of CP leaf methanolic extract [15]. Besides the leaves, other parts of CP such as the seeds, latex and epicarp also exhibited wound-healing properties in in vitro and in vivo studies [16,17,18]. The exact mechanisms of CP’s wound healing potential are still obscure; however, diminished oxidative stress may play a role [19,20]. The leaves contain multiple bioactive components such as manghaslin, clitorin, rutin, nicotiflorin, papain, chymopapain, cystatin, α-tocopherol, ρ-coumaric acid, and caffeic acid that may contribute to the defense against oxidative stress [10,21,22].
In the pursuit of finding the best mixture of bioactive compounds through the extraction process, CP leaf extracts (CPEs) obtained from several extraction methods were suggested for testing in a targeted bioassay [14]. Conventional (maceration, soxhlet, juicing, and sonication) and non-conventional scCO2 green extraction methods have previously been employed to extract bioactive compounds from CP leaves [21,23,24,25]. The advantages of non-conventional scCO2 extraction include the need for little or no organic solvent, non-toxicity, and the resulting lack of solvent residues, making this extraction technique environmentally friendly [26]. However, scCO2 is only beneficial for the extraction of small non-polar molecules, while polar molecules remain unextracted. The incorporation of co-solvents such as ethanol or water during scCO2 extraction can resolve this problem and result in inclusion of polar molecules, thus enhancing extraction range of targeted bioactive compounds [26].
In this study, the phytochemical profiling of CPEs extracted by conventional and non-conventional green extraction was performed using GCMS and HPLC analysis. Following this, the antioxidant potential of CPEs was evaluated using cell-free and cell-based assays for the first time. Specifically, the cytoprotective effect of CPEs in non-cancerous Hs27 skin fibroblast cell lines was assessed under oxidative stress conditions.
2. Results
2.1. GC-MS Analysis of Lipophilic Constituents in CP Leaf Extracts
All CP leaf extracts (5 mg/mL) resulting from both supercritical carbon dioxide and conventional extraction methods were evaluated for their lipophilic constituents using GC-MS. The details of the identified compounds are provided in Table 1. In general, CPSC and C. papaya leaf scCO2 extract with 5% ethanol (CPSCE) were found to contain a greater variety and number of lipophilic constituents as compared to the conventional leaf extracts (C. papaya leaf hexane extract (CPHE), C. papaya leaf ethanol extract (CPEE), and C. papaya leaf juice freeze-dried extract (CPFD)). CPSC and CPSCE were rich in lipophilic constituents such as essential fatty acids, phytosterols, and triterpenes (Figure 1a). By contrast, the conventional leaf extracts (CPHE, CPEE, and CPFD) contained relatively fewer constituents (Figure 1b). A total of 11 lipophilic constituents were identified in the CPSCE extract (Figure 1a). The identified major constituents were α-tocopherol (5), squalene (3), phytol (2), and a mixture of phytosterols such as campesterol (6), stigmasterol (7), and β-sitosterol (8), consistent with those reported in our previous study (Figure 2) [27]. A number of minor constituents such as hexadecanoic acid (1), γ-tocopherol (4), olean-12-ene (9), 13,17-cycloursan-3-one (10), and cycloartenol (11) were also detected in the CPSC extract. Interestingly, greater constituent concentration was observed in the CPSCE extract, which was extracted with supercritical fluid and a co-solvent (ethanol (5% v/v)). The extraction yields of the major constituents—(3) and (5)—in CPSCE were found to be 2- and 5-fold higher than those of the CPSC extract. Both CPSC and CPSCE extracts contained higher amounts and a greater variety of phytol (2), triterpenes (9, 10), and phytosterols (6, 7, 8, 11) compared to the conventional solvent extracts (CPHE, CPE, and CPFD), indicating the efficiency of supercritical fluids in extracting lipophilic constituents. In summary, the strength of extraction methods in extracting lipophilic constituents was as follows: CPSCE > CPSC > CPHE > CPEE > CPFD. One component that was found to be exclusive to CPEE extract was carpaine (12), a major papaya alkaloid [28].

Table 1.
Relative amounts of lipophilic constituents in CP extracts.
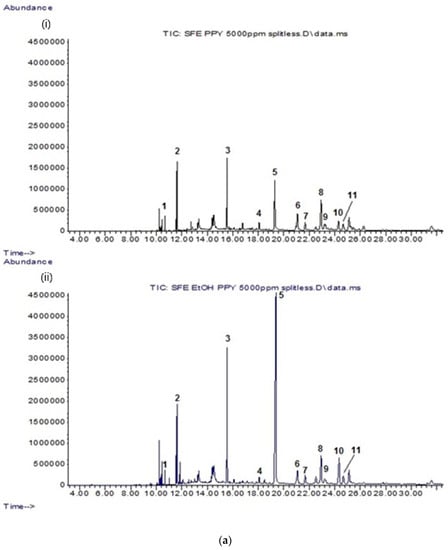
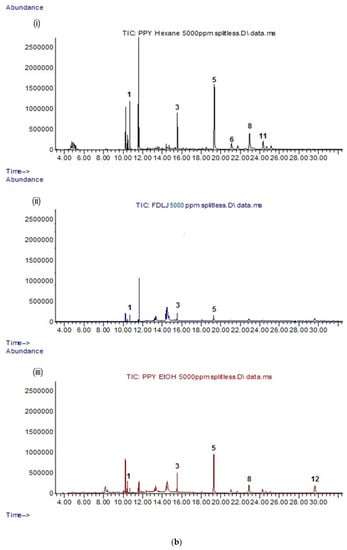
Figure 1.
(a) Total ion chromatograms (TIC) of (i) CPSC extract; (ii) CPSCE extract at a fixed concentration of 5 mg/mL. The identified compounds (1–11) are listed in Table 1. (b) TIC chromatograms of conventional solvent extracts: (i) CPHE extract; (ii) CPFD extract; (iii) CPEE extract at a fixed concentration of 5 mg/mL. The identified compounds (1–12) are listed in Table 1. CPHE: Carica papaya leaf hexane extract; CPFD: C. papaya leaf juice freeze-dried extract; CPEE: C. papaya leaf ethanol extract; CPSC: C. papaya leaf scCO2 extract; CPSCE: C. papaya leaf scCO2 extract with 5% ethanol.
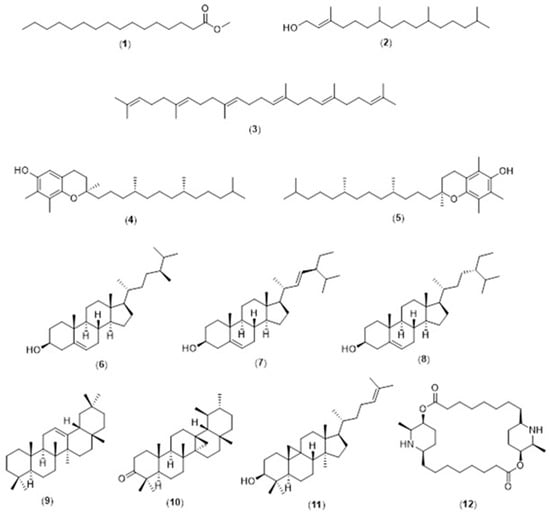
Figure 2.
Chemical structures of identified lipophilic compounds (1–12) in Carica papaya (CP) extracts. The identified compounds are listed in Table 1.
2.2. HPLC Analysis of Hydrophilic Constituents in CP Leaf Extracts
All the CPEs were subjected to HPLC-DAD analysis at a fixed concentration of 1 mg/mL following the programmed gradient method as described in Section 4.4. (b). Rutin, a major flavonoid found in CP leaves, was used as the chemical marker for quantification purposes [21,29]. However, only hydrophilic extracts (CPFD and CPEE) showed the separation (presence) of phenolic constituents in HPLC. The HPLC chromatograms for CPEE and CPFD extracts and rutin are given in Figure 3. The chromatographic peak of rutin in both extracts was identified by comparing its retention time, 14.27 min, with that of the rutin standard. The developed method was found to be selective due to its ability to separate rutin from other phytochemicals, and the targeted peak was free from interferences in the tested extract based on the UV spectrum (Figure 3). A linear calibration curve of rutin standard was established between 1.56 and 25.0 μg/mL, with a mean equation of:
and a correlation coefficient (R2) of 0.9996. CPEE extract was found to contain 22.07 ± 0.93 mg of rutin (per g of dry extract), which was approximately 3-fold higher than that of the CPFD extract (8.58 ± 0.12 mg per g of dry extract).
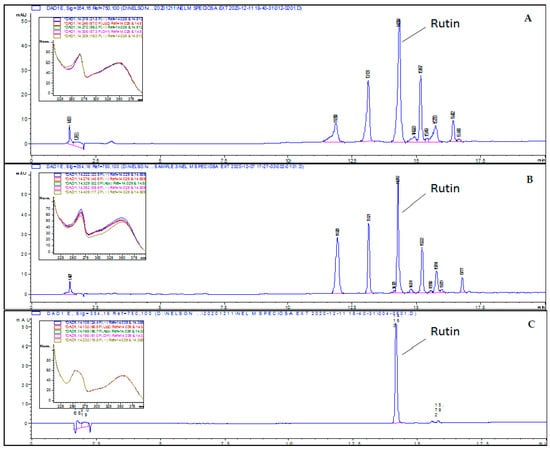
Figure 3.
HPLC chromatogram of: (A) CPEE extract, (B) CPFD extract, and (C) the rutin standard at 14.2 min.
2.3. Antioxidant Activity of CP Leaf Extract
- (a)
- 1,1-diphenyl-2-picryl-hydrazyl (DPPH) scavenging activity
To assess the free radical-scavenging ability of CP leaf extracts, DPPH assays were performed. Table 2 shows DPPH scavenging of CPEs. All CPEs demonstrated DPPH scavenging activity, with 50% inhibitory concentration (IC50) values ranging from 69.05 to 459.86 μg/mL. Among the CPEs, CPSCE had the most promising DPPH free radical scavenging activity, which was comparable with that of the standard compound butylated hydroxytoluene. On the contrary, CPHE had the lowest DPPH scavenging activity (6.6-fold lower than that of CPSCE).

Table 2.
50% inhibitory concentration (IC50) of DPPH scavenging activity of CPE.
- (b)
- Total phenolic content (TPC)
The antioxidant properties for natural products are largely attributed to their total phenolic contents. Spectrophotometric assays were therefore employed to assess TPC levels. The TPC levels for CPEE and CPFD were 10 to 16-fold higher than those of CPHE, CPSE, and CPSC. CPEE (29.35 ± 1.72 mg gallic acid equivalents (GAE)/g) and CPFD (28.92 ± 1.43 mg GAE/g) showed the highest phenolic content, followed by CPHE (2.92 ± 0.41 mg GA/g), CPSCE (2.42 ± 0.46 mg GAE/g), and CPSC (1.85 ± 0.08 mg GAE/g). The results are presented in Figure 4.
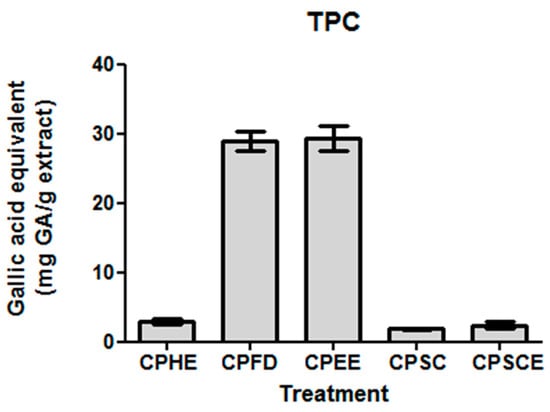
Figure 4.
Total phenolic content of CPEs. The data are presented as the mean ± SEM of five independent experiments (n = 5).
- (c)
- Ferric-reduced antioxidative power (FRAP)
To further investigate the antioxidant potential of CPEs, FRAP assays were performed by which the assay was used to assess the reducing power of the samples (Figure 5). In this assay, CPEs with high reducing power reduced the Fe3+–2,4,6-tris(2-pyridyl)-s-triazine complex to a blue Fe2+–TPTZ complex. CPEE exhibited the highest FRAP reducing power at 30.88 ± 0.87 mg ascorbic acid equivalents (AAE)/g, followed by CPFD (24.24 ± 1.21 mg AAE/g), CPSCE (13.63 ± 1.24 mg AAE/g), CPSC (7.29 ± 1.03 mg AAE/g), and CPHE (3.04 ± 0.33 mg AAE/g).
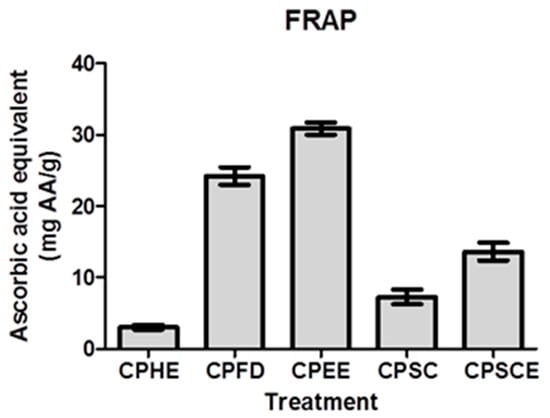
Figure 5.
Ferric-reduced antioxidative power (FRAP) activity of CPEs. The data are presented as the mean ± SEM of five independent experiments (n = 5).
2.4. Cytotoxicity of CP Leaf Extracts to Hs27 Human Skin Fibroblasts
Hs27 human skin fibroblasts were employed to assess the effect of CPEs on cell viability. As shown in Figure 6, none of the CPEs (4 to 125 μg/mL) exhibited significant toxicity to skin fibroblasts after the 48-h incubation period. All CPEs triggered fibroblast proliferation except for CPFD. CPHE, CPSC, CPSCE, and CPEE showed a proliferation-enhancing effect between 4 to 125 μg/mL. This indicates that the safety and therapeutic range of C. papaya leaf extracts was below 125 μg/mL. Thus, treatment doses between 25 to 100 μg/mL were selected for the pre- and post-treatment assays. Cells treated with H2O2 showed a significant dose-dependent reduction in viability. Cells treated with 700–800 μM of H2O2 resulted in a fibroblast killing effect of between 30% and 60%. Based on calculations, the IC50 value of H2O2 was approximately 750 µM. Thus, in the following assay, 750 μM H2O2 was used to induce 50% cell toxicity in the pre- and post-treatment assays.
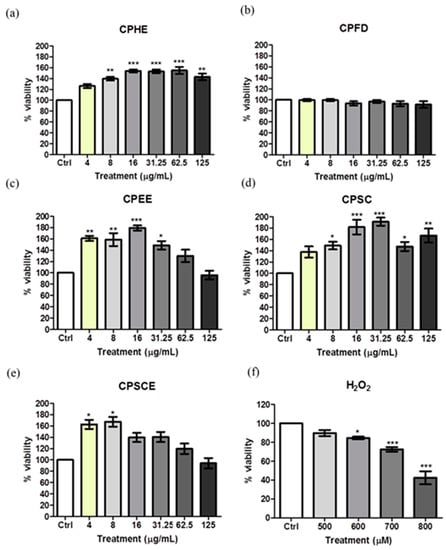
Figure 6.
The viability of Hs27 fibroblast cells after exposure to different concentrations of extracts and H2O2. (a) CPHE, (b) CPFD, (c) CPEE, (d) CPSC, (e) CPSCE, and (f) H2O2. The data are presented as the mean ± SEM (n = 6). The significant difference between the groups was calculated using one-way ANOVA with Tukey’s post-hoc test. * represents p < 0.05, ** represents p < 0.01, and *** represents p < 0.001 as compared to the control.
2.5. Protective Effect of CP Leaf Extracts towards H2O2-Induced Oxidative Damage
The antioxidant effect of the CPEs in the cell model was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Figure 7 shows Hs27 cell viabilities when exposed to CPEs. The CPEs were pre-treated with CPEs for 24 h, followed by another 24-h period of exposure of H2O2. Incubation of H2O2 in fibroblast cells reduced the cell viability in a dose-dependent manner. After exposure of 750 μM H2O2, cell viability was reduced to 50% as compared to untreated cells (100% viability). Meanwhile, pre-treatment with CPFD extract (ranging from 25 to 100 μg/mL) protected cells from oxidative damage significantly. Cells pre-treated with CPEE at 50 μg/mL showed statistical significance with regard to the proliferation of Hs27 cells. CPHE, CPSC, and CPSCE did not have promising effects on H2O2 induced oxidative damage.
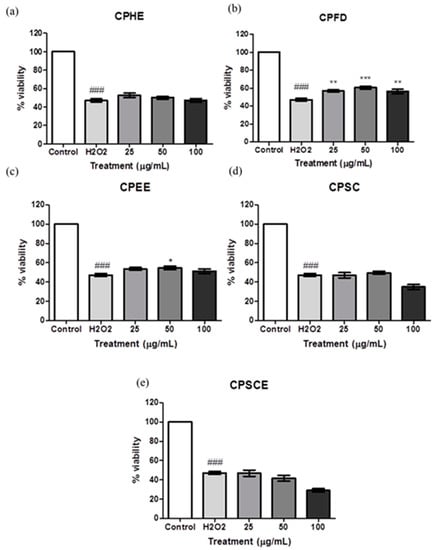
Figure 7.
The viability of Hs27 fibroblast cells pre-treated with (a) CPHE, (b) CPFD, (c) CPEE, (d) CPSC, and (e) CPSCE after exposure to H2O2. The data are presented as means ± SEM (n = 6). The significant difference between the groups was calculated using one-way ANOVA with Tukey´s post-hoc test. * represents p < 0.05, ** represents p < 0.01 and *** represents p < 0.001 compared to H2O2; ### represents p < 0.001 compared to the control.
The ability of CPEs with regard to cell recovery from oxidative damage by H2O2 on skin fibroblasts was evaluated by the MTT assays. CPEs (25 to 100 ug/mL) were introduced after the exposure to 750 μM H2O2 for 24 h. Figure 8 shows Hs27 cell viabilities when exposed to CPEs. CPFD and CPSCE at concentrations of 25 μg/mL and 50 μg/mL showed statistical significance in the proliferation of Hs27 cells when exposed to H2O2-induced oxidative stress. Approximately 10% of cells proliferated after treatment with 25 to 100 μg/mL of CPFD. Interestingly, up to 30% of Hs27 cells proliferated after treatment with 50 μg/mL of CPSCE.
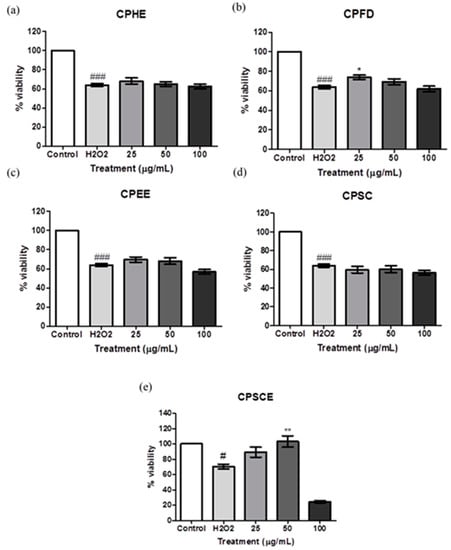
Figure 8.
The viability of Hs27 fibroblast cells post-treated with (a) CPHE, (b) CPFD, (c) CPEE, (d) CPSC and (e) CPSCE after being exposed to H2O2. The data are presented as means ± SEM (n = 6). The significant difference between the groups was calculated using one-way ANOVA with Tukey´s post-hoc test. * represents p < 0.05, and ** represents p < 0.01 compared to H2O2; # represents p < 0.05, and ### represents p < 0.001 compared to the control.
3. Discussion
The potential use of CP leaves in therapeutic skin care has been of great interest due to its abundance of natural antioxidants such as vitamin E, phenolics, flavonoids, etc. Previous reports revealed that CP leaf extract improved wound healing in in vivo models and exhibited a protective effect in the skin against UV radiation [30,31]. However, the phytochemicals which are responsible for the skin protection have not been well-identified. In this study, green and conventional extraction methods were employed to extract the bioactive compounds and evaluate their potential antioxidant and skin-protective effects.
Our results showed that scCO2 extraction of CP leaves with 5% of ethanol as a co-solvent increased lipophilic bioactive compounds, as revealed by GCMS analysis. The addition of a small amount of ethanol or methanol as co-solvent is encouraged to lower the activation energy and enhance the transportation of metabolites to the fluid, thus increasing the extraction yield [26]. Consistent with this theory, the α-tocopherol and squalene contents extracted by scCO2 + 5% ethanol (CPSCE) were 5- and 2- fold higher than scCO2 (CPSC) alone and extracts extracted by conventional extraction methods. In addition, the extraction yield of CPSCE extracted by scCO2 + co-solvent was 150% higher than that of CPSC extracted by scCO2 alone. Interestingly, the scCO2 extraction yield of papaya leaves was 1.5–3 fold lower than those found in previous study, despite the use of an additional co-solvent [27]. It might be reasoned that in different geographical locations the soil pH promotes the biosynthesis of bioactive compounds. Next, all CPEs were evaluated for their antioxidant and cytoprotective potentials in vitro.
The potential of an extract in attenuating oxidative stress is based on two main criteria: (1) the scavenging ability and (2) the possibility of activation of oxidative genes. In this study, the antioxidant capability of CPEs was evaluated by DPPH, FRAP, and TPC. CPSCE had the highest free radical scavenging activity as compared with CPSC, CPHE, and CPFD. This might be attributed to the presence of the α-tocopherol as a major component in the CPSCE. α-tocopherol is a well-known antioxidant exhibiting excellent scavenging activity and cytoprotection in various in vitro and in vivo oxidative stress models [32,33,34]. Besides, the presence of phytosterols in the lipophilic extracts might enhance the total radical scavenging activity through the formation of an allylic free radical. This allylic radical will then isomerize the existing radicals to other relatively stable free radicals [35]. Interestingly, CPSCE also significantly improved (~30%) the recovery of Hs27 cells after treatment with hydrogen peroxide. This can be attributed to the presence of active secondary metabolites including squalene and α-tocopherol in CPSCE for cell regeneration [33]. In addition, topical application of α-tocopherol and squalene have been reported to improve wound healing and skin regeneration [36,37,38].
On the other hand, phenolic-rich extracts (CPFD and CPEE) exhibited stronger ferric-reducing activity, with moderate protective effects against H2O2-induced oxidative stress in CPE-pretreated skin fibroblasts. The antioxidant capacity of phenolic compounds is mainly due to their redox properties. These properties allow them to act as excellent reducing agents, hydrogen donors, singlet oxygen quenchers, or metal chelators. Hypothetically, the structure of phenolic compounds, which consists of hydroxyl groups in ring B and the presence of carbon 2 and carbon 3 double bonds connected with the carbon 3 hydroxyl group and carbon 4 carbonyl group are important in both reducing power and radical scavenging effect. These essential structures are found in bioactive components such as rutin and phenolic acids in hydrophilic extracts (CPFD and CPEE) [39,40,41,42]. Through HPLC analysis, rutin (a flavonol glycoside) was found to be one of the major flavonoids in CPFD and CPEE, in accordance with the literature [20,43]. Rutin is well known for its wound-healing and antioxidant properties, which may partly explain the reason why CPFD and CPEE enhanced fibroblast proliferation after post-treatment with H2O2 [44,45]. Rutin has been proven to enhance the production and accumulation of extracellular matrices during the fibroblast healing process [44]. However, the possible skin-protective effects of other unidentified constituents remain unknown and inconclusive. Besides, plant primary metabolites such as polysaccharides and peptides might also contribute to the total antioxidant activities of CPFD and CPEE in skin fibroblast protection (pretreatment). In addition, a previous study showed that polysaccharides from Alfafa and Tremella fuciformis improved survival and reduced oxidative stress in skin fibroblast cells [46,47].
4. Materials and Methods
4.1. Chemical and Reagent
Rutin hydrate (HPLC grade; purity >94%), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, Missouri, United States). Formic acid (98–100%), acetonitrile (HPLC grade), methanol (HPLC grade), hexane (analytical (AR) grade), and ethanol (AR grade) were purchased from Merck (Darmstadt, Germany). High-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin, penicillin/streptomycin, and fetal bovine serum (FBS) were obtained from Invitrogen (Life Technologies, Mulgrave, VIC, Australia). The human skin cell line (Hs27 ATCC CRL-1634) was purchased from the American Type Culture Collection (Manassas, VA, USA).
4.2. Plant Materials
In total, 10 kg of fresh mature C. papaya leaves were collected from a papaya tree growing in a local fruit farm at Muar, Johor, Malaysia. The collected leaves were washed with tap water to remove contaminants. The leaf material was then separated into two portions, where (a) air-dried leaves were used for the extraction with hexane (CPHE), ethanol (CPEE), and supercritical fluids (CPSC and CPSCE), and (b) fresh leaves were used for leaf juice (CPFD) extraction. The air-dried leaf material was kept in an air-tight container and stored at −20 °C prior to extraction procedures.
4.3. Extraction Methods
- (a)
- Supercritical fluid extraction
The scCO2 extraction of air-dried CPL was performed using Supercritical Fluid Preparative scale CO2 extraction equipment (Taiwan Supercritical Technology), and extraction parameters in accordance with a previously optimized method [27]. The scCO2 extraction was operated with the following parameters: pressure 250 bar, temperature 35 °C, extraction time: 3 h, and with or without 5% ethanol as co-solvent. All extracts were kept in a freezer (−20 °C) until further experimentation. The extraction yields for CPSC and CPSCE were 1.12 and 1.54%, respectively.
- (b)
- Maceration
In total, 2 kg of air-dried CPL were extracted with hexane and ethanol, respectively, according to the procedures described previously [14]. All extracts were kept in a −20 °C freezer until further evaluation. The extraction yields of hexane and ethanol extracts were approximately 5.2 and 15.6%, respectively.
- (c)
- Leaf juice extraction
In total, 2 kg of fresh CPL were blended with a juice extractor (Panasonic, Kobe, Japan). The leaf juice was filtered and then freeze-dried to produce the lyophilized leaf juice extract (CPFD). The CPFD was kept at −20 °C until further experimentation. The overall extraction yield was about 14%.
4.4. Chemical Analysis
- (a)
- GC-MS analysis
Lipophilic constituents of C. papaya extracts were determined by a hyphenated Agilent 6890N Network GC system coupled to an Agilent 5973i mass selective detector (Agilent Technologies, Germany) as described previously by Chear et al. (2016) [48]. An aliquot of 10 mg/mL extract (in methanol) was separated on a HP-5MS column (30 m × 0.25 mm, 0.25-µm film thickness; Agilent Technologies, Waldbronn, Germany) with helium gas flowing at 1.2 mL/min. The injection volume was 1 µL with a splitless mode. The initial column temperature was set at 70 °C for 2 min, and then slowly increased to 280 °C at a constant rate of 20 °C/min. The column temperature was maintained at the final temperature of 280 °C for another 20 min. The total run time was 32.5 min. The injector, detector, and interface temperatures were set at 250 °C, 280 °C, and 300 °C, respectively. Mass acquisition was performed in the range of 40–550 m/z using electron impact ionization at 70 eV. The detected compounds were identified by performing spectral database matching against the National Institute of Standards and Technology database (NIST 02; Gaithersburg, MD, USA). The identity of the detected compound was determined by comparing the mass of their molecular ions, base ions, and fragment ions, as well as their peak intensities with those reference standards in the database. The detected compounds with >90% spectral matching quality were considered acceptable.
- (b)
- HPLC profiling of the polar extracts
A simple and selective high-performance liquid chromatography (HPLC) method was developed and validated for the analysis of CPL extracts. The HPLC analysis was performed on an Agilent 1200 series HPLC system coupled to a photodiode array detector (Agilent, Santa Clara, CA, USA). Briefly, a stock solution of CPL sample was prepared at 500 µg/mL in a mixture of methanol and water (80:20 v/v) and centrifuged to remove the undissolved particles. The chromatographic separation was achieved on an Eclipse C18 reversed phase column (4.6 mm × 150 mm, 3.5 µm) (Agilent Technologies, Santa Clara, CA, USA) at an adjusted temperature of 30 °C. The employed mobile phase was a mixture of 0.1% formic acid in water (pH 2.65) (A) and acetonitrile (B) running at a gradient method with a flow rate of 1 mL/min. The detailed gradient method is provided in Table 3. Twenty-five microliters of CPL extract were injected to the system, and the total run time was 30 min. The detection and quantification of a major flavonoid marker (rutin) were achieved using an Agilent photodiode array detector at λmax of 354 nm. Identification of rutin was done by comparing the HPLC retention time and UV spectrum of the analyte with that of reference standard. ChemStation LC3D software (Rev. B.03.01 317) was used for the data analysis. A 1000 μg/mL stock solution of rutin standard was prepared in methanol and then diluted into a series of 5 working standard solutions of 1.56, 3.125, 6.25, 12.5, and 25 μg/mL. Calibration curve was constructed by plotting the peak area against its corresponding concentration. Each individual standard/extract at a fixed concentration was consecutively injected three times.

Table 3.
Mobile phase gradient program.
4.5. Antioxidant Assay
- (a)
- DPPH scavenging activity
The DPPH radical scavenging activity of the CPEs was evaluated using prior references [49,50]. The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging capacity assay was determined by the decolourisation of the DPPH solution. Ten microliters of each sample with final concentrations in the range of 25 to 1000 ug/mL and 25 to 200 ug/mL for the standard were added to 96-well microtiter plate, followed by the addition of DPPH solution. Upon reaction in the dark for 30 min, the optical density of the solution was evaluated at 517 nm. The assay was done in triplicate.
- (b)
- Total phenolic content
The total phenolic content (TPC) was performed using the Folin-–Ciocalteu method [51]. A total of 20 μL of 5 mg/mL CPEs in 25% (v/v) DMSO were mixed with 100 μL of 1:4 diluted Folin–Ciocalteu reagent and shaken for 1 min in a flat-bottom 96-well microplate. The mixture was left for 2 min followed by the addition of 75 μL of sodium carbonate solution (100 mg/mL) and the mixture was shaken at medium-continuous speed for 1 min. After 2 h at room temperature, the absorbance was measured at λ = 750 nm. The absorbance of the same reaction with water instead of the extract was subtracted from the absorbance of the reaction with CPE. Gallic acid (0.05–0.5 mg/mL) in 25% (v/v) DMSO were used as standards for calibration. The TPC was calculated as gallic acid equivalents (GAE) in mg per g of CPE (mg GAE/g).
- (c)
- Ferric-reducing antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) of CPEs were quantified using the method proposed previously [52]. An aliquot of 280 µL of the freshly prepared FRAP reagent and 20 µL of 1 mg/mL CPE in 5% DMSO were added to each well, and after 30 min reaction the absorbance was read at λ = 593 nm. The FRAP reagent was prepared fresh by mixing sodium acetate buffer (300 mM, pH 3.6), a solution of TPTZ (10 mM) in 40 mM HCl, and 20 mM FeCl3.6H2O using the proportion 10:1:1 (v/v/v). An analytical curve with different concentrations of ascorbic acid (0.01–0.1 mg/mL) was plotted to quantify the ferric reducing antioxidant power of the selected extracts. The results were expressed in mg ascorbic acid equivalents (AAE) per g of CPE (mg AAE/g).
4.6. Cell Culture and Maintenance
Hs27 skin fibroblasts were cultured in Dulbecco’s Modified Eagle’s Medium high glucose (ATCC) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C incubator supplied with 5% CO2.
4.7. Cytotoxicity of C. papaya Extracts in Hs27 Human Skin Fibroblasts
MTT assay was used to evaluate the competency of CPEs. In brief, 5000/100 µL of Hs27 cells were seeded into a 96-well microtiter plate and incubated for 24 h. After that, the medium of each well was removed and replaced with CPEs prepared in various concentrations (4, 8, 16, 31.25, 62.5, and 125 µg/mL). The treated cells were incubated for 48 h at 37 °C in an incubator with 5% CO2. All CPEs were prepared in DMEM medium with reduced serum (1% FBS). For the growth control group, Hs27 cells were maintained in DMEM medium with 1% FBS. After 48 h, 10 μL MTT solution was added into each well. After 4 h of incubation, the medium in each well was removed and added with 100 μL DMSO to access cell viability. The optical density (OD) of each well was evaluated at 570 nm adjacent to the reference wavelength of 650 nm. Cell viability was calculated using the formula below:
4.8. Protective Effect of C. papaya Leaf Extracts Against H2O2 Induced Toxicity
The protective studies of CPEs towards H2O2-induced oxidative stress were carried out as reported previously [53]. Hs27 human skin fibroblasts were seeded in 7.5 × 103 cells per well in a 96-well plate and allowed to grow until a monolayer formed. To determine the protective effects of extracts, cells were either pre-treated or post-treated with CPEs. For the pre-treatment assay, cells were initially treated with different doses of extracts (25, 50, and 100 μg/mL) for 24 h, and then discharged and replaced with medium containing H2O2 (750 μM, IC50 of H2O2) for another 24 h.
For post-treatment assay, cells were treated with H2O2 (750 μM) for 24 h to induce oxidative damage. After that, the medium containing H2O2 was removed, and fresh medium containing different doses of extracts (25, 50, and 100 μg/mL) was added into the wells for another 24 h. The viability of the cells was determined by MTT assay. MTT was added to each well and subsequently incubated for 3 h. Upon incubation, the medium was removed and DMSO was added to dissolve the formed tetrazolium salt. The absorbance was measured at 490 nm by a Multiskan Go UV microplate reader. Cell viability was calculated as previously mentioned.
5. Conclusions
Overall, scCO2 with the addition of co-solvent was shown to improve the α-tocopherol and squalene contents and total extraction yield. In addition, CPSCE showed the most potent free radical-scavenging activity in DPPH assay, while also protecting Hs27 cells against H2O2-induced cytotoxicity in the post-treatment assay. These activities are mainly attributed to its high α-tocopherol content. On the other hand, CPEE and CPFD exhibited significant reducing power in FRAP assay, and CPFD moderately protected Hs27 cells in pre- and post-treatment assays due to its high phenolic content (particularly rutin). However, future studies to investigate the wound-healing effect of single compounds from these extracts are warranted for the better understanding of the underlying skin-protective mechanisms.
Author Contributions
Conceptualization, K.-Y.K. and J.A.; methodology, N.J.-Y.C., J.A., K.-Y.K.; writing—original draft preparation, B.-K.K., N.J.-Y.C., J.A., K.-Y.K.; writing—review and editing, J.A., K.-Y.K.; funding acquisition, K.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Maestro Laboratories Sdn Bhd (grant number MMRD-SOP-002) and USM short-term grant (grant number 304.CDADAH.6315221).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Maestro Laboratories Sdn Bhd for the financial support of this research.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds are available from the authors.
Abbreviations
Carica papaya leaf extracts (CPEs); Carica papaya leaf hexane extract (CPHE); Carica papaya leaf juice freeze-dried extract (CPFD); Carica papaya leaf ethanol extract (CPEE); Carica papaya leaf scCO2 extract (CPSC); Carica papaya leaf scCO2 extract with 5% ethanol (CPSCE).
References
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Beacham, D.A.; Amatangelo, M.D.; Cukierman, E. Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr. Protoc. Cell Biol. 2006, 33, 10.9.1–10.9.21. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef]
- Milkovic, L.; Gasparovic, A.C.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Calafato, S.; Puleo, E.; Cornelius, C.; Sapienza, M.; Morganti, P.; Mancuso, C. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: Role of vitagenes. Clin. Dermatol. 2008, 26, 358–363. [Google Scholar] [CrossRef]
- Ozbek, E. Induction of Oxidative Stress in Kidney. Int. J. Nephrol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Birch-Machin, M.A. Oxidative Stress and Ageing: The Influence of Environmental Pollution, Sunlight and Diet on Skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Canini, A.; Alesiani, D.; D’Arcangelo, G.; Tagliatesta, P. Gas chromatography–mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J. Food Compos. Anal. 2007, 20, 584–590. [Google Scholar] [CrossRef]
- Starley, I.F.; Mohammed, P.; Schneider, G.; Bickler, S.W. The treatment of paediatric burns using topical papaya. Burns 1999, 25, 636–639. [Google Scholar] [CrossRef]
- Zunjar, V.; Mammen, D.; Trivedi, B.M.; Daniel, M. Pharmacognostic, Physicochemical and Phytochemical Studies on Carica papaya Linn. Leaves. Pharmacogn. J. 2011, 3, 5–8. [Google Scholar] [CrossRef]
- Nugroho, A.; Heryani, H.; Choi, J.S.; Park, H.-J. Identification and quantification of flavonoids in Carica papaya leaf and peroxynitrite-scavenging activity. Asian Pac. J. Trop. Biomed. 2017, 7, 208–213. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Shaw, P.N.; Parat, M.-O.; Pandey, S.; Falconer, J.R. Compound Identification and In Vitro Cytotoxicity of the Supercritical Carbon Dioxide Extract of Papaya Freeze-Dried Leaf Juice. Processes 2020, 8, 610. [Google Scholar] [CrossRef]
- Soib, H.H.; Ismail, H.F.; Husin, F.; Abu Bakar, M.H.; Yaakob, H.; Sarmidi, M.R. Bioassay-Guided Different Extraction Techniques of Carica papaya (Linn.) Leaves on In Vitro Wound-Healing Activities. Molecules 2020, 25, 517. [Google Scholar] [CrossRef]
- Nayak, B.S.; Ramdeen, R.; Adogwa, A.; Marshall, J.R.; Ramsubhag, A. Wound-healing potential of an ethanol extract of Carica papaya (Caricaceae) seeds. Int. Wound J. 2012, 9, 650–655. [Google Scholar] [CrossRef]
- Gurung, S.; Škalko-Basnet, N. Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. J. Ethnopharmacol. 2009, 121, 338–341. [Google Scholar] [CrossRef]
- Anuar, N.S.; Zahari, S.S.; Taib, I.A.; Rahman, M.T. Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food Chem. Toxicol. 2008, 46, 2384–2389. [Google Scholar] [CrossRef]
- Fitzmaurice, S.; Sivamani, R.; Isseroff, R. Antioxidant Therapies for Wound Healing: A Clinical Guide to Currently Commercially Available Products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Norahmad, N.A.; Razak, M.R.M.A.; Misnan, N.M.; Jelas, N.H.M.; Sastu, U.R.; Muhammad, A.; Ho, T.C.D.; Jusoh, B.; Zolkifli, N.A.; Thayan, R.; et al. Effect of freeze-dried Carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in AG129 mice. BMC Complement. Altern. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Aguilar-Domínguez, D.E.; La Fuente, L.F.R.-D.; Lobato-García, C.E.; Ble-Castillo, J.L.; López-Meraz, L.; Diaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef]
- Ilham, R.; Lelo, A.; Harahap, U.; Widyawati, T.; Siahaan, L. The Effectivity of Ethanolic Extract from Papaya Leaves (Carica papaya L.) as an Alternative Larvacide to Aedes spp. Open Access Maced. J. Med. Sci. 2019, 7, 3395–3399. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.H.; Chong, K.P. The potential of papaya leaf extract in controlling Ganoderma boninense. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Chemical and Bioprocess Engineering, Kota Kinabalu, Malaysia, 9–12 December 2015; IOP Publishing Ltd.: Bristol, UK, 2016. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Nguyen, T.T.T.; Pandey, S.; Thurecht, K.J.; Falconer, J.R. Factorial design-assisted supercritical carbon-dioxide extraction of cytotoxic active principles from Carica papaya leaf juice. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Chear, N.J.Y.; Maran, S.; Yeong, K.Y.; Ong, Y.S.; Goh, B.H. Butyrylcholinesterase inhibitory activity and GC-MS analysis of Carica papaya leaves. Nat. Prod. Sci. 2020, 26, 165–170. [Google Scholar]
- Subenthiran, S.; Choon, T.C.; Cheong, K.C.; Thayan, R.; Teck, M.B.; Muniandy, P.K.; Afzan, A.; Abdullah, N.R.; Ismail, Z. Carica papaya Leaves Juice Significantly Accelerates the Rate of Increase in Platelet Count among Patients with Dengue Fever and Dengue Haemorrhagic Fever. Evid. Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- A Seo, S.; Ngo, H.T.; Hwang, E.; Park, B.; Yi, T.-H. Protective effects of Carica papaya leaf against skin photodamage by blocking production of matrix metalloproteinases and collagen degradation in UVB-irradiated normal human dermal fibroblasts. S. Afr. J. Bot. 2020, 131, 398–405. [Google Scholar] [CrossRef]
- Mahmood, A.A.; Sidik, K.; Salmah, I. Wound Healing Activity of Carica papaya L. Aqueous Leaf Extract in Rats. Int. J. Mol. Med. Adv. Sci. 2005, 1, 398–401. [Google Scholar]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Bilgic, M.B.; Lacin, N.T.; Berber, H.; Mansuroglu, B. In vitro evaluation of alpha-tocopherol loaded carboxymethylcellulose chitosan copolymers as wound dressing materials. Mater. Technol. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Lopez-Torres, M.; Thiele, J.J.; Shindo, Y.; Han, D.; Packer, L. Topical application of α-tocopherol modulates the antioxidant network and diminishes ultraviolet-induced oxidative damage in murine skin. Br. J. Dermatol. 1998, 138, 207–215. [Google Scholar] [CrossRef]
- Gordon, M.H.; Magos, P. The effect of sterols on the oxidation of edible oils. Food Chem. 1983, 10, 141–147. [Google Scholar] [CrossRef]
- Lucero, M.; Vigo, J.; Leon, M.; Martin, F.; Sánchez, J. Therapeutic efficacy of hydrophilic gels of α-tocopherol and tretinoin in skin ulcers induced by adriamycin hydrochloride. Int. J. Pharm. 1996, 127, 73–83. [Google Scholar] [CrossRef]
- Zahid, S.; Khalid, H.; Ikram, F.; Iqbal, H.; Samie, M.; Shahzadi, L.; Shah, A.T.; Yar, M.; Chaudhry, A.A.; Awan, S.J.; et al. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater. Sci. Eng. C 2019, 101, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a Step toward Sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Liang, T.; Yue, W.; Li, Q. Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Malaysian Herbal Monograph: Carica papaya L. Available online: https://www.globinmed.com/index.php?option=com_content&view=article&id=105958:carica-papaya-l-105958&catid=209&Itemid=143 (accessed on 30 January 2021).
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In Situ Forming and Rutin-Releasing Chitosan Hydrogels as Injectable Dressings for Dermal Wound Healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, Y.; Yang, W.; Yang, Z.; Jiang, S.; Zhang, C.; Zhang, G. Alfalfa polysaccharide prevents H2O2-induced oxidative damage in MEFs by activating MAPK/Nrf2 signaling pathways and suppressing NF-κB signaling pathways. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Chear, N.J.-Y.; Khaw, K.-Y.; Murugaiyah, V.; Lai, C.-S. Cholinesterase inhibitory activity and chemical constituents of Stenochlaena palustris fronds at two different stages of maturity. J. Food Drug Anal. 2016, 24, 358–366. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chear, N.J.-Y.; Fauzi, A.N.; Khaw, K.-Y.; Choi, S.-B.; Yaacob, N.S.; Lai, C.-S. Free Radical Scavenging and Cytotoxic Properties of Acylated and Non-Acylated Kaempferol Glycosides from Stenochlaena Palustris: A Perspective on Their Structure – Activity Relationships. Pharm. Chem. J. 2019, 53, 188–193. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Ponnusamy, Y.; Chear, N.J.-Y.; Ramanathan, S.; Lai, C.-S. Polyphenols rich fraction of Dicranopteris linearis promotes fibroblast cell migration and proliferation in vitro. J. Ethnopharmacol. 2015, 168, 305–314. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).