Meloxicam and Study of Their Antimicrobial Effects against Phyto- and Human Pathogens
Abstract
1. Introduction
2. Results and Discussion
2.1. Vibrational Spectra
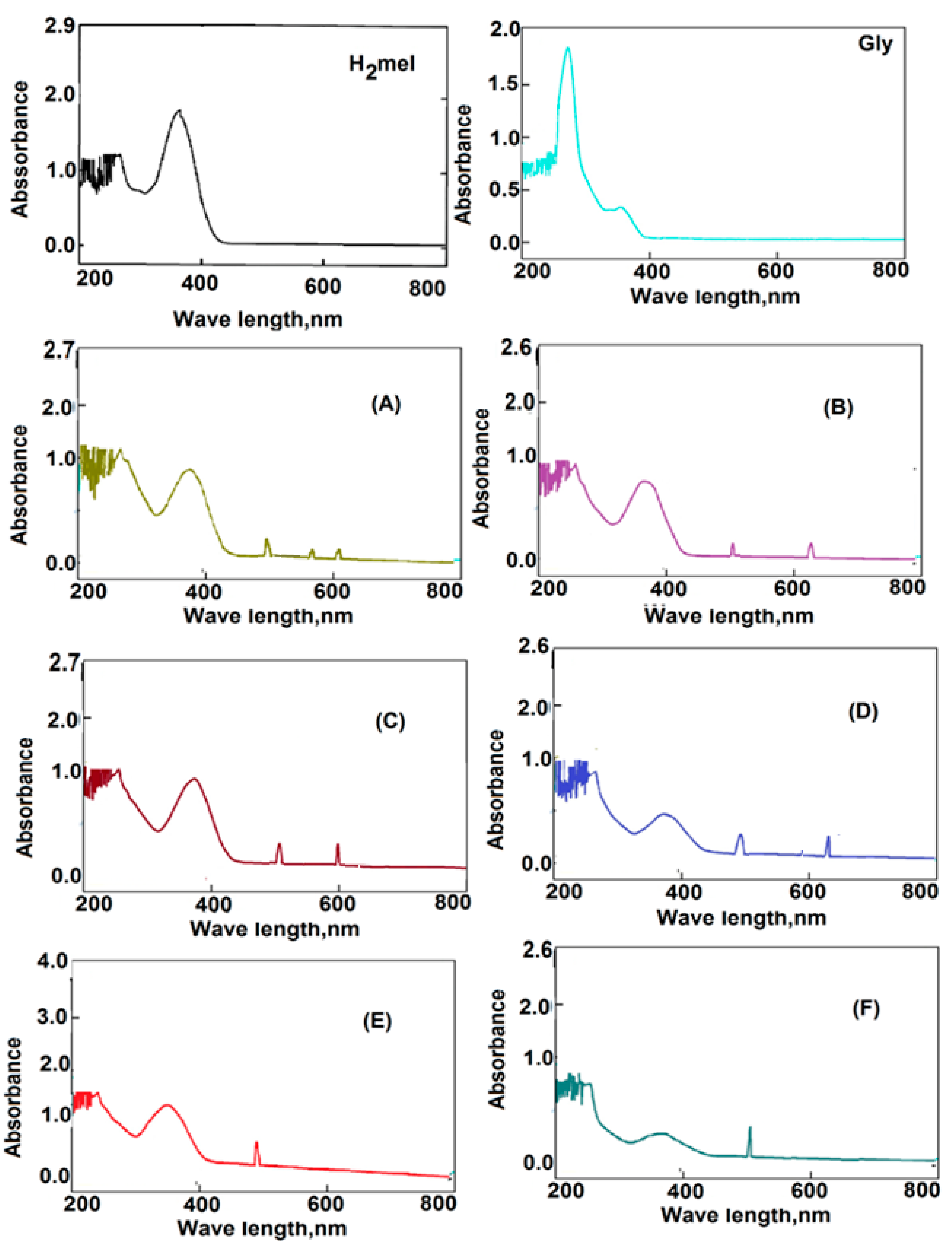
2.2. UV-Visible Spectra
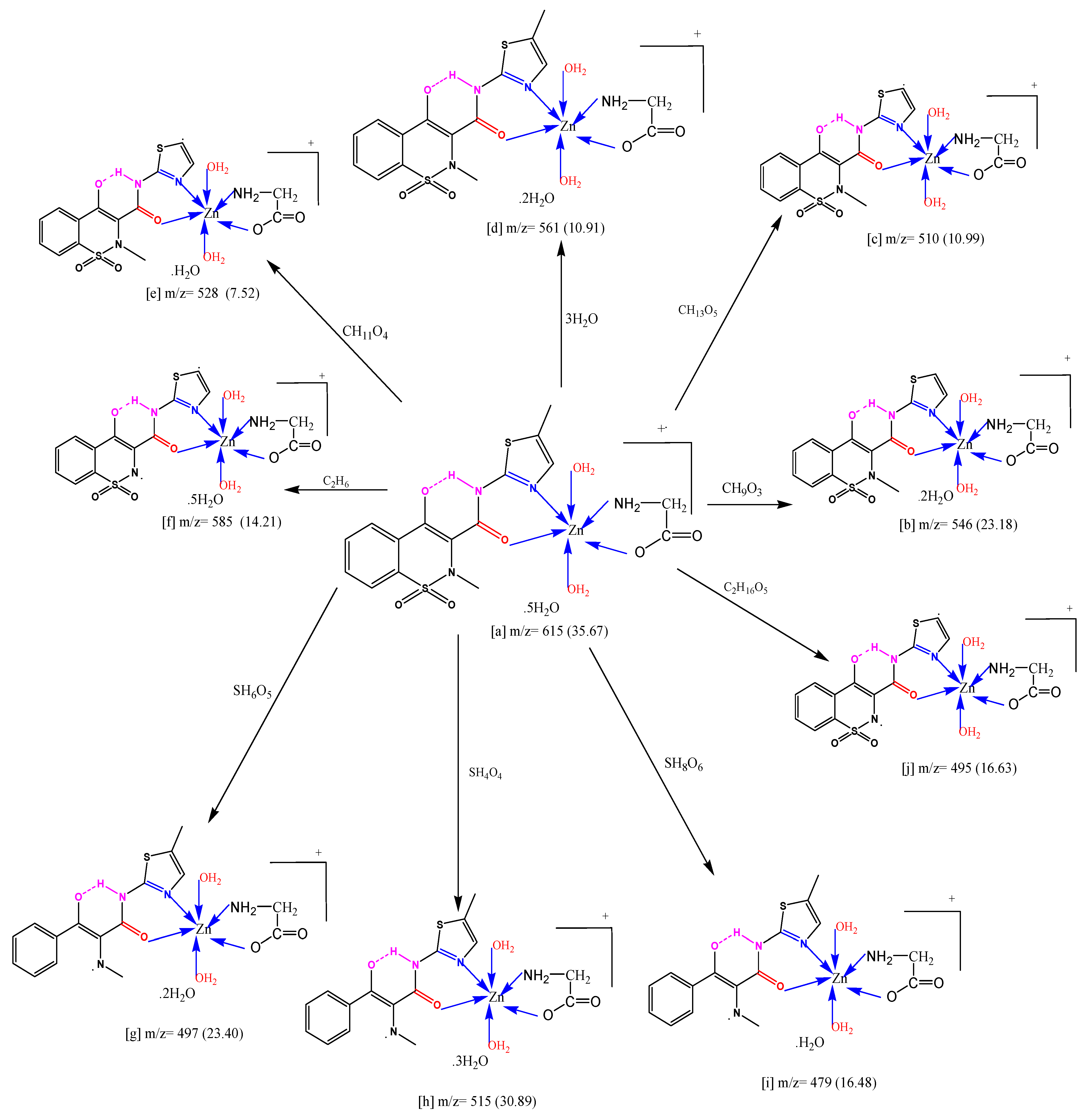
2.3. Mass Spectra (GC-MS)
2.4. Thermal Analysis Studies
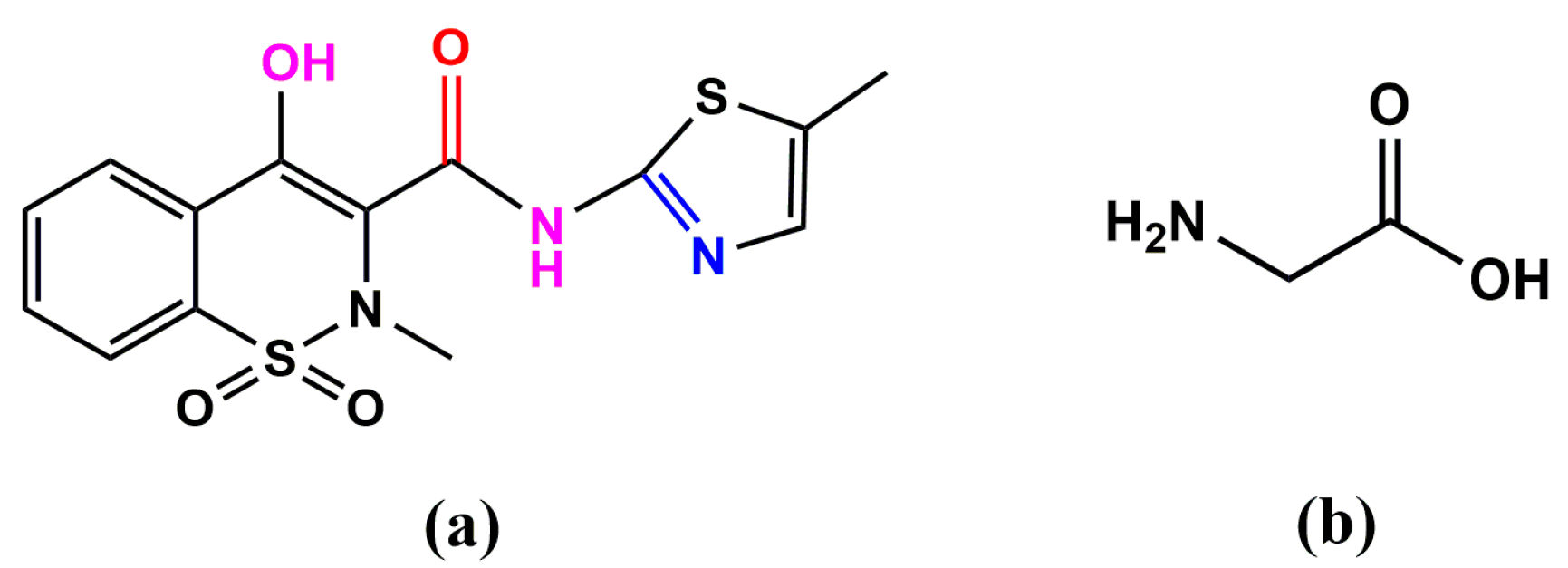
2.5. Geometrical Structure of H2mel
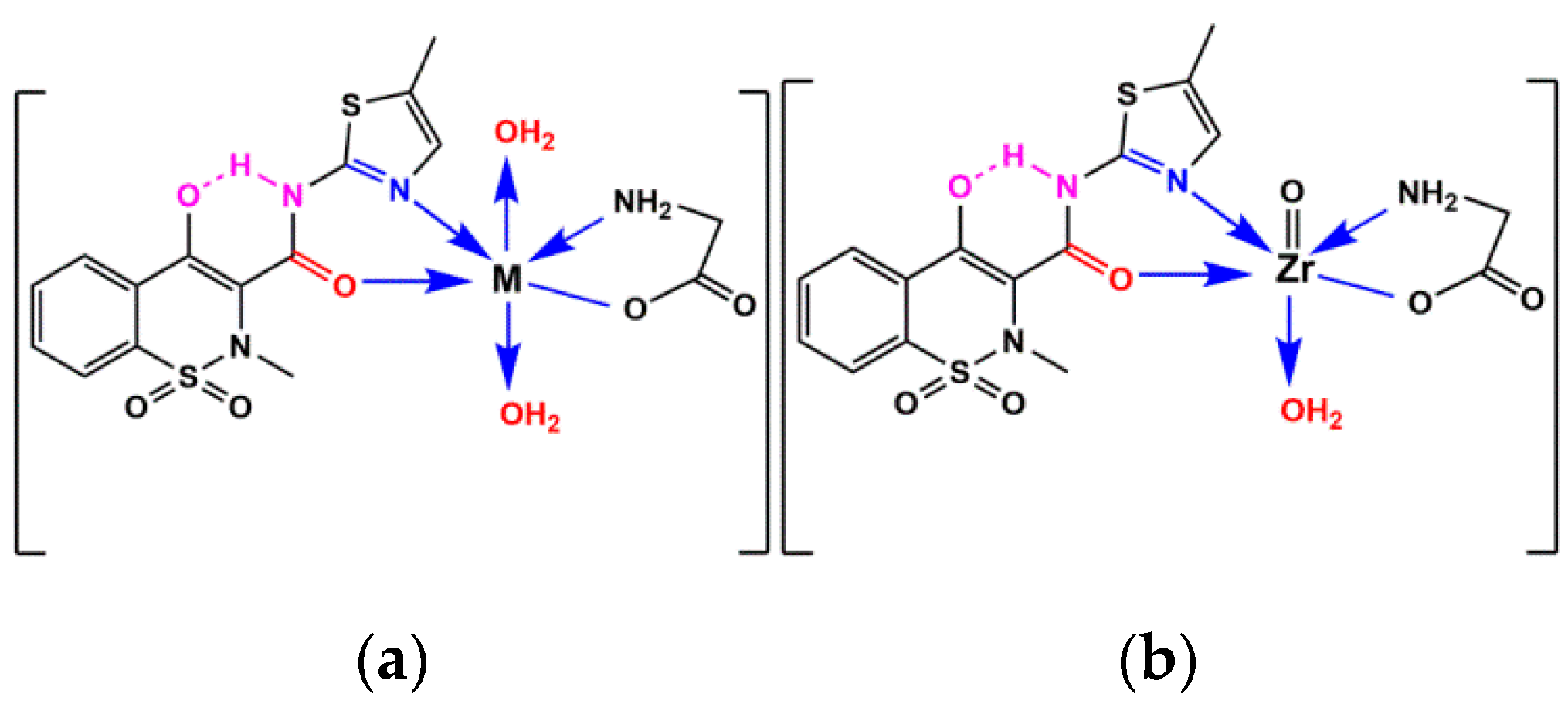
2.6. Geometrical Structure of Complexes
2.7. Charge Distribution Analysis
2.8. Frontier and Molecular Orbitals
2.9. Excited State
2.10. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Metal-Ligand Complexes
3.3. Computational Studies
3.4. Instruments
3.5. Antimicrobial Investigation
3.6. Minimum Inhibitory Concentration (96-Well Microplate Method)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stadler, P.; Armstrong, D.; Margalith, D.; Saraga, E.; Stolte, M.; Lualdi, P.; Mautone, G.; Blum, A.L. Diclofenac Delays Healing of Gastroduodenal Mucosal Lesions. Dig. Dis. Sci. 1991, 36, 594–600. [Google Scholar] [CrossRef]
- Gerli, R.; Paolucci, C.; Gresele, P.; Bistoni, O.; Fiorucci, S.; Muscat, C.; Belia, S.; Bertotto, A.; Constantini, V. Salicylates Inhibit Adhesion and Transmigration of T Lymphocytes by Preventing Integrin Activation Induced by Contact With Endothelial Cells. Blood 1998, 92, 2389–2398. [Google Scholar] [CrossRef]
- Mutschler, E.; Geisslinger, G.; Kroemer, H.K.; Korting, M.S. Arzneimittel Wirkungen (In German), 8th ed.; Wissens chaftlicheVerlagsgesellschaft: Stuttgart, Germany, 2001; p. 233. [Google Scholar]
- Baran, E.J. Quimica Bioinorganica; McGraw-Hill Interamericana: Madrid, Spain, 1995. [Google Scholar]
- Defazio, S.; Cini, R. Synthesis, X-ray structural characterization and solution studies of metal complexes containing the anti-inflammatory drugs meloxicam and tenoxicam. Polyhedron 2003, 22, 1355–1366. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Badawy, M.A.; Omar, M.M.; Nassar, M.M.; Kame, A.B. Synthesis, spectroscopic, thermal and biological activity studies on triazine metal complexes. Spectrochim. Acta A 2010, 77, 773–781. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Abd El-Halim, H.F.; El- Dessouky, M.M.; Mahmoud, W.H. Synthesis and characterization of mixed ligand complexes of lomefloxacin drug and glycine with transition metals. Antibacterial, antifungal and cytotoxicity studies. J. Mol. Struct. 2001, 999, 29–38. [Google Scholar] [CrossRef]
- Sakr, S.H.; Elshafie, H.S.; Camele, I.; Sadeek, S.A. Synthesis, Spectroscopic, and Biological Studies of Mixed Ligand Complexes of Gemifloxacin and Glycine with Zn(II), Sn(II), and Ce(III). Molecules 2018, 23, 1182. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Awad, H.M.; Mohamed, A.A. Biological and Spectroscopic Investigations of New Tenoxicam and 1.10-Phenthroline Metal Complexes. Molecules 2020, 25, 1207. [Google Scholar] [CrossRef]
- Donadu, M.G.; Le, N.T.; HO, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, M.; Marchetti, M.; Sanna, G.; Madeddu, S. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of Hornstedtia bella Škorničk. Antibiotics 2020, 9, 334. [Google Scholar] [CrossRef]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef]
- Roncevic, T.; Vukicevic, D.; Ilic, N.; Krce, L.; Gajski, G.; Tonkic, M.; Goic-Barisič, I.; Zoranic, L.; Sonavane, Y.; Benincasa, M.; et al. Antibacterial activity affected by the conformational flexibility in glycine-lysine based α-helical antimicrobial peptides. J. Med. Chem. 2018, 61, 2924–2936. [Google Scholar] [CrossRef]
- Prakash, O.; Kumar, R.; Kumar, R.; Tyagi, P.; Kuhad, R.C. Organoiodine(III) mediated synthesis of 3,9-diaryl- and 3,9-difuryl-bis-1,2,4-triazolo[4,3-a][4,3-c]pyrimidines as antibacterial agents. Eur. J. Med. Chem. 2007, 42, 868–872. [Google Scholar] [CrossRef]
- Fallik, E.; Klein, J.; Grinberg, S.; Lomaniee, E.; Lurie, S.; Lalazar, A. Effect of postharvest heat treatment of tomatoes on fruit ripening and decay caused by Botrytis cinerea. Plant Dis. 1993, 77, 985–988. [Google Scholar] [CrossRef]
- Gamil, M.A.; Sadeek, S.A.; Zordok, W.A.; El Shwiniy, W.H. Spectroscopic, DFT modeling and biological study of some new mixed ligand metal complexes derived from gatifloxacin and pregabalin. J. Mol. Struct. 2020, 1209, 127941. [Google Scholar] [CrossRef]
- El Shwiniy, W.H.; Gamil, M.A.; Sadeek, S.A.; Zordok, W.A.; El-farargy, A.F. Ligational, DFT modeling and biological properties of some new metal complexes with 3-(bromoacetyl)coumarin and 1,10-phenanthroline. Appl. Organometal. Chem. 2020, 34, e5696. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, R.V. Ferrocenyl-substituted Schiff base complexes of boron: Synthesis, structural, physico-chemical and biochemical aspects. Spectrochim. Acta A 2011, 78, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G.; Abd El-Wahab, Z.H. Salisaldehyde-2-aminobenzimidazole schiff base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II). J. Therm. Anal. 2003, 73, 347–359. [Google Scholar] [CrossRef]
- Santi, E.; Torre, M.H.; Kremer, E.; Etcheverry, S.B.; Baran, E.J. Vibrational spectra of the copper(II) and nickel(II) complexes of piroxicam. Vib. Spect. 1993, 5, 285–293. [Google Scholar] [CrossRef]
- Riley, C.M.; Ross, D.L.; Vander, D.V.; Takusagawa, F. Characterization of the complexation of fluoroquinolone antimicrobials with metal ions by nuclear magnetic resonance spectroscopy. J. Pharm. Biomed. Anal. 1993, 11, 49–59. [Google Scholar] [CrossRef]
- Florence, A.J.; Kennedy, A.R.; Shankland, N.; Wright, E.; Al-Rubayi, A. Norfloxacin dihydrate. Acta Crystallogr. 2000, 56, 1372–1373. [Google Scholar] [CrossRef]
- Turel, I.; Bukovec, P.; Quiroś, M. Crystal structure of ciprofloxacin hexahydrate and its characterization. Int. J. Pharm. 1997, 152, 59–65. [Google Scholar] [CrossRef]
- Serafin, A.; Stańczak, A. The complexes of metal ions with fluoroquinolones. Rus. J. Coord. Chem. 2009, 35, 81–95. [Google Scholar] [CrossRef]
- Shen, L.L.; Pernet, A.G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: The target of the drugs is DNA. Natl. Acad. Sci. 1985, 82, 307–311. [Google Scholar] [CrossRef]
- Gellert, M.; Mizuuchi, K.; O’Dea, M.H.; Nash, H. DNA gyrase: An enzyme that introduces superhelical turns into DNA. Natl. Acad. Sci. 1976, 73, 3872–3876. [Google Scholar] [CrossRef]
- Wallis, S.C.; Gahan, L.R.; Charles, B.G.; Hambley, T.W. 13C N.M.R. and Single-Crystal X-Ray Structural Investigation of the Fluoroquinolone Antimicrobial Drug Norfloxacin 2DCl.D2O. Aust. J. Chem. 1994, 47, 799–806. [Google Scholar] [CrossRef]
- Czugler, M.; Argay, G.; Frank, J.; Meśzaŕos, Z.; Kutschabsky, L.; Reck, G. 1-Ethyl-1,4-dihydro-4-oxo-5-amino-6,7-methylenedioxy-3-quinolinecarboxyli acid (aminooxolinic acid). Acta Crystallogr. B 1976, 32, 3124–3126. [Google Scholar] [CrossRef]
- Refat, M.S.; Mohamed, G.G.; de Farias, R.F.; Powell, A.K.; El-Garib, M.S.; ElKorashy, S.A.; Hussien, M.A. Spectroscopic, thermal and kinetic studies of coordination compounds of Zn(II), Cd(II) and Hg(II) with norfloxacin. J. Therm. Anal. Calor. 2010, 102, 225–232. [Google Scholar] [CrossRef]
- Kasselouri, S.; Hadjiliadis, N. Mixed nucleobase, amino acid complexes of Pt(II). Preparation and x-ray structure of trans-[(CH3NH2)2Pt(1-MeC-N3)(gly-N)]NO3·2H2O and its precursor trans-[(CH3NH2)2Pt(1-MeC-N3)Cl]Cl·H2O. Inorg. Chim. Acta. 1990, 169, 195–200. [Google Scholar]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall: London, UK, 1975. [Google Scholar]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds, 2nd ed.; “A Wiley-Interscience puplication”; Wiley: New York, NY, USA, 1970; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/bbpc.19710750622 (accessed on 3 March 2021).
- Defazio, S.; Cini, R. Synthesis, X-ray structure and molecular modelling analysis of cobalt(II), nickel(II), zinc(II) and cadmium(II) complexes of the widely used anti-inflammatory drug meloxicam. J. Chem. Soc. Dalton Trans. 2002, 22, 1888–1897. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Mohamed, A.A.; El-Sayed, H.A.; El-Attar, M.S. Spectroscopic characterization, thermogravimetric and antimicrobial studies of some new metal complexes derived from 4-(4-Isopropyl phenyl)-2-oxo-6-phenyl 1,2-dihyropyridine-3-carbonitrile (L). Appl. Organometal. Chem. 2019, 34, e5334. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H. Preparation, structural characterization and biological studies of some new levofloxacin metal complexes. J. Iran. Chem. Soc. 2017, 14, 1711–1723. [Google Scholar] [CrossRef]
- Sultana, N.; Naz, A.; Arayne, M.S.; Mesaik, M.A. Synthesis, characterization, antibacterial, antifungal and immunomodulating activities of gatifloxacin–metal complexes. J. Mol. Struct. 2010, 969, 17–24. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 1999; p. 1054. [Google Scholar]
- Sadeek, S.A.; Refat, M.S.; Hashem, H.A. Complexation and thermogravimetric investigation on tin(II) and tin(IV) with norfloxacin as antibacterial agent. J. Coord. Chem. 2006, 59, 759–775. [Google Scholar] [CrossRef]
- Sadeek, S.A.; EL-Shwiniy, W.H. Metal complexes of the third generation quinolone antibacterial drug sparfloxacin: Preparation, structure, and microbial evaluation. J. Coord. Chem. 2010, 63, 3471–3482. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.H.; Sadeek, S.A.; Camele, I. Biological investigations and spectroscopic studies of new Moxifloxacin/Glycine-Metal complexes. Chem. Biodiver. 2019, 16, e1800633. [Google Scholar] [CrossRef] [PubMed]
- Macias, B.; Martinez, M.; Sanchez, A.; Dominguez-Gil, A. A physico-chemical study of the interaction of ciprofloxacin and ofloxacin with polivaient cations. Int. J. Pharm. 1994, 106, 229–235. [Google Scholar]
- Sadeek, S.A.; Abd El-Hamid, S.M.; Zordok, W.A. Spectroscopic, DFT and antimicrobial activity of Zn(II), Zr(IV), Ce(IV) and U(VI) complexes of N,N-chelated 4,6-bis (4-chlorophenyl)-2-amino-1,2-dihydropyridine-3. Appl. Organometal. Chem. 2018, 32, e4457. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Horowitz, H.W.; Metzger, G.A. New Analysis of Thermogravimetric Traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Moore, J.W.; Pearson, R.G. Kinetic and Mechanism, 3rd ed.; John Wiley& Sons: New York, NY, USA, 1981. [Google Scholar]
- Change, R. Physical Chemistry for Chemical and Biological Sciences; University Science Books: Mill Valley, CA, USA, 2000. [Google Scholar]
- Tamasi, G.; Serinelli, F.; Consumi, M.; Magnani, A.; Casolaro, M.; Cini, R. Release studies from smart hydrogels as carriers for piroxicam and copper(II)–oxicam complexes as anti-inflammatory and anti-cancer drugs. X-ray structures of new copper(II)–piroxicam and –isoxicam complex Molecules. J. Inoorg. Biochem. 2008, 102, 1862–1873. [Google Scholar] [CrossRef]
- Abu-Eittah, R.H.; Zordok, W.A. A molecular orbital treatment of piroxicam and its M(II)-complexes: The change of the drug configuration in a time of bond formation. J. Mol. Struct. 2010, 951, 14–20. [Google Scholar] [CrossRef]
- Cini, R.; Tamasi, G.; Defazio, S.; Hursthouse, M.B. Unusual coordinating behavior by three non-steroidal anti-inflammatory drugs from the oxicam family towards copper(II). Synthesis, X-ray structure for copper(II)-isoxicam, -meloxicam and -cinnoxicam-derivative complexes, and cytotoxic activity for a copper(II)-piroxicam complex. J. Inorg. Biochem. 2007, 101, 1140–1152. [Google Scholar]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: London, UK, 1976. [Google Scholar]
- Kurtaran, R.; Odabasoglu, S.; Azizoglu, A.; Kara, H.; Atakol, O. Experimental and computational study on [2,6-bis(3,5-dimethyl-N-pyrazolyl)pyridine]-(dithiocyanato)mercury(II). Polyhedron 2007, 26, 5069–5074. [Google Scholar] [CrossRef]
- Krogmann, K.; Anorg, Z. Die Kristallstruktur von K2[Pd(C2O4)2]4H2O. Allg. Chem. 1966, 346, 188–202. [Google Scholar] [CrossRef]
- Ciofini, I.; Laine, P.P.; Bedioui, F.; Admo, C. Photoinduced Intramolecular Electron Transfer in Ruthenium and Osmium Polyads: Insights from Theory. J. Am. Chem. Soc. 2004, 126, 10763–11077. [Google Scholar] [CrossRef]
- Ciofini, I.I.; Daul, C.; Adamo, C.J. Phototriggered Linkage Isomerization in Ruthenium–Dimethylsulfoxyde Complexes: Insights from Theory. J. Phys. Chem. A 2003, 107, 11182–11190. [Google Scholar] [CrossRef]
- Casida, F.M. Recent Advances in Density Functional Methods, Part 1; Chong, D.P., Ed.; World Scientific: Singapore, 1995. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Sharaby, C.M. Metal complexes of Schiff base derived from sulphametrole and o-vanilin: Synthesis, spectral, thermal characterization and biological activity. Spectrochim. Acta A 2007, 66, 949–958. [Google Scholar] [CrossRef]
- Sengupta, S.K.; Pandey, O.P.; Srivastava, B.K.; Sharma, V.K. Synthesis, structural and biochemical aspects of titanocene and zirconocene chelates of acetylferrocenyl thiosemicarbazones. Trans. Met. Chem. 1998, 23, 349–353. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 98; Revision A.6, Inc.: Pittsburgh, PA, USA, 1998. [Google Scholar]
- Stevens, W.J.; Krauss, M.; Bosch, H.; Jasien, P.G. Relativistic compact effective potentials and efficient, shared-exponent basis sets for the third-, fourth-, and fifth-row atoms. Can. J. Chem. 1992, 70, 612–630. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Aliberti, L.; Amato, M.; De Feo, V.; Camele, I. Chemical composition and antimicrobial activity of chia (Salvia hispanica L.) essential oil. Europ. Food Res. Technol. 2018, 244, 1675–1682. [Google Scholar] [CrossRef]


| Compounds | ν(O-H); Enolate; H2O | ν(N-H) | ν(NH2) | ν(C=O); Amide | ν(C=O) COOH | νas(COO−) | ν(C=N) Thiazoyl Ring | νs(COO−) | νas(so2) | νs(so2) | ν(Zr=O) | ν(M-O) and ν(M-N) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2mel | 3438 br | 3289 s,sh | 1620 s | - | ---- | 1550 vs | 1346 s | 1183 s | ---- | ---- | ||
| Gly | ----- | ----- | 3420 br 3169 m | 1604 vs | --- | ----- | ----- | ---- | ||||
| (A) | 3459 m | ------ | 3376 w 3165 m | 1608 vs | ------- | 1597 m | 1515 m | 1394 m | 1327 s | 1168 vs | --- | 652 m, 610 m, 574 m and 530 m |
| (B) | 3403 m | ------ | 3380 vw 3100 vw | 1614 vs | -------- | 1599 m | 1514 s | 1398 vs | 1331 vs | 1165 s | ---- | 671 w, 623 w and 576 m |
| (C) | 3395 m, br | ---- | 3256 w 3105 vw | 1606 vs | ------- | 1599 m | 1510 s | 1398 s | 1335 s | 1174 s | ----- | 628 m, 573 m and 526 vw |
| (D) | 3449 w | ----- | 3265 w 3106 vw | 1593 vs | ---- | 1590 m | 1509 m | 1389 s | 1336 s | 1167 s | ---- | 664 w, 625 m, 574 m and 529 w |
| (E) | 3400 w | ----- | 3379 w 3182 m | 1610 vs | ---- | 1597 m | 1511 s | 1396 s | 1334 s | 1175 s | ------ | 670 m, 624 m, 571 m and 526 m |
| (F) | 3422 s | ---- | 3200 w 3100 w | 1612 vs | ----- | 1568 sh | 1523 m | 1365 w | 1348 s | 1171 s | 856 m | 697 m, 659 m, 579 m and 525 m |
| Lost Species | Weight Loss (%) | Tmax (°C) | Decomposition | Compounds | |
|---|---|---|---|---|---|
| Found | Calc. | ||||
| 6C2H2 + 2SO2 + 1.5N2 + 0.5H2 | 93.00 | 93.10 | 265 | First step | H2mel |
| (C14H13N3O4S2) | |||||
| 93.00 | 93.10 | Total loss | |||
| 2C | 7.00 | 6.80 | Residue | ||
| CO + NH3 + H2O | 83.38 | 84.01 | 255 | First step | Gly |
| 83.38 | 84.01 | Total loss | (C2H5NO2) | ||
| C | 16.62 | 15.99 | Residue | ||
| 5H2O | 15.00 | 14.87 | 136 | First step | (A) |
| C2H4 + NO2 + 2H2O | 18.00 | 18.18 | 298 | Second step | (MnC16H30N4O13S2) |
| 6C2H2 + C2N2 + NO + 2SO | 55.14 | 55.24 | 488, 773 | Third step | |
| 88.14 | 88.29 | Total loss | |||
| MnO | 11.86 | 11.71 | Residue | ||
| 2H2O | 7.00 | 6.49 | 105 | First step | (B) |
| C2H4 + NO2 + 2H2O | 20.00 | 19.82 | 374 | Second step | (CoC16H24N4O10S2) |
| 6C2H2 + C2N2 + NO + 2SO | 58.63 | 60.20 | 553, 798 | Third step | |
| 85.63 | 86.51 | Total loss | |||
| CoO | 14.37 | 13.49 | Residue | ||
| H2O | 3.30 | 3.35 | 100 | First step | (C) |
| 2H2O | 6.74 | 6.71 | 198 | Second step | (NiC16H22N4O9S2) |
| 3C2H4 + CO2 + H2S + 1.5N2 | 37.00 | 37.24 | 309 | Third step | |
| 5C2H2 | 24.17 | 24.20 | 561 | Fourth step | |
| C2H2 + C2N2 | 14.17 | 14.60 | 814 | Fifth step | |
| 85.38 | 86.10 | Total loss | |||
| NiO | 14.62 | 13.90 | Residue | ||
| 4H2O + C2H4 + NO2 | 26.10 | 26.09 | 185 | First step | (D) |
| 2C2H2 + NH3 + HCNS | 23.00 | 22.86 | 366 | Second step | (CuC16H24N4O10S2) |
| 3C2H2 | 13.82 | 13.92 | 552 | Third step | |
| HCN + SO2 + CO | 23.75 | 23.64 | 790 | Fourth step | |
| 86.67 | 86.51 | Total loss | |||
| Cu + C | 13.33 | 13.49 | Residue | ||
| 2H2O | 5.30 | 5.85 | 109 | First step | (E) |
| 6C2H2 + C2N2 + C2H4 + 2NO2 + 2SO + 5H2O | 84.46 | 83.53 | 411, 734 | Second step | (ZnC16H30N4O13S2) |
| 89.76 | 89.38 | Total loss | |||
| Zn | 10.24 | 10.62 | Residue | ||
| 3H2O | 8.10 | 8.95 | 100 | First step | (F) |
| C2H4 + NO2 + H2O | 15.00 | 15.25 | 198 | Second step | (ZrC16H24N4O11S2) |
| 6C2H2 + CO + 2SO + NO2 + N2 | 59.27 | 58.71 | 411, 707 | Third step | |
| 82.38 | 82.91 | Total loss | |||
| Zr + C | 17.62 | 17.09 | Residue | ||
| Compounds | Decomposition Range (K) | Ts (K) | Method | Parameters | R a | SD b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| E* (KJ/mol) | A (s−1) | ΔS* (KJ/mol·K) | ΔH* (KJ/mol) | ΔG* (KJ/mol) | ||||||
| H2mel (C14H13N3O4S2) | 467–1273 | 538 | CR | 71.39 | 3.99 × 103 | −0.1809 | 66.92 | 164.28 | 0.967 | 0.324 |
| HM | 55.49 | 9.35 × 102 | −0.1929 | 51.01 | 154.82 | 0.953 | 0.386 | |||
| Gly (C2H5NO2) | 468–588 | 528 | CR | 68.88 | 1.41 × 104 | −0.1701 | 64.49 | 154.34 | 0.980 | 0.223 |
| HM | 65.80 | 1.52 × 104 | −0.1695 | 61.41 | 150.94 | 0.971 | 0.271 | |||
| (A) (MnC16H30N4O13S2) | 669–826 | 731 | CR | 362.765 | 2.09 × 1023 | 0.1940 | 356.68 | 214.80 | 0.974 | 0.287 |
| HM | 348.03 | 9.65 × 1022 | 0.1876 | 341.96 | 204.79 | 0.972 | 0.303 | |||
| 826–1273 | 1046 | CR | 406.68 | 9.11 × 1017 | 0.0884 | 397.98 | 305.44 | 0.995 | 0.084 | |
| HM | 401.42 | 8.16 × 1017 | 0.0875 | 392.73 | 301.14 | 0.995 | 0.087 | |||
| (B) (Co C16H24N4O10S2) | 464–748 | 647 | CR | 96.26 | 2.08 × 106 | −0.13038 | 90.88 | 175.24 | 0.962 | 0.335 |
| HM | 126.54 | 9.94 × 107 | −0.0982 | 121.16 | 184.73 | 0.950 | 0.377 | |||
| 948–1273 | 1071 | CR | 359.69 | 3.58 × 1014 | 0.0230 | 27.06 | 23.40 | 0.977 | 0.220 | |
| HM | 421.60 | 2.87 × 102 | 0.1552 | 412.70 | 246.41 | 0.977 | 0.236 | |||
| (C) (Ni C16H22N4O9S2) | 514–765 | 583 | CR | 131.42 | 1.37 × 108 | −0.0946 | 126.58 | 181.76 | 0.961 | 0.336 |
| HM | 88.55 | 4.47 × 105 | −0.1423 | 83.70 | 166.67 | 0.958 | 0.363 | |||
| 766–944 | 834 | CR | 349.18 | 6.30 × 1022 | 0.1830 | 342.24 | 189.61 | 0.970 | 0.308 | |
| HM | 358.42 | 2.89 × 1020 | 0.1382 | 351.48 | 236.18 | 0.960 | 0.340 | |||
| 944–1273 | 1087 | CR | 983.30 | 1.07 × 1046 | 0.6255 | 974.26 | 294.31 | 0.975 | 0.279 | |
| HM | 101.71 | 1.30 × 1047 | 0.6462 | 1008.09 | 305.59 | 0.973 | 0.287 | |||
| (D) (Cu C16H24N4O10S2) | 558–750 | 639 | CR | 172.48 | 2.74 × 1011 | −0.0322 | 167.16 | 187.78 | 0.985 | 0.194 |
| HM | 162.47 | 1.52 × 1011 | −0.0371 | 157.16 | 180.92 | 0.981 | 0.215 | |||
| 751–949 | 825 | CR | 694.12 | 2.58 × 1043 | 0.5777 | 687.26 | 210.65 | 0.995 | 0.995 0.100 | |
| HM | 736.991 | 9.97 × 1044 | 0.6080 | 730.13 | 228.46 | 0.995 | ||||
| 950–1273 | 1063 | CR | 412.43 | 9.23 × 1017 | 0.0884 | 403.59 | 309.57 | 0.977 | 0.221 | |
| HM | 415.33 | 1.88 × 1018 | 0.0943 | 406.49 | 306.16 | 0.974 | 0.236 | |||
| (E) (Zn C16H30N4O13S2)) | 426–827 | 684 | CR | 35.79 | 15.30 | −0.2291 | 30.11 | 186.83 | 0.987 | 0.176 |
| HM | 83.31 | 8.20 × 103 | −0.1768 | 77.63 | 198.62 | 0.987 | 0.176 | |||
| 827–1273 | 1007 | CR | 756.40 | 3.9 × 1036 | 0.4455 | 748.03 | 299.36 | 0.956 | 0.312 | |
| HM | 745.78 | 7.1 × 1036 | 0.4504 | 737.41 | 283.77 | 0.953 | 0.329 | |||
| (F) (ZrC16H26N4O10S2) | 414–552 | 471 | CR | 120.00 | 1.42 × 1011 | −0.0351 | 116.09 | 132.65 | 0.988 | 0.163 |
| HM | 120.08 | 2.24 × 1011 | −0.0313 | 116.17 | 130.95 | 0.984 | 0.185 | |||
| 553–847 | 684 | CR | 279.65 | 2.88 × 1014 | 0.0249 | 273.95 | 256.83 | 0.986 | 0.181 | |
| HM | 174.96 | 1.63 × 1013 | 0.0011 | 169.27 | 168.48 | 0.984 | 0.297 | |||
| 848–1267 | 980 | CR | 229.81 | 3.53 × 1017 | 0.0811 | 221.66 | 142.14 | 0.971 | 0.297 | |
| HM | 600.29 | 1.24 × 1030 | 0.3213 | 592.14 | 277.27 | 0.967 | 0.317 | |||
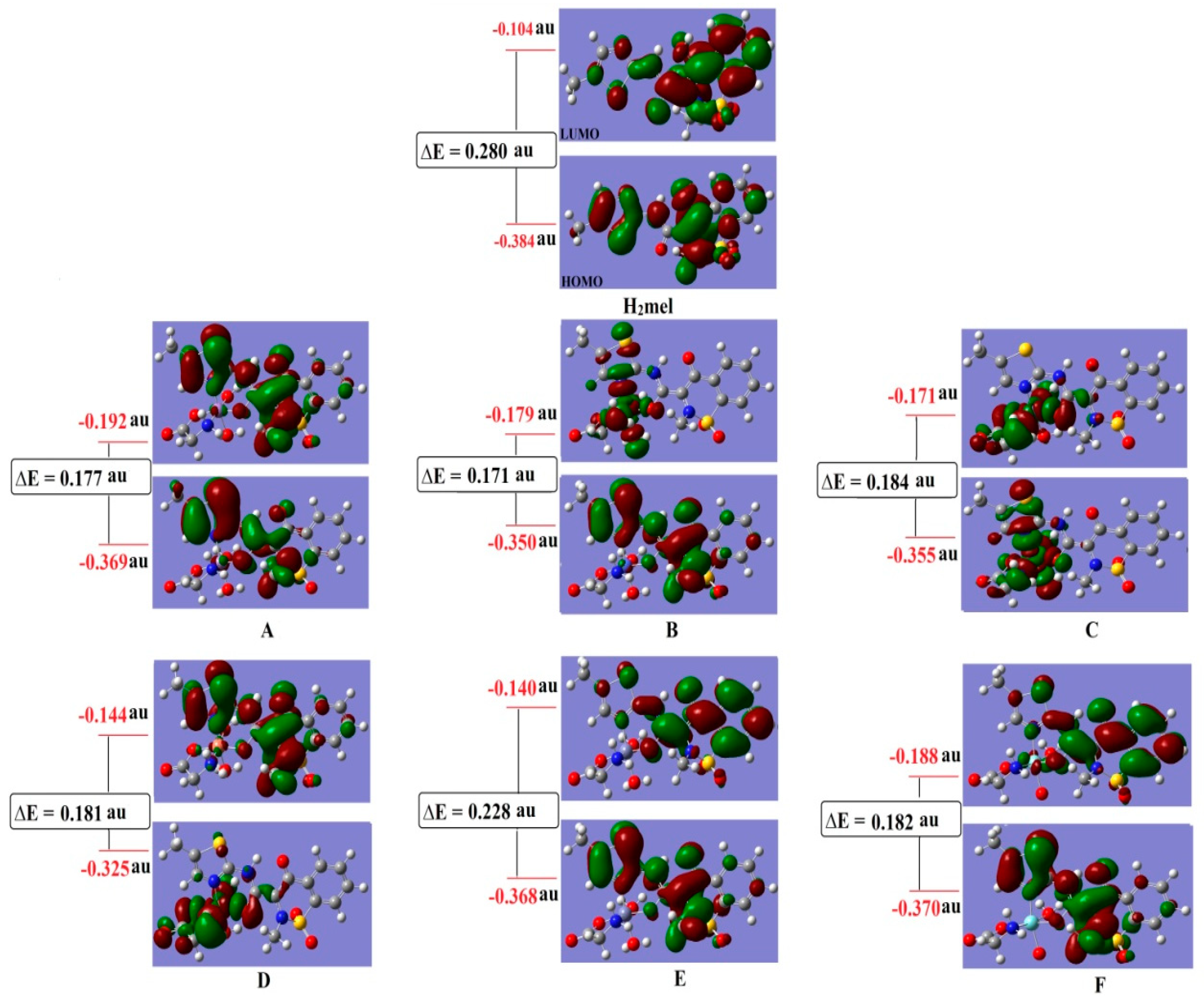
| Parameters | H2mel | (A) | (B) | (C) | (D) | (E) | (F) |
|---|---|---|---|---|---|---|---|
| M | - | −0.153 | −0.052 | 0.095 | −0.117 | 0.111 | 0.367 |
| Nthiazole | −0.318 | −0.174 | −0.139 | −0.153 | −0.133 | −0.147 | −0.158 |
| Ocarbimide | −0.592 | −0.418 | −0.416 | −0.481 | −0.384 | −0.461 | −0.387 |
| N6Gly | - | −0.172 | −0.178 | −0.185 | −0.179 | −0.185 | −0.173 |
| O9Gly | - | −0.444 | −0.438 | −0.513 | −0.393 | −0.495 | −0.408 |
| HOMO, H | −0.384 | −0.369 | −0.350 | −0.355 | −0.325 | −0.368 | −0.370 |
| LUMO, L | −0.104 | −0.192 | −0.179 | −0.171 | −0.144 | −0.140 | −0.188 |
| I = −H | 0.384 | 0.369 | 0.350 | 0.355 | 0.325 | 0.368 | 0.370 |
| A = −L | 0.104 | 0.192 | 0.179 | 0.171 | 0.144 | 0.140 | 0.188 |
| ΔE = L − H | 0.28 | 0.177 | 0.171 | 0.184 | 0.181 | 0.228 | 0.182 |
| η = (I − A)/2 | 0.140 | 0.089 | 0.086 | 0.092 | 0.091 | 0.114 | 0.091 |
| χ = −(H − L/2) | 0.244 | 0.281 | 0.265 | 0.263 | 0.235 | 0.254 | 0.279 |
| σ = 1/η | 7.14 | 11.236 | 11.628 | 10.869 | 10.989 | 8.772 | 10.989 |
| S = 1/2 η | 3.57 | 5.618 | 5.814 | 5.435 | 5.495 | 4.386 | 5.495 |
| Pi = −χ | −0.244 | −0.281 | −0.265 | −0.263 | −0.235 | −0.254 | −0.279 |
| ω = (Pi)2/2 η | 0.213 | 0.444 | 0.775 | 0.376 | 0.303 | 0.283 | 0.428 |
| ΔNmax = χ/η | 1.74 | 3.16 | 3.081 | 2.859 | 2.582 | 2.228 | 3.066 |
| Tested Compounds | Tested Microorganism | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | |||||||||||||
| S. aureus | MIC | Listeria | MIC | E. coli | MIC | Coliform | MIC | Citrobacter | MIC | S. typhimurium | MIC | A. niger. | MIC | |
| H2mel | 0 | - | 0 | - | 7.3+2 ± 0.8 | 50.0 ± 0.02 | 0 | - | 4.6+3 ± 0.3 | 30.0 ± 0.1 | 0 | - | 0 | |
| Gly | 0 | - | 5+2 ± 0.8 | 12.8 ± 1.3 | 4+2 ± 0.9 | 25.6 ± 2.3 | 6+2 ± 1.2 | 12.8 ± 1.2 | 0 | - | 0 | - | 0 | - |
| (A) | 0 | - | 4+2 ± 0.5 | 10.8 ± 1.1 | 2+2 ± 0.2 | 10.8 ± 1.2 | 6+2 ± 1.1 | 21.6 ± 1.8 | 0 | - | 0 | - | 0 | - |
| (B) | 0 | - | 3+2 ± 0.2 | 44.32 ± 2.3 | 7+2 ± 1.1 | 22.16 ± 1.5 | 7+2 ± 1.3 | 22.16 ± 1.6 | 0 | - | 0 | - | 0 | - |
| (C) | 0 | - | 1 ± 0.1+2 | 32.16 ± 2.1 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - |
| (D) | 0 | - | 2+2 ± 0.4 | 20.96 ± 1.8 | 5+2 ± 0.9 | 20.96 ± 1.9 | 7+2 ± 1.2 | 20.96 ± 2.1 | 0 | - | 0 | - | 0 | - |
| (E) | 0 | - | 2+2 ± 0.2 | 24.632 ± 1.2 | 6+2 ± 1.1 | 36.94 ± 2.3 | 0 | - | 0 | - | 0 | - | 0 | - |
| (F) | 0 | - | 0 | - | 2+3 ± 0.1 | 36.21 ± 2.5 | 0 | - | 0 | - | 0 | - | 0 | |
| Control (DMF) | 0.26 ± 0.012 | 0.14 ± 0.01 | 0.26 ± 0.02 | 0.35 ± 0.022 | 0.35 ± 0.022 | 0 | - | 0 | ||||||
| A/C | 1.9 ± 0.4 | 1 ± 0.1 | 1.7 ± 0.2 | 1.4 ± 0.3 | 0 | 0 | 0 | |||||||
| Cetaxime | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshafie, H.S.; Sadeek, S.A.; Zordok, W.A.; Mohamed, A.A. Meloxicam and Study of Their Antimicrobial Effects against Phyto- and Human Pathogens. Molecules 2021, 26, 1480. https://doi.org/10.3390/molecules26051480
Elshafie HS, Sadeek SA, Zordok WA, Mohamed AA. Meloxicam and Study of Their Antimicrobial Effects against Phyto- and Human Pathogens. Molecules. 2021; 26(5):1480. https://doi.org/10.3390/molecules26051480
Chicago/Turabian StyleElshafie, Hazem S., Sadeek A. Sadeek, Wael A. Zordok, and Amira A. Mohamed. 2021. "Meloxicam and Study of Their Antimicrobial Effects against Phyto- and Human Pathogens" Molecules 26, no. 5: 1480. https://doi.org/10.3390/molecules26051480
APA StyleElshafie, H. S., Sadeek, S. A., Zordok, W. A., & Mohamed, A. A. (2021). Meloxicam and Study of Their Antimicrobial Effects against Phyto- and Human Pathogens. Molecules, 26(5), 1480. https://doi.org/10.3390/molecules26051480









