Co-Opting Host Receptors for Targeted Delivery of Bioconjugates—From Drugs to Bugs
Abstract
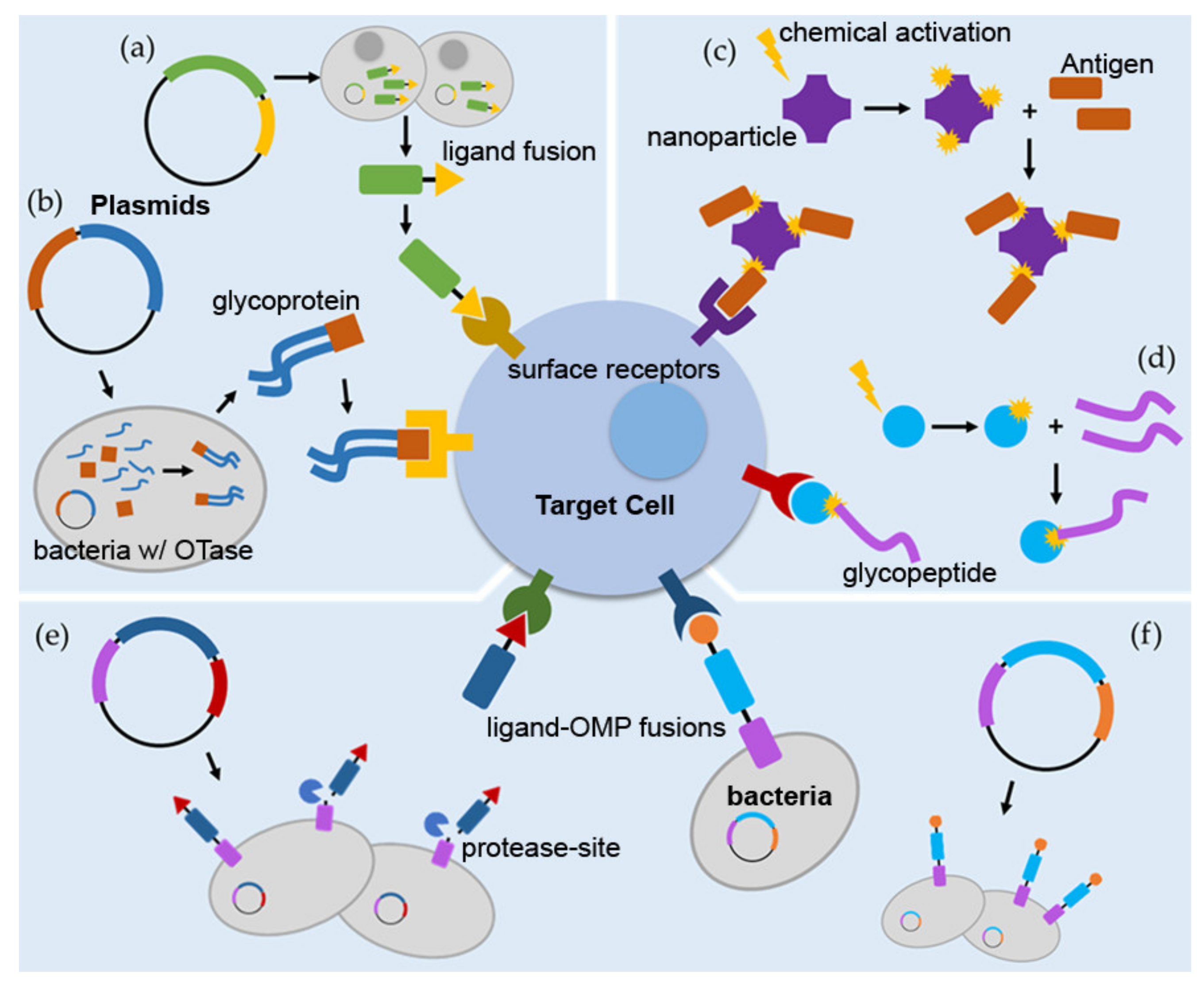
1. Introduction
2. Targeted Therapies
2.1. Cancer Therapies
2.2. Central Nervous System Therapies
2.3. Antimicrobials
3. Targeted Vaccinations
3.1. Protein Subunit Vaccine Conjugates
3.2. Glyconjugate Vaccines
3.3. Conjugated Nanoparticle-Based Vaccines
3.4. Targeting and Conjugation Involving Whole-Cell Vaccines
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.; Delamarre, L. Dendritic cell-targeted vaccines. Front. Immunol. 2014, 5, 255. [Google Scholar] [CrossRef] [PubMed]
- Goyvaerts, C.; Breckpot, K. Pros and Cons of Antigen-Presenting Cell Targeted Tumor Vaccines. J. Immunol Res. 2015, 2015, 785634. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, S.; Lyu, H.; Riker, A.I.; Zhang, Y.; Liu, B. Development of Effective Therapeutics Targeting HER3 for Cancer Treatment. Biol. Proced. Online 2019, 21, 5. [Google Scholar] [CrossRef]
- Sancho, D.; Mourao-Sa, D.; Joffre, O.P.; Schulz, O.; Rogers, N.C.; Pennington, D.J.; Carlyle, J.R.; Reis e Sousa, C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J. Clin. Investig. 2008, 118, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Cedazo-Minguez, A.; Kenigsberg, P.; Page, G.; Duarte, A.; Giusti, P.; Zusso, M.; Robert, P.; Frisoni, G.; Cattaneo, A.; et al. Current and emerging avenues for Alzheimer’s disease drug targets. J. Intern. Med. 2019, 286, 398–437. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J. Parkinson’s disease: A review. Front. Biosci. (Sch. Ed.) 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Nguyen, L.; Garcia, J.; Gruenberg, K.; MacDougall, C. Multidrug-Resistant Pseudomonas Infections: Hard to Treat, But Hope on the Horizon? Curr. Infect. Dis. Rep. 2018, 20, 23. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- DeVita, V.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Chari, R.; Miller, M.; Widdison, W. Antibody-drug conjugates: An emerging concept in cancer therapy. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 3796–3827. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J. Site-specific antibody drug conjugates for cancer therapy. In mAbs; Taylor Francis: Abingdon-on-Thames, UK, 2014; Volume 6. [Google Scholar] [CrossRef] [PubMed]
- Grattan, B.; Freake, H. Zinc and cancer: Implications for LIV-1 in breast cancer. Nutrients 2012, 4, 648–675. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T. Targeted Therapies for Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Pusztai, L.; Forero, A.; Mita, M.; Miller, K.; Weise, A.; Krop, I.; Burris, H.; Kalinsky, K.; Tsai, M.; et al. Abstract PD3-14: Phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Cancer Res. 2018. [Google Scholar] [CrossRef]
- McGuinness, J.; Kalinsky, K. Antibody-drug conjugates in metastatic triple negative breast cancer: A spotlight on sacituzumab govitecan, ladiratuzumab vedotin, and trastuzumab deruxtecan. Expert Opin. Biol. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, D.; Jiang, Z.; Tong, R.; Jiang, P.; Bai, L.; Chen, L.; Zhu, Y.; Guo, C.; Shi, J.; et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur. J. Med. Chem. 2019, 183, 111682. [Google Scholar] [CrossRef] [PubMed]
- De Propris, M.; Raponi, S.; Diverio, D.; Milani, M.; Meloni, G.; Falini, B.; Foà, R.; Guarini, A. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica 2011, 96, 1548. [Google Scholar] [CrossRef]
- Hughes, B. Antibody-drug conjugates for cancer: Poised to deliver? Nat. Rev. Drug Discov. 2010, 9, 665. [Google Scholar] [CrossRef]
- Gomes, I.; Arinto, P.; Lopes, C.; Santos, C.; Maia, C. STEAP1 is overexpressed in prostate cancer and prostatic intraepithelial neoplasia lesions, and it is positively associated with Gleason score. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Hoboken, NJ, USA, 2014; Volume 32. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.; Kasamon, Y.; Chen, H.; de Claro, R.; Ye, J.; Blumenthal, G.; Farrell, A.; Pazdur, R. FDA Approval Summary: Brentuximab Vedotin in First-Line Treatment of Peripheral T-Cell Lymphoma. Oncology 2019, 24, e180. [Google Scholar] [CrossRef]
- Wedam, S.; Fashoyin-Aje, L.; Gao, X.; Bloomquist, E.; Tang, S.; Sridhara, R.; Goldberg, K.; King-Kallimanis, B.; Theoret, M.; Ibrahim, A.; et al. FDA Approval Summary: Ado-Trastuzumab Emtansine for the Adjuvant Treatment of HER2-positive Early Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4180–4185. [Google Scholar] [CrossRef]
- Norsworthy, K.; Ko, C.; Lee, J.; Liu, J.; John, C.; Przepiorka, D.; Farrell, A.; Pazdur, R. FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. Oncology 2018, 23, 1103. [Google Scholar] [CrossRef]
- Lamb, Y. Inotuzumab Ozogamicin: First Global Approval. Drugs 2017, 77, 1603–1610. [Google Scholar] [CrossRef]
- Lambert, J.; Morris, C. Antibody-Drug Conjugates (ADCs) for Personalized Treatment of Solid Tumors: A Review. Adv. Ther. 2017, 34, 1015–1035. [Google Scholar] [CrossRef]
- Versteegen, R.; Rossin, R.; ten Hoeve, W.; Janssen, H.; Robillard, M. Click to release: Instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. (Int. Ed. Engl.) 2013, 52, 14362–14366. [Google Scholar] [CrossRef]
- Oliveira, B.; Guo, Z.; Bernardes, G. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed]
- Van Onzen, A.; Versteegen, R.; Hoeben, F.; Filot, I.; Rossin, R.; Zhu, T.; Wu, J.; Hudson, P.; Janssen, H.; Ten Hoeve, W.; et al. Bioorthogonal Tetrazine Carbamate Cleavage by Highly Reactive trans-Cyclooctene. J. Am. Chem. Soc. 2020, 142, 10955–10963. [Google Scholar] [CrossRef]
- Rossin, R.; Versteegen, R.; Wu, J.; Khasanov, A.; Wessels, H.; Steenbergen, E.; Ten Hoeve, W.; Janssen, H.; van Onzen, A.; Hudson, P.; et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Xu, M.; Parvez, S.; Peterson, R.; Franzini, R. Bioorthogonal Removal of 3-Isocyanopropyl Groups Enables the Controlled Release of Fluorophores and Drugs in Vivo. J. Am. Chem. Soc. 2018, 140, 8410–8414. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Deb, T.; Tu, J.; Franzini, R. Tuning Isonitrile/Tetrazine Chemistry for Accelerated Deprotection and Formation of Stable Conjugates. J. Org. Chem. 2019, 84, 15520–15529. [Google Scholar] [CrossRef]
- Tu, J.; Svatunek, D.; Parvez, S.; Eckvahl, H.; Xu, M.; Peterson, R.; Houk, K.; Franzini, R. Isonitrile-responsive and bioorthogonally removable tetrazine protecting groups. Chem. Sci. 2019, 11, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Sussman, D.; Smith, L.; Anderson, M.; Duniho, S.; Hunter, J.; Kostner, H.; Miyamoto, J.; Nesterova, A.; Westendorf, L.; Van Epps, H.; et al. SGN-LIV1A: A novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol. Cancer Ther. 2014, 13, 2991–3000. [Google Scholar] [CrossRef]
- Park, H.Y.; Tan, P.S.; Kavishna, R.; Ker, A.; Lu, J.; Chan, C.E.Z.; Hanson, B.J.; MacAry, P.A.; Caminschi, I.; Shortman, K.; et al. Enhancing vaccine antibody responses by targeting Clec9A on dendritic cells. NPJ Vaccines 2017, 2, 31. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef]
- Rusiecka, I.; Ruczyński, J.; Kozłowska, A.; Backtrog, E.; Mucha, P.; Kocić, I.; Rekowski, P. TP10-Dopamine Conjugate as a Potential Therapeutic Agent in the Treatment of Parkinson’s Disease. Bioconjugate Chem. 2019, 30, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Shamili, F.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Mahmoudi, M.; Kalantari, M.; Taghdisi, S.; Ramezani, M. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J. Control. Release Off. J. Control. Release Soc. 2019, 299, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Domanskyi, A.; Renko, J.; Sarparanta, M.; Wang, C.; Correia, A.; Mäkilä, E.; Alanen, O.; Salonen, J.; Airaksinen, A.; et al. Engineered antibody-functionalized porous silicon nanoparticles for therapeutic targeting of pro-survival pathway in endogenous neuroblasts after stroke. Biomaterials 2020, 227, 119556. [Google Scholar] [CrossRef]
- Lehar, S.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.; Morisaki, J.; et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.; Zhang, L. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J. Am. Chem. Soc. 2011, 133, 4132–4139. [Google Scholar] [CrossRef] [PubMed]
- Grødeland, G.; Mjaaland, S.; Tunheim, G.; Fredriksen, A.; Bogen, B. The specificity of targeted vaccines for APC surface molecules influences the immune response phenotype. PLoS ONE 2013, 8, e0080008. [Google Scholar] [CrossRef][Green Version]
- Lysen, A.; Braathen, R.; Gudjonsson, A.; Tesfaye, D.Y.; Bogen, B.; Fossum, E. Dendritic cell targeted Ccl3- and Xcl1-fusion DNA vaccines differ in induced immune responses and optimal delivery site. Sci. Rep. 2019, 9, 1820. [Google Scholar] [CrossRef] [PubMed]
- Fossum, E.; Grødeland, G.; Terhorst, D.; Tveita, A.; Vikse, E.; Mjaaland, S.; Henri, S.; Malissen, B.; Bogen, B. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus. Eur. J. Immunol. 2015, 45, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Micoli, F. Improving efficacy of glycoconjugate vaccines: From chemical conjugates to next generation constructs. Curr. Opin. Immunol. 2020, 65, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.F.; Mayer Bridwell, A.E.; Scott, N.E.; Vinogradov, E.; McKee, S.R.; Chavez, S.M.; Twentyman, J.; Stallings, C.L.; Rosen, D.A.; Harding, C.M. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2019, 116, 18655–18663. [Google Scholar] [CrossRef]
- Leneghan, D.B.; Miura, K.; Taylor, I.J.; Li, Y.; Jin, J.; Brune, K.D.; Bachmann, M.F.; Howarth, M.; Long, C.A.; Biswas, S. Nanoassembly routes stimulate conflicting antibody quantity and quality for transmission-blocking malaria vaccines. Nature 2017. [Google Scholar] [CrossRef]
- Layek, B.; Lipp, L.; Singh, J. APC targeted micelle for enhanced intradermal delivery of hepatitis B DNA vaccine. J. Control. Release 2015, 207, 143–153. [Google Scholar] [CrossRef]
- Musa, H.H.; Zhang, W.J.; Lv, J.; Duan, X.L.; Yang, Y.; Zhu, C.H.; Li, H.F.; Chen, K.W.; Meng, X.; Zhu, G.Q. The molecular adjuvant mC3d enhances the immunogenicity of FimA from type I fimbriae of Salmonella enterica serovar Enteritidis. J. Microbiol. Immunol. Infect. 2014, 47, 57–62. [Google Scholar] [CrossRef]
- Green, T.D.; Montefiori, D.C.; Ross, T.M. Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d. J. Virol. 2003, 77, 2046–2055. [Google Scholar] [CrossRef][Green Version]
- Holland-Tummillo, K.M.; Shoudy, L.E.; Steiner, D.; Kumar, S.; Rosa, S.J.; Namjoshi, P.; Singh, A.; Sellati, T.J.; Gosselin, E.J.; Hazlett, K.R. Autotransporter-Mediated Display of Complement Receptor Ligands by Gram-Negative Bacteria Increases Antibody Responses and Limits Disease Severity. Pathogens 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Pompa-Mera, E.N.; Arroyo-Matus, P.; Ocana-Mondragon, A.; Gonzalez-Bonilla, C.R.; Yepez-Mulia, L. Protective immunity against enteral stages of Trichinella spiralis elicited in mice by live attenuated Salmonella vaccine that secretes a 30-mer parasite epitope fused to the molecular adjuvant C3d-P28. Res. Vet. Sci. 2014, 97, 533–545. [Google Scholar] [CrossRef] [PubMed]
- 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12. [CrossRef]
- Xia, H.; Anderson, B.; Mao, Q.; Davidson, B. Recombinant human adenovirus: Targeting to the human transferrin receptor improves gene transfer to brain microcapillary endothelium. J. Virol. 2000, 74, 11359–11366. [Google Scholar] [CrossRef] [PubMed]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef]
- Johnsen, K.; Burkhart, A.; Thomsen, L.; Andresen, T.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Savitt, J. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Yandek, L.; Pokorny, A.; Florén, A.; Knoelke, K.; Langel, U.; Almeida, P. Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys. J. 2007, 92, 2434–2444. [Google Scholar] [CrossRef]
- Nastasijevic, B.; Wright, B.; Smestad, J.; Warrington, A.; Rodriguez, M.; Maher, L. Remyelination induced by a DNA aptamer in a mouse model of multiple sclerosis. PLoS ONE 2012, 7, e0039595. [Google Scholar] [CrossRef]
- Khardori, N.; Stevaux, C.; Ripley, K. Antibiotics: From the Beginning to the Future: Part 1. Indian J. Pediatrics 2020, 87, 39–42. [Google Scholar] [CrossRef]
- Mohr, K. History of Antibiotics Research. Curr. Top. Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Mudshinge, S.; Deore, A.; Patil, S.; Bhalgat, C. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–4392. [Google Scholar] [CrossRef]
- Crooke, S.; Schimer, J.; Raji, I.; Wu, B.; Oyelere, A.; Finn, M. Lung Tissue Delivery of Virus-Like Particles Mediated by Macrolide Antibiotics. Mol. Pharm. 2019, 16, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Pageni, P.; Rahman, M.; Bam, M.; Zhu, T.; Chen, Y.; Nagarkatti, M.; Decho, A.; Tang, C. Gold Nanoparticles with Antibiotic-Metallopolymers toward Broad-Spectrum Antibacterial Effects. Adv. Healthc. Mater. 2019, 8, 1800854. [Google Scholar] [CrossRef] [PubMed]
- Bagga, P.; Siddiqui, H.; Akhtar, J.; Mahmood, T.; Zahera, M.; Khan, M. Gold Nanoparticles Conjugated Levofloxacin: For Improved Antibacterial Activity over Levofloxacin Alone. Curr. Drug Deliv. 2017, 14, 1114–1119. [Google Scholar] [CrossRef]
- Palmieri, G.; Tatè, R.; Gogliettino, M.; Balestrieri, M.; Rea, I.; Terracciano, M.; Proroga, Y.; Capuano, F.; Anastasio, A.; De Stefano, L. Small Synthetic Peptides Bioconjugated to Hybrid Gold Nanoparticles Destroy Potentially Deadly Bacteria at Submicromolar Concentrations. Bioconjugate Chem. 2018, 29, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, K. Global Eradication of Small-Pox: Historical Fact, Experiences and Enlightenment. Zhonghua Liu Xing Bing Xue Za Zhi 1999, 20, 67–70. [Google Scholar]
- Baxby, D. Edward Jenner’s Inquiry; A Bicentenary Analysis. Vaccine 1999, 17, 301–307. [Google Scholar] [CrossRef]
- Buckland, B.C. The development and manufacture of influenza vaccines. Hum. Vaccin Immunother. 2015, 11, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B. Cholera: Immunity and Prospects in Vaccine Development. J. Infect. Dis. 2018, 218, S141–S146. [Google Scholar] [CrossRef]
- Gerlich, W.H. Medical Virology of Hepatitis B: How it began and where we are now. Virol. J. 2013, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Ogden, S.A.; Ludlow, J.T.; Alsayouri, K. Diphtheria Tetanus Pertussis (DTaP) Vaccine. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545173/ (accessed on 18 December 2020).
- Harandi, A.M. Systems Analysis of Human Vaccine Adjuvants. Semin. Immunol. 2018, 39, 30–34. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garcon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B. Alum Adjuvant: Some of the Tricks of the Oldest Adjuvant. J. Med Microbiol. 2012, 61. [Google Scholar] [CrossRef]
- Rawool, D.B.; Bitsaktsis, C.; Li, Y.; Gosselin, D.R.; Lin, Y.; Kurkure, N.V.; Metzger, D.W.; Gosselin, E.J. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J. Immunol. 2008, 180, 5548–5557. [Google Scholar] [CrossRef]
- Gosselin, E.J.; Bitsaktsis, C.; Li, Y.; Iglesias, B.V. Fc receptor-targeted mucosal vaccination as a novel strategy for the generation of enhanced immunity against mucosal and non-mucosal pathogens. Arch. Immunol. Ther. Exp. 2009, 57, 311–323. [Google Scholar] [CrossRef]
- Iglesias, B.; Bitsaktsis, C.; Pham, G.; Drake, J.; Hazlett, K.; Porter, K.; Gosselin, E. Multiple mechanisms mediate enhanced immunity generated by mAb-inactivated F. tularensis immunogen. Immunol. Cell Biol. 2013, 91, 139–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haas, K.M.; Toapanta, F.R.; Oliver, J.A.; Poe, J.C.; Weis, J.H.; Karp, D.R.; Bower, J.F.; Ross, T.M.; Tedder, T.F. Cutting edge: C3d functions as a molecular adjuvant in the absence of CD21/35 expression. J. Immunol 2004, 172, 5833–5837. [Google Scholar] [CrossRef]
- Bower, J.F.; Ross, T.M. A minimum CR2 binding domain of C3d enhances immunity following vaccination. Adv. Exp. Med. Biol. 2006, 586, 249–264. [Google Scholar]
- Dempsey, P.W.; Allison, M.E.; Akkaraju, S.; Goodnow, C.C.; Fearon, D.T. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science 1996, 271, 348–350. [Google Scholar] [CrossRef]
- García-Machorro, J.; López-González, M.; Barrios-Rojas, O.; Fernández-Pomares, C.; Sandoval-Montes, C.; Santos-Argumedo, L.; Villegas-Sepúlveda, N.; Gutiérrez-Castañeda, B.; Cedillo-Barrón, L. DENV-2 Subunit Proteins Fused to CR2 Receptor-Binding Domain (P28)-induces Specific and Neutralizing Antibodies to the Dengue Virus in Mice. Hum. Vaccines Immunother. 2013, 9, 2326–2335. [Google Scholar] [CrossRef]
- Dunn, M.D.; Rossi, S.L.; Carter, D.M.; Vogt, M.R.; Mehlhop, E.; Diamond, M.S.; Ross, T.M. Enhancement of anti-DIII Antibodies by the C3d Derivative P28 Results in Lower Viral Titers and Augments Protection in Mice. Virol. J. 2010, 7, 95. [Google Scholar] [CrossRef]
- Wang, L.X.; Xu, W.; Guan, Q.D.; Chu, Y.W.; Wang, Y.; Xiong, S.D. Contribution of C3d-P28 Repeats to Enhancement of Immune Responses Against HBV-preS2/S Induced by Gene Immunization. World J. Gastroenterol. 2004, 10, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xia, Q.; Wu, J.; Liu, D.; Wang, X.; Niu, Z. Construction and Immunogenicity of DNA Vaccines Encoding Fusion Protein of Murine Complement C3d-p28 and GP5 Gene of Porcine Reproductive and Respiratory Syndrome Virus. Vaccine 2011, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Mettu, R.; Chen, C.; Wu, C. Synthetic carbohydrate-based vaccines: Challenges and opportunities. J. Biomed. Sci. 2020, 27. [Google Scholar] [CrossRef]
- Barton, G.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; McLoughlin, R.; Cobb, B.; Charrel-Dennis, M.; Zaleski, K.; Golenbock, D.; Tzianabos, A.; Kasper, D. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 2006, 203, 2853–2863. [Google Scholar] [CrossRef]
- Test, S.T.; Mitsuyoshi, J.; Connolly, C.C.; Lucas, A.H. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect. Immun. 2001, 69, 3031–3040. [Google Scholar] [CrossRef]
- Riddle, M.; Kaminski, R.; Di Paolo, C.; Porter, C.; Gutierrez, R.; Clarkson, K.; Weerts, H.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: A Single-Blind, Randomized Phase I Study. Clin. Vaccine Immunol. Civ. 2016, 23, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R. Glycoconjugate vaccines: Principles and mechanisms. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Wacker, M.; Linton, D.; Hitchen, P.; Nita-Lazar, M.; Haslam, S.; North, S.; Panico, M.; Morris, H.; Dell, A.; Wren, B.; et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 2002, 298, 1790–1793. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, N.; Haeuptle, M.; Kowarik, M.; Fernandez, F.; Carranza, P.; Brunner, A.; Steffen, M.; Wetter, M.; Keller, S.; Ruch, C.; et al. Purification and characterization of a Shigella conjugate vaccine, produced by glycoengineering Escherichia coli. Glycobiology 2016, 26, 51–62. [Google Scholar] [CrossRef]
- Feldman, M.; Wacker, M.; Hernandez, M.; Hitchen, P.; Marolda, C.; Kowarik, M.; Morris, H.; Dell, A.; Valvano, M.; Aebi, M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. USA 2005, 102, 3016–3021. [Google Scholar] [CrossRef]
- Doorduijn, D.J.; Rooijakkers, S.H.; van Schaik, W.; Bardoel, B.W. Complement Resistance Mechanisms of Klebsiella Pneumoniae. Immunobiology 2016, 221, 1102–1109. [Google Scholar] [CrossRef]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.; Dane, E.L.; et al. Innate Immune Recognition of Glycans Targets HIV Nanoparticle Immunogens to Germinal Centers. Science 2019, 363, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.C.; Wang, A.Z. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. Quant. Biosci. Nano Macro 2014, 6, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.; Care, A.; Sunna, A. Bioengineering Strategies for Protein-Based Nanoparticles. Genes 2018, 9, 370. [Google Scholar] [CrossRef]
- Liébana, S.; Drago, G. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Scally, S.W.; McLeod, B.; Bosch, A.; Miura, K.; Liang, Q.; Carroll, S.; Reponen, S.; Nguyen, N.; Giladi, E.; Rämisch, S.; et al. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schudel, A.; Chapman, A.; Yau, M.; Higginson, C.; Francis, D.; Manspeaker, M.; Avecilla, A.; Rohner, N.; Finn, M.; Thomas, S. Programmable multistage drug delivery to lymph nodes. Nat. Nanotechnol. 2020, 15, 491–499. [Google Scholar] [CrossRef]
- Kwon, Y.; James, E.; Shastri, N.; Fréchet, J. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc. Natl. Acad. Sci. USA 2005, 102, 18264–18268. [Google Scholar] [CrossRef] [PubMed]
| Targeting Unit | Cargo | Targeted Disease/Treatment/Effect | Stage of Use |
|---|---|---|---|
| Cancer Therapies | |||
| α-LIV-1 Ab | Monomethyl auristatin E | Metastatic breast cancer [15,16,17,34] | Phase 2 trials |
| α-HER2 Ab | Deruxtecan | HER2-positive breast & stomach cancer [15,18] | Clinical use |
| α-HER2 Ab | Mertansine | HER2-positive early breast cancer [23] | Clinical use |
| α-CD33 Ab | N-acetyl γ calicheamicin | Acute myeloid leukemia [24,25,35] | Clinical use |
| α-CD33 Ab | Calicheamicin | Relapsed/refractory acute myeloid leukaemia [24] | Clinical use |
| α-CD30 Ab | Monomethyl auristatin E | Hodgkin’s lymphoma [22] | Clinical use |
| α-CD22 Ab | Calicheamicin | Relapsed/refractory acute lymphoblastic leukaemia [25] | Clinical use |
| α-gp72 Ab | Monomethyl auristatin E | Models of ovarian and colon carcinoma [30] | Pre-clinical |
| Central Nervous System Therapies | |||
| B6 peptide | Curcumin | Alzheimer’s disease. Delivery of loaded NPs to TfRs on the BBB, improved memory & learning [36] | Pre-clinical |
| TP10 peptide | Dopamine | Parkinson’s disease. Delivery of dopamine to the brain [37] | Pre-clinical |
| LJM3064 aptamer | Exosomes | Multiple sclerosis (EAE) associated demyelination [38] | Pre-clinical |
| α-PSA-NECM Ab | SC-79 | Post-stroke neuro-regeneration. Delivery of SC-79 loaded NPs to neuroblasts to enhance pro-survival signaling [39] | Pre-clinical |
| Antimicrobials | |||
| α-teichoic acid Ab | Rifalogue | Enhanced killing of intracellular MRSA [40] | Pre-clinical |
| α toxin-reactive NPs | Vancomycin | Controlled release of antibiotic at the site of infection [41] | Pre-clinical |
| Targeted Vaccinations | |||
| α-Clec9A Ab | Influenza M2e | Target influenza Ag to DCs to enhance responses & protection [35] | Pre-clinical |
| α-MHC-II Ab | Influenza HA | Increased α-HA Ab & Th2 responses, protecting against influenza [42] | Pre-clinical |
| α-CCR1/3/5 Ab | Influenza HA | Increased CD8+ & Th1 responses, protecting against influenza [42] | Pre-clinical |
| Xcl1 | Influenza HA | Increased proliferation of CD4+ & CD8+ T cells against influenza [43,44] | Pre-clinical |
| Ccl3 | Influenza HA | Target HA to CCR1/3/5 to induce CD4+ T cells against influenza [43] | Pre-clinical |
| Diptheria toxoid | Nm PS | Increased α-Nm PS Abs, protection for meningococcal disease [45] | Clinical use |
| Pa exotoxin protein A | Kp PS | Increased α-Kp PS Abs, protection against Kp infection [46] | Pre-clinical |
| Qβ VLPs | Pfs25 | Increased transmission-blocking Abs against malaria [47] | Pre-clinical |
| Mannose | hepB DNA | APC transfection via MR, stimulating α-HepB responses [48] | Pre-clinical |
| C3d | Se FimA | Increased immunogenicity of FimA, protection against Se [49] | Pre-clinical |
| C3d | HIV1 Env | Increased neutralizing Ab production against HIV1 [50] | Pre-clinical |
| C3d | Ft whole cells | Increased Ag binding to APCs, protection against tularemia [51] | Pre-clinical |
| C3d p28 | Ts Ag30 | Increased Ab production, protection against trichinosis [52] | Pre-clinical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummillo, K.M.; Hazlett, K.R.O. Co-Opting Host Receptors for Targeted Delivery of Bioconjugates—From Drugs to Bugs. Molecules 2021, 26, 1479. https://doi.org/10.3390/molecules26051479
Tummillo KM, Hazlett KRO. Co-Opting Host Receptors for Targeted Delivery of Bioconjugates—From Drugs to Bugs. Molecules. 2021; 26(5):1479. https://doi.org/10.3390/molecules26051479
Chicago/Turabian StyleTummillo, Kristen M., and Karsten R.O. Hazlett. 2021. "Co-Opting Host Receptors for Targeted Delivery of Bioconjugates—From Drugs to Bugs" Molecules 26, no. 5: 1479. https://doi.org/10.3390/molecules26051479
APA StyleTummillo, K. M., & Hazlett, K. R. O. (2021). Co-Opting Host Receptors for Targeted Delivery of Bioconjugates—From Drugs to Bugs. Molecules, 26(5), 1479. https://doi.org/10.3390/molecules26051479







