Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical and Ultrasound-Assisted Pectin Extraction
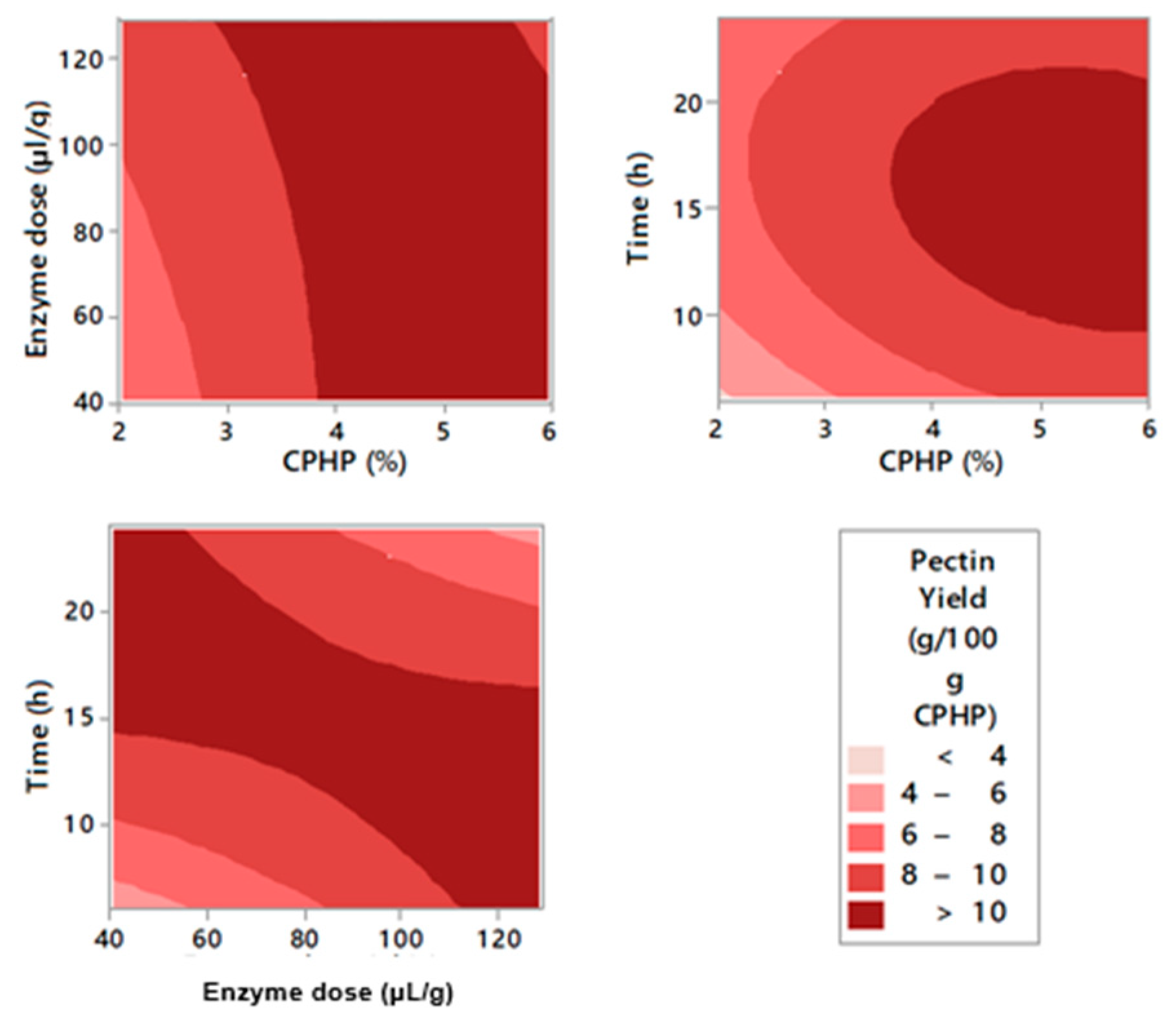
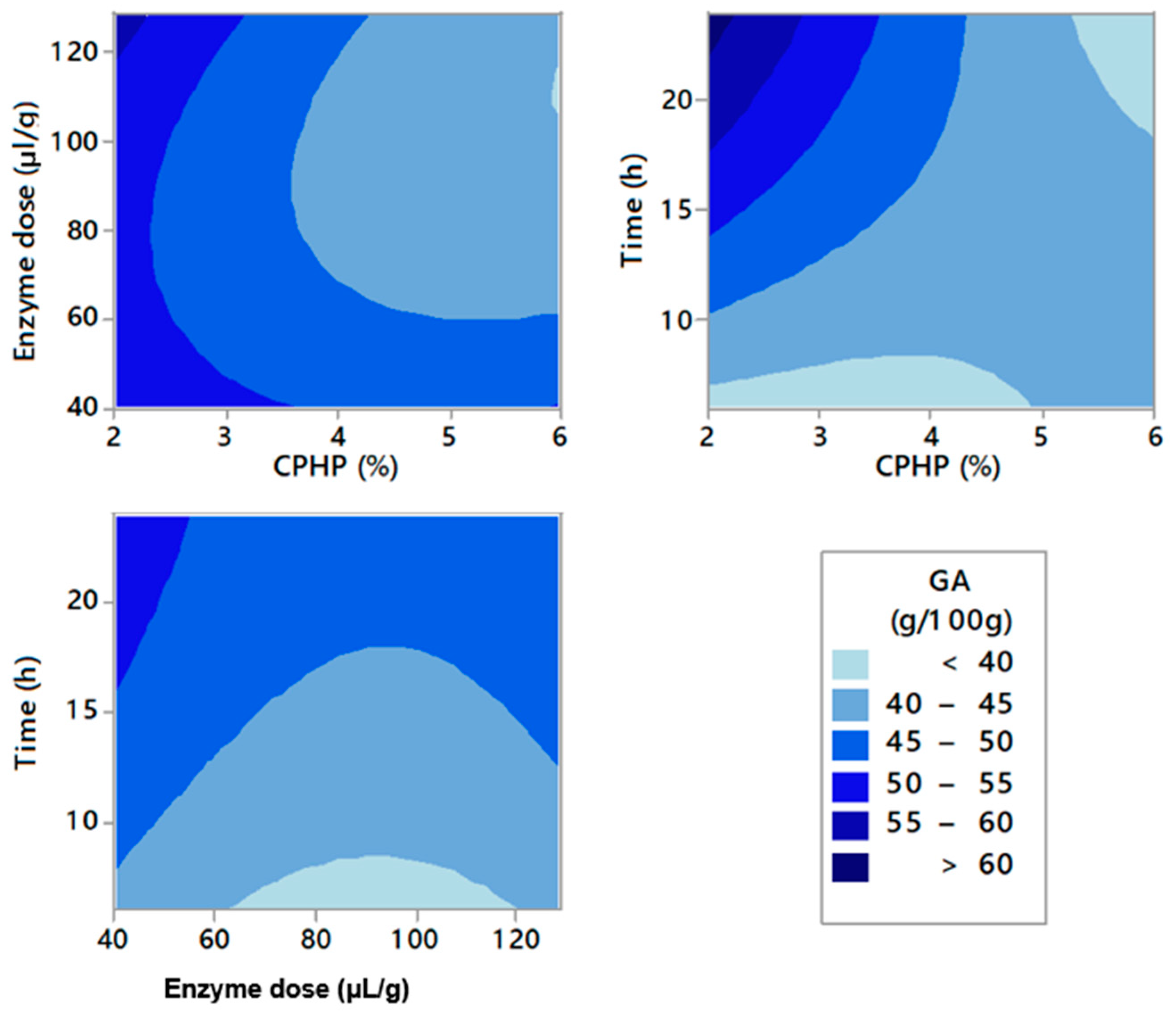
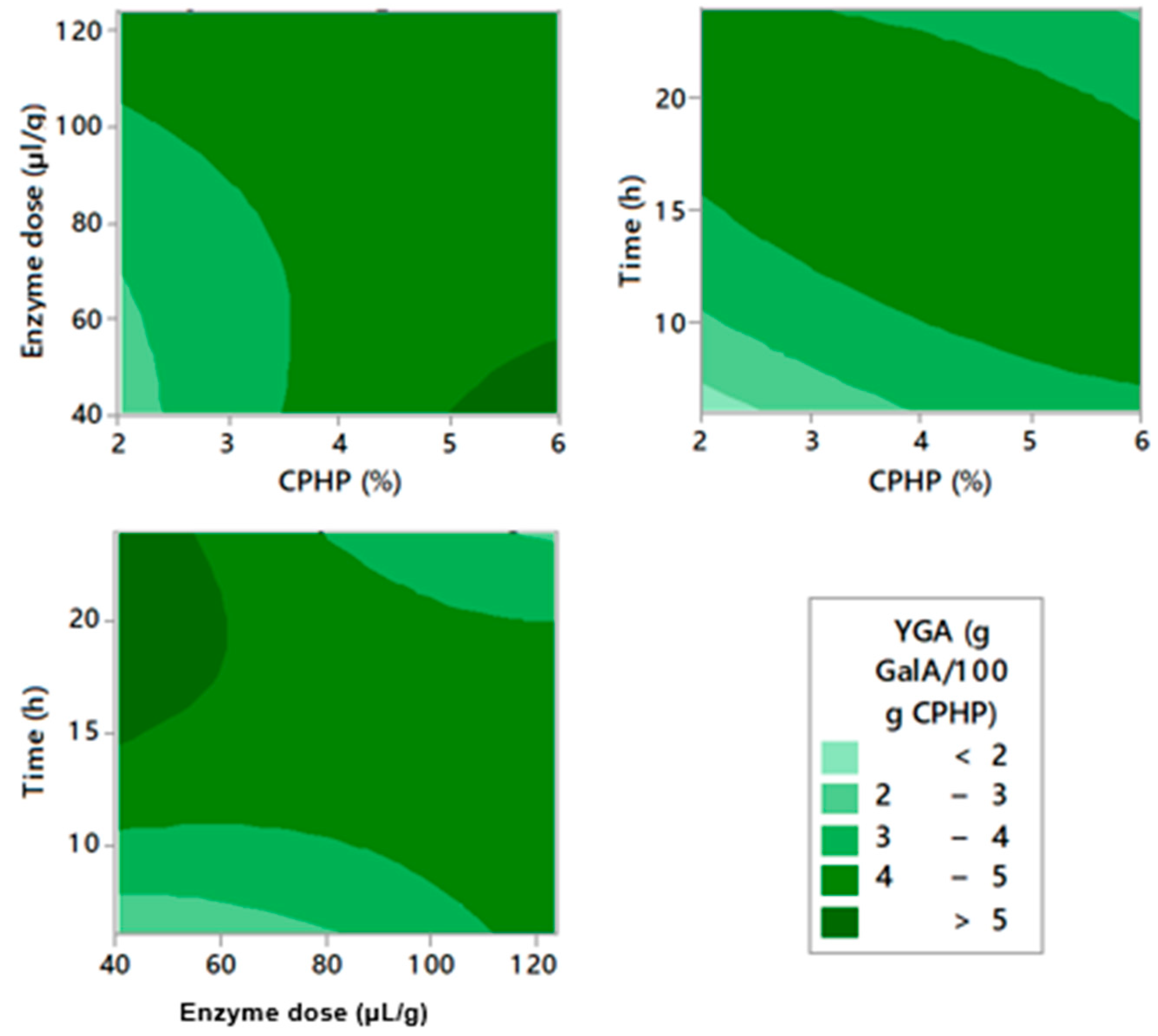
2.2. Enzymatic Pectin Extraction
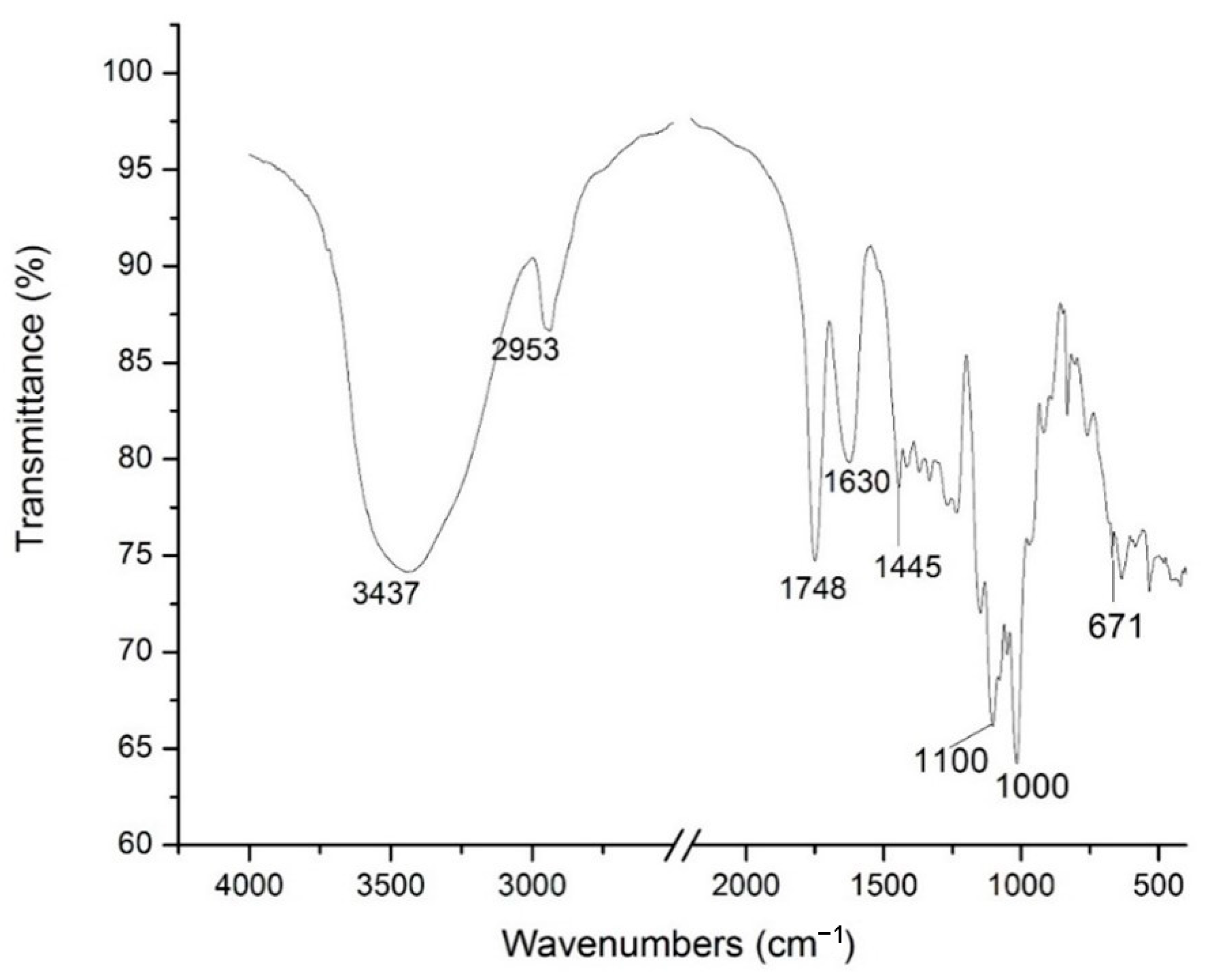
2.3. FTIR Spectra, Degree of Esterfication and Total Sugars of Enzymatically Extracted Pectin
2.4. Rheological Characterization and Gelling Test
2.5. Colorimetry of Pectin and Phenolic Content of CPHP
3. Materials and Methods
3.1. Feedstock
3.2. Enzymatic Pectin Extraction
3.3. Acid Pectin Extraction
3.4. Ultrasound-Assisted Pectin Extraction
3.5. Pectin Characterization
3.6. Color Characterization
3.7. Determination of Rheological Properties and Gelling Test
3.8. CPHP Phenolic Content Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Run | Independent Variables | Responses | ||||
|---|---|---|---|---|---|---|
| Cacao Pod Husk Powder Concentration CPHP (%) | Enzyme Dose (µL/g) | Extraction Time (h) | EY (g/100 g CPHP) | GA (g/100 g Pectin) | YGA (g/100 g CPHP) | |
| 1 | 2.81 (−1) | 110.96 (1) | 20.35(1) | 8.23 | 54.39 | 4.47 |
| 2 | 2.81 (−1) | 58.04 (−1) | 9.65 (−1) | 6.46 | 43.28 | 2.80 |
| 3 | 2.00 (−1.68) | 84.50 (0) | 15.00 (0) | 7.49 | 52.46 | 3.93 |
| 4 | 5.19 (1) | 110.96 (1) | 9.65 (−1) | 11.12 | 41.16 | 4.58 |
| 5 | 4.00 (0) | 84.50(0) | 15.00 (0) | 10.80 | 44.23 | 4.78 |
| 6 | 4.00 (0) | 129.00 (1.68) | 15.00 (0) | 10.44 | 46.64 | 4.87 |
| 7 | 4.00 (0) | 84.50 (0) | 24.00 (1.68) | 8.19 | 46.37 | 3.80 |
| 8 | 4.00 (0) | 84.50 (0) | 15.00 (0) | 10.21 | 44.23 | 4.52 |
| 9 | 5.19 (1) | 110.96 (1) | 20.35(1) | 8.26 | 40.06 | 3.31 |
| 10 | 5.19 (1) | 58.04 (−1) | 9.64 (−1) | 9.18 | 44.03 | 4.04 |
| 11 | 4.00 (0) | 84.50 (0) | 15.00 (0) | 10.27 | 44.23 | 4.54 |
| 12 | 6.00 (1.68) | 84.50 (0) | 15.00 (0) | 11.08 | 41.06 | 4.55 |
| 13 | 4.00 (0) | 84.50 (0) | 15.00 (0) | 10.66 | 44.23 | 4.72 |
| 14 | 4.00 (0) | 84.50 (0) | 6.00 (−1.68) | 8.09 | 39.43 | 3.19 |
| 15 | 2.81 (−1) | 110.96 (1) | 9.65 (−1) | 9.46 | 42.39 | 4.01 |
| 16 | 4.00 (0) | 84.50 (0) | 15.00 (0) | 10.00 | 44.23 | 4.42 |
| 17 | 4.00 (0) | 40.00 (−1.68) | 15.00 (0) | 10.55 | 49.58 | 5.23 |
| 18 | 5.19 (1) | 58.04 (−1) | 20.35(1) | 11.31 | 45.91 | 5.19 |
| 19 | 4.00 (0) | 84.50 (0) | 15.00 (0) | 10.32 | 42.13 | 4.35 |
| 20 | 2.81 (−1) | 58.04 (−1) | 20.35(1) | 8.69 | 53.40 | 4.64 |
| Variables | Model Equation | R2(%) | R2 Adjusted (%) |
|---|---|---|---|
| Pectin extraction yield (EY g/100 g CPHP) | EY = −20.73 + 4.909 X1 + 0.1713 X2 + 1.635 X3 − 0.2963 X12 + 0.000012 X22 − 0.02873 X32 − 0.01447 X1X2 − 0.0341 X1X3 − 0.007464 X2X3 | 97.02 | 94.33 |
| GA content (g/100 g pectin) | GA = 33.08 + 1.50 X1 − 0.2193 X2 + 2.699 X3 + 0.650 X12 + 0.001992 X22 − 0.01557 X32 − 0.0351 X1X2 − 0.4191 X1X3 − 0.00097 X2X3 | 96.90 | 94.10 |
| Yield of GA (YGA g/100 g CPHP) | YGA = −9.89 + 2.423 X1 + 0.0494 X2 + 0.9356 X3 − 0.0956 X12 + 0.000216 X22 − 0.01391 X32 − 0.00952 X1X2 − 0.0477 X1X3 − 0.003354 X2X3 | 95.35 | 91.16 |

References
- Cely, L. Oferta productiva del cacao colombiano en el posconflicto. Estrategias para el aprovechamiento de oportunidades comerciales en el marco del acuerdo comercial Colombia-Unión Europea. Equidad Y Desarro. 2017, 167–195. [Google Scholar] [CrossRef]
- ICCO Quarterly Bulletin of Cocoa Statistics, Vol. XLVI, No.1, Cocoa Year 2019/20. Available online: https://www.icco.org/about-us/international-cocoa-agreements/cat_view/30-related-documents/46-statistics-production.html (accessed on 22 August 2019).
- Vriesmann, L.C.; Amboni, R.D.d.M.C.; Petkowicz, C.L.d.O. Cacao pod husks (Theobroma cacao L.): Composition and hot-water-soluble pectins. Ind. Crop. Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Betancourt Latorre, L.D.; Llano Moreno, J.E. Extracción de Pectinas a Partir de los Subproductos del Beneficio del Cacao. Bachelor’s Thesis, Universidad EAFIT, Medellin, Colombia, 2009. [Google Scholar]
- Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Cacao pod husks as a source of low-methoxyl, highly acetylated pectins able to gel in acidic media. Int. J. Biol. Macromol. 2017, 101, 146–152. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.; Figueroa, J.; Pérez-Álvarez, J.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Tambunan, B.; Pambudi, N. A preliminary study on use of cocoa pod husk as a renewable source of energy in Indonesia. Energy Sustain. Dev. 2012, 16, 74–77. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; Mason, S.M.; Gomez, L. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Cabarcas, E.; Guerra, A.; Henao, C.; Acevedo Morantes, M.T.D. Extracción y Caracterización de Pectina a Partir de Cáscaras de Plátano Para Desarrollar un Diseño General del Proceso de Producción. Bachelor’s Thesis, Universidad de Cartagena, Cartagena, Colombia, 2012. [Google Scholar]
- Li, J.-M.; Nie, S.-P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Zapata, A.D.; Escobar, C.A.; Cavalitto, S.F.; Hours, R.A. Evaluación de la capacidad de solubilización de pectina de cáscara de limón usando protopectinasa-SE. Vitae 2009, 16, 67–74. [Google Scholar]
- Chan, S.-Y.; Choo, W.-S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef]
- Vriesmann, L.; Teófilo, R.; Petkowicz, C. Optimization of nitric acid-mediated extraction of pectin from cacao pod husks (Theobroma cacao L.) using response surface methodology. Carbohydr. Polym. 2011, 84, 1230–1236. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Teófilo, R.F.; de Oliveira Petkowicz, C.L. Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. LWT-Food Sci. Technol. 2012, 49, 108–116. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Muneeswari, S.; Naganyashree, S.; Shivamathi, C. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef]
- Priyangini, F.; Walde, S.G.; Chidambaram, R. Extraction optimization of pectin from cocoa pod husks (Theobroma cacao L.) with ascorbic acid using response surface methodology. Carbohydr. Polym. 2018, 202, 497–503. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef]
- Mendoza-Vargas, L.; Jiménez-Forero, J.; Ramírez-Niño, M. Evaluación de la pectina extraída enzimáticamente a partir de las cáscaras del fruto de cacao (Theobroma cacao L.). Rev. U.D.C.A Actual. Divulg. Científica 2017, 20, 131–138. [Google Scholar] [CrossRef]
- Contreras Esquivel, J.; Hours, R.; Aguilar, C.; Reyes Vega, M.; Romero, J. Revisión: Extracción microbiológica y enzimática de pectina. Arch. latinoam. Nutr. 1997, 47, 208–216. [Google Scholar]
- Vasco-Correa, J.; Zapata, A.D. Enzymatic extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) at laboratory and bench scale. LWT-Food Sci. Technol. 2017, 80, 280–285. [Google Scholar] [CrossRef]
- Rosales, E.; Pazos, M.; Ángeles Sanromán, M. Chapter 15—Solid-State Fermentation for Food Applications. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 319–355. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochemistry 2020, 60, 104726. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Pectin Market by Type (HM Pectin, LM Pectin), Raw Material (Citrus fruits, Apples, Sugar beet), Function, Application (Food & beverages, Pharmaceutical & Personal Care Products, Industrial Applications), and Region-Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/pectin-market-139129149.html (accessed on 21 July 2020).
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Development of complete hydrolysis of pectins from apple pomace. Food Chem. 2015, 172, 675–680. [Google Scholar] [CrossRef]
- Sabater, C.; Corzo, N.; Olano, A.; Montilla, A. Enzymatic extraction of pectin from artichoke (Cynara scolymus L.) by-products using Celluclast®1.5L. Carbohydr. Polym. 2018, 190, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Hu, D.; Xiao, K.; Wu, J.-Y. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of Pectin Enzymatic Extraction from Malus domestica ‘Fălticeni’Apple Pomace with Celluclast 1.5 L. Molecules 2019, 24, 2158. [Google Scholar] [CrossRef]
- Guerrero, G.; Suárez, D.; Orozco, D. Implementation of a method of extraction conditions of pectin obtained from agroindustrial by-product cocoa husks. Rev. Temas. Agrar. 2017, 22, 85–90. [Google Scholar] [CrossRef][Green Version]
- Marsiglia, D.; Ojeda, K.; Ramirez, M.; Sánchez, E. Pectin extraction from cocoa pod husk (Theobroma Cacao L.) by hydrolysis with citric and acetic acid. Int. J. Chem. Tech. Res. 2016, 9, 497–507. [Google Scholar]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Sundari, N. Extrication of pectin from waste peels: A Review. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1842–1848. [Google Scholar]
- Prakash Maran, J.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box–Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Application of Celluclast 1.5 L in apple pectin extraction. Carbohydr. Polym. 2015, 134, 251–257. [Google Scholar] [CrossRef]
- Liew, S.; Chin, N.; Yusof, Y.; Sowndhararajan, K. Comparison of acidic and enzymatic pectin extraction from passion fruit peels and its gel properties. J. Food Process. Eng. 2016, 39, 501–511. [Google Scholar] [CrossRef]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A. Extraction and characterization of pectin from passion fruit peels. Agric. Agric. Sci. Procedia 2014, 2, e236. [Google Scholar] [CrossRef]
- Ismail, N.S.M.; Ramli, N.; Hani, N.M.; Meon, Z. Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) using various extraction conditions. Sains Malays. 2012, 41, 41–45. [Google Scholar]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Lim, J.; Yoo, J.; Ko, S.; Lee, S. Extraction and characterization of pectin from Yuza (Citrus junos) pomace: A comparison of conventional-chemical and combined physical–enzymatic extractions. Food Hydrocoll. 2012, 29, 160–165. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Garna, H.; Paquot, M.; Deleu, M. Purification of pectin from apple pomace juice by using sodium caseinate and characterisation of their binding by isothermal titration calorimetry. Food Hydrocoll. 2012, 29, 211–218. [Google Scholar] [CrossRef]
- Garna, H.; Mabon, N.; Robert, C.; Cornet, C.; Nott, K.; Legros, H.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield and purity of apple pomace pectin precipitated but not washed by alcohol. J. Food Sci. 2007, 72, C001–C009. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.G.; Espeleta, A.F.; Branco, A.; de Assis, S.A. Aqueous extraction of pectin from sisal waste. Carbohydr. Polym. 2013, 92, 1997–2001. [Google Scholar] [CrossRef]
- Monsoor, M.; Kalapathy, U.; Proctor, A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2001, 74, 233–238. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Ardila, S.F. Pectinas: Aislamiento, Caracterización y Producción a Partir de Frutas Tropicales y de los Residuos de su Procesamiento Industrial; Faculdad de Ciencias, Departamento de Farmacia, Universidad Nacional de Colombia: Bogotá, Colombia, 2007. [Google Scholar]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef]
- Farina, J.; Sineriz, F.; Molina, O.; Perotti, N. Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. Rheological properties, molecular weight and conformational characteristics. Carbohydr. Polym. 2001, 44, 41–50. [Google Scholar] [CrossRef]
- Bello-Lara, J.E.; Balois-Morales, R.; Sumaya-Martínez, M.T.; Juárez-López, P.; Rodríguez-Hernández, A.I.; Sánchez-Herrera, L.M.; Jiménez-Ruíz, E.I. Extraction and rheological characterization of starch and pectin in ‘Pera’ (Musa ABB) banana fruits. Rev. Mex. Cienc. Agrícolas 2014, 5, 1501–1507. [Google Scholar]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Guayabo, G.T. Evaluation of a colorant and oil extracted from avocado waste as functional components of a liquid soap formulation. Rev. Fac. Nac. Agron. Medellín 2019, 72, 8855–8862. [Google Scholar] [CrossRef]
- Obón, J.; Castellar, M.; Alacid, M.; Fernández-López, J. Production of a red–purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Baississe, S.; Ghannem, H.; Fahloul, D.; Lekbir, A. Comparison of structure and emulsifying activity of pectin extracted from apple pomace and apricot pulp. World J. Dairy Food Sci. 2010, 5, 79–84. [Google Scholar]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hunter Associates Laboratory. Measuring Color Using Hunter L, a, b versus CIE 1976 L*a*b*. Available online: https://support.hunterlab.com/hc/en-us/articles/204137825-Measuring-Color-using-Hunter-L-a-b-versus-CIE-1976-L-a-b-AN-1005b (accessed on 12 December 2020).
- Resolution Number 3929 of 2013. 2013. Available online: https://www.icbf.gov.co/cargues/avance/docs/resolucion_minsaludps_3929_2013.htm (accessed on 14 July 2020).
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. A hydrocolloid based biorefinery approach to the valorisation of mango peel waste. Food Hydrocoll. 2018, 77, 142–151. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Bodoira, R.; Velez, A.; Andreatta, A.E.; Martínez, M.; Maestri, D. Extraction of bioactive compounds from sesame (Sesamum indicum L.) defatted seeds using water and ethanol under sub-critical conditions. Food Chem. 2017, 237, 114–120. [Google Scholar] [CrossRef] [PubMed]

| FTIR Wavenumber (cm−1) | Assignments | |
|---|---|---|
| CPHP Pectin | Commercial Pectin | |
| 3382 | 3437 | O-H |
| 2932 | 2953 | C-H |
| 1730 | 1748 | C=O |
| 1612–1421 | 1630–1445 | C=O from free carboxyl groups |
| 950–1200 | Fingerprint | Fingerprint |
| Concentration (%) | K (Pa·sn) | n | R2 |
|---|---|---|---|
| 0.5 | 0.00549 | 0.83 | 0.95 |
| 1 | 0.02064 | 0.81 | 0.95 |
| 2 | 0.09957 | 0.78 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennessey-Ramos, L.; Murillo-Arango, W.; Vasco-Correa, J.; Paz Astudillo, I.C. Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L. Molecules 2021, 26, 1473. https://doi.org/10.3390/molecules26051473
Hennessey-Ramos L, Murillo-Arango W, Vasco-Correa J, Paz Astudillo IC. Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L. Molecules. 2021; 26(5):1473. https://doi.org/10.3390/molecules26051473
Chicago/Turabian StyleHennessey-Ramos, Licelander, Walter Murillo-Arango, Juliana Vasco-Correa, and Isabel Cristina Paz Astudillo. 2021. "Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L" Molecules 26, no. 5: 1473. https://doi.org/10.3390/molecules26051473
APA StyleHennessey-Ramos, L., Murillo-Arango, W., Vasco-Correa, J., & Paz Astudillo, I. C. (2021). Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L. Molecules, 26(5), 1473. https://doi.org/10.3390/molecules26051473








