Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect
Abstract
1. Introduction
2. Results and Discussion
- (a)
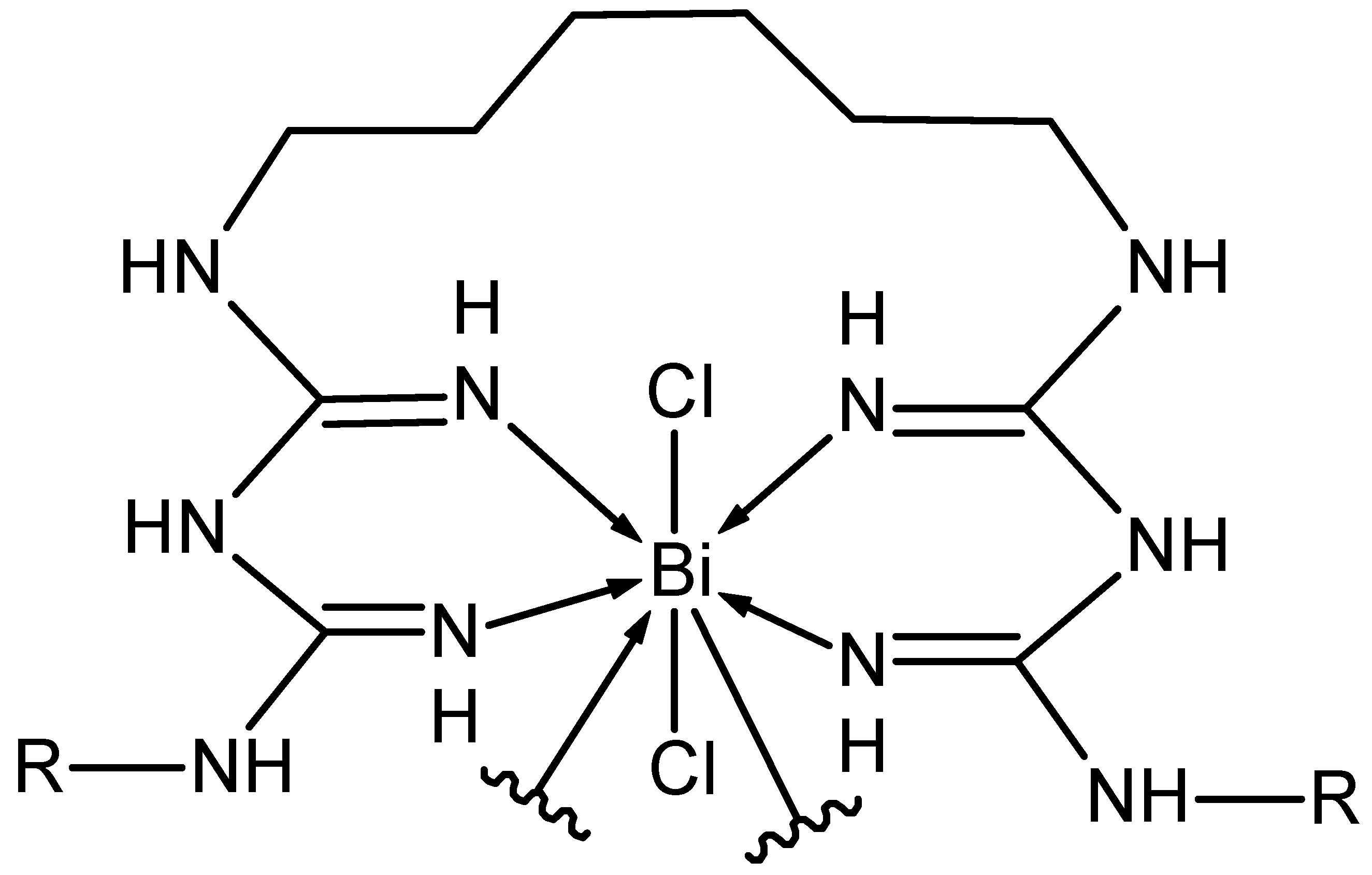
- Bi(CHX)2NH3: C(35.45%) H(4.46%) Bi(28.03%) Cl(9.51%) N(22.55%),
- (b)
- Bi(CIP)(2NH3)2Cl: C(31.69%) H(3.60%) Bi(32.44%) Cl(11.01%) F(2.95%) N(10.87%) O(7.45%),
- (c)
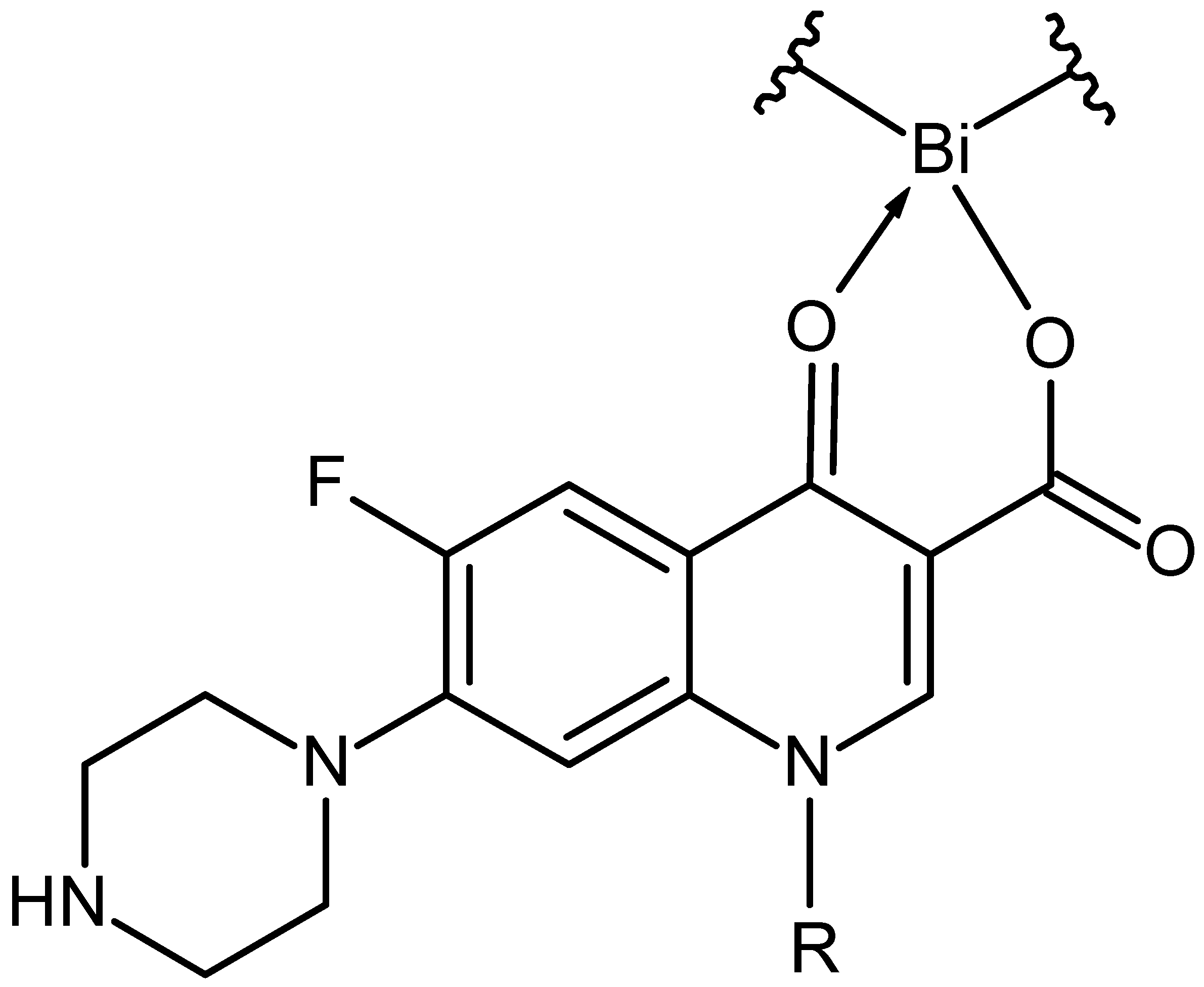
- Bi(CIP)(CHX): C(44.92%) H(4.35%) Bi(20.04%) Cl(6.80%) F(1.82%) N(17.46%) O(4.60%),
- (d)
- Bi(CIP)(CHX)2CI: C(41.99%) H(4.25%) Bi(18.73%) Cl(12.71%) F(1.70%) N(16.32%) O(4.30%).
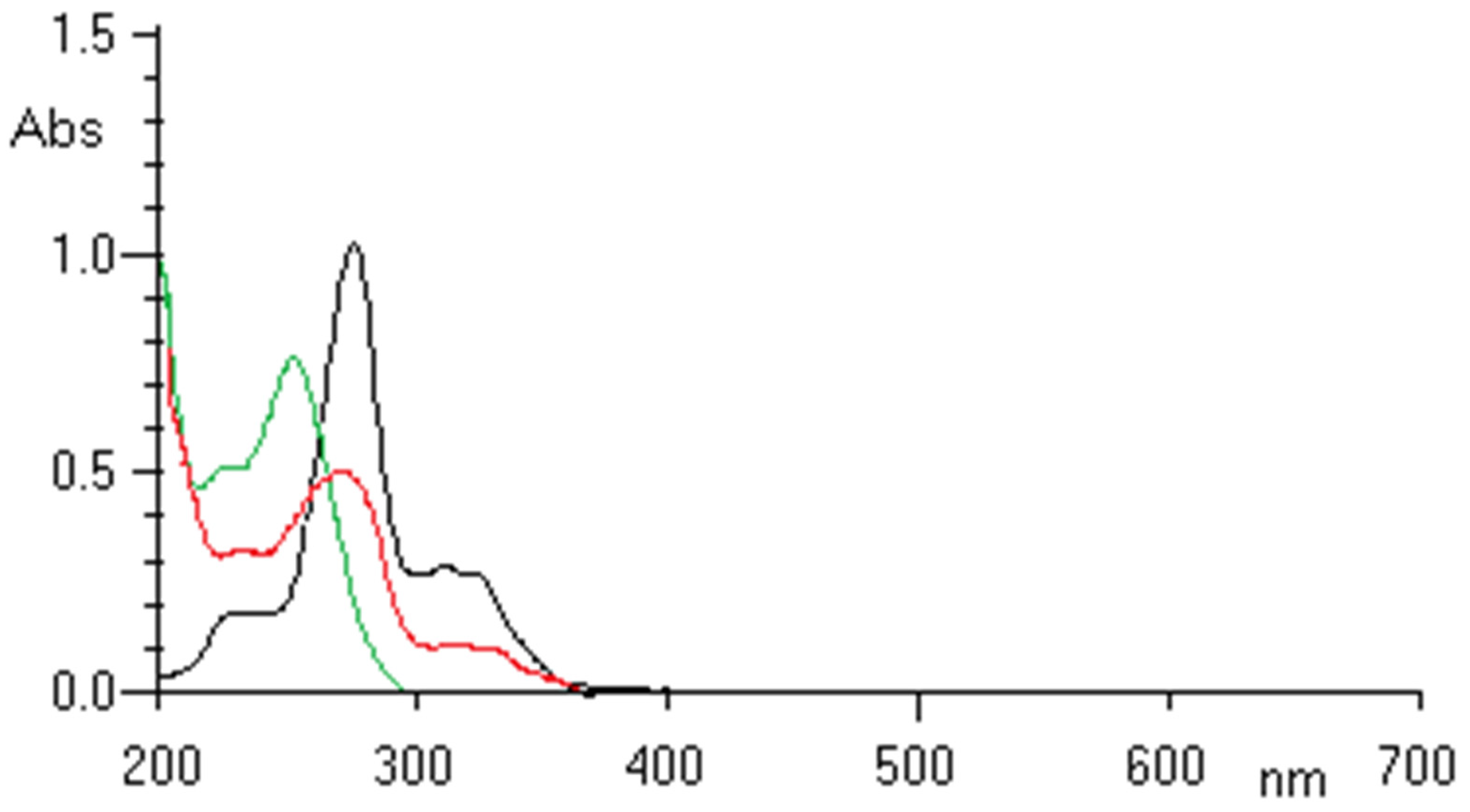
2.1. UV-VIS Analysis
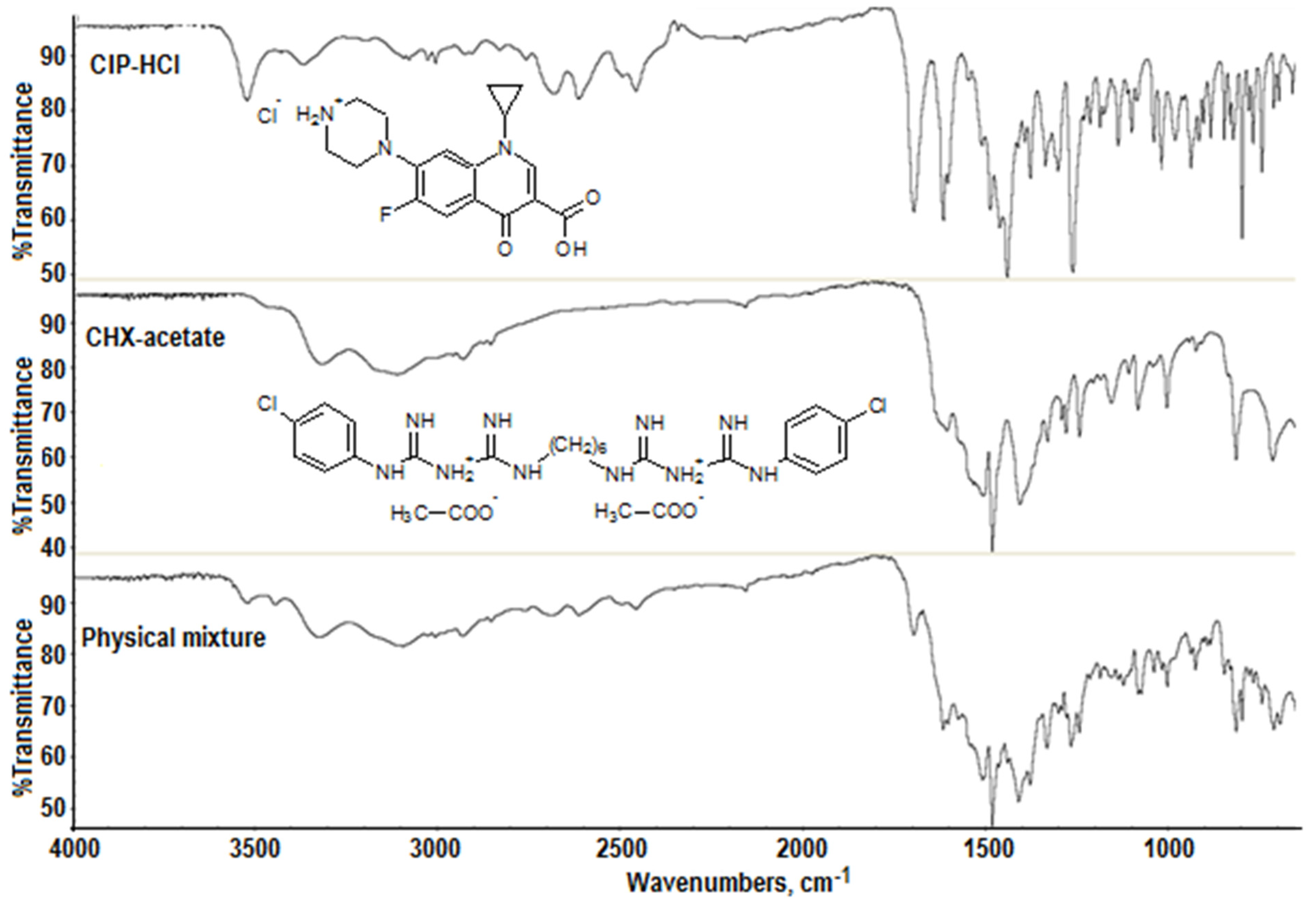
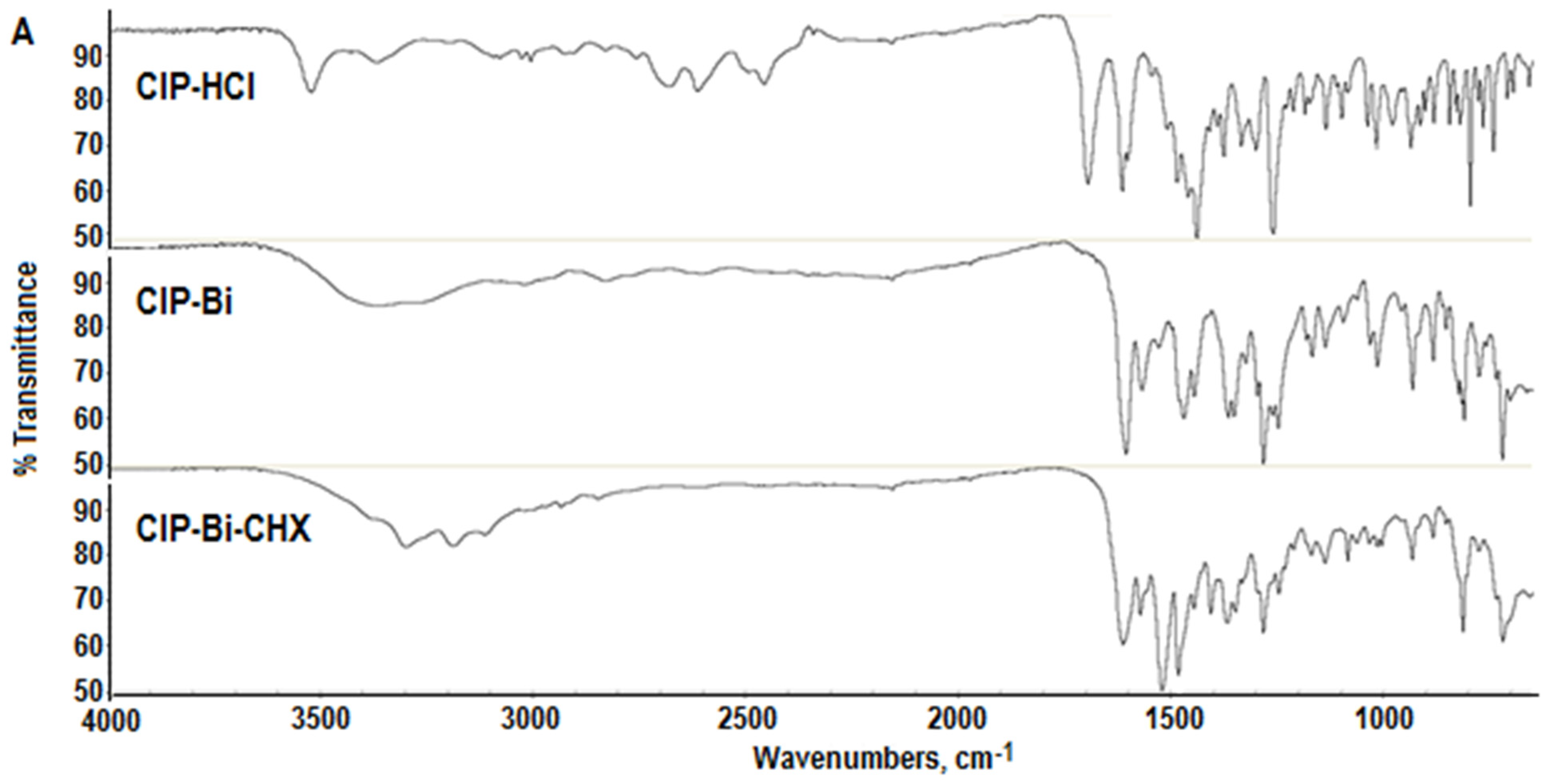
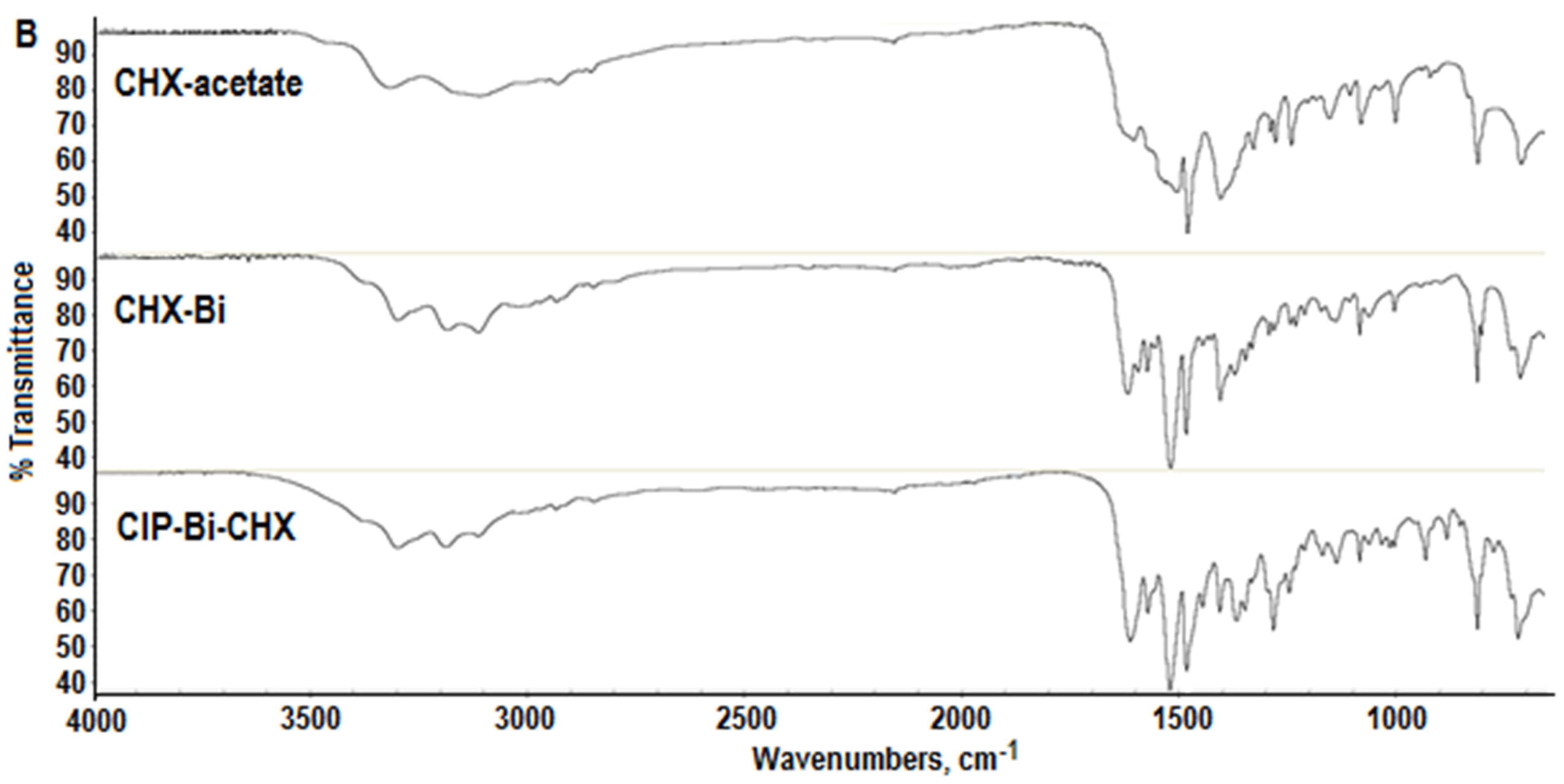
2.2. FTIR Analysis
- The disappearance of the stretching vibration band of the OH group at 3528 cm−1;
- The disappearance of the stretching vibration band of C=O in the COOH group at 1702 cm−1, and the appearance of the stretching vibration band of C=O in the COO− group at 1580 cm−1;
- The shift and significant intensification of the stretching vibration band of the ketone group >C=O at 1621 cm−1 due to interference with the ν C=N band of chlorhexidine.
- A positive shift (towards higher frequencies) from 3178 cm−1 in the CHX molecule to 3196 cm−1 in the composite molecule and an intensification of the stretching vibration band of the =NH group;
- A positive shift from 1611 cm−1 in the CHX molecule to 1621 cm−1 in the composite molecule, and a significant intensification of the stretching vibration band of the C=N group, probably indicating a loosening of the bond due to its coordination to the metal ion [29];
- A positive shift from 1515 cm−1 in the CHX molecule to 1520 cm−1 in the composite molecule, and a significant intensification of the stretching vibration band of the N=H group.
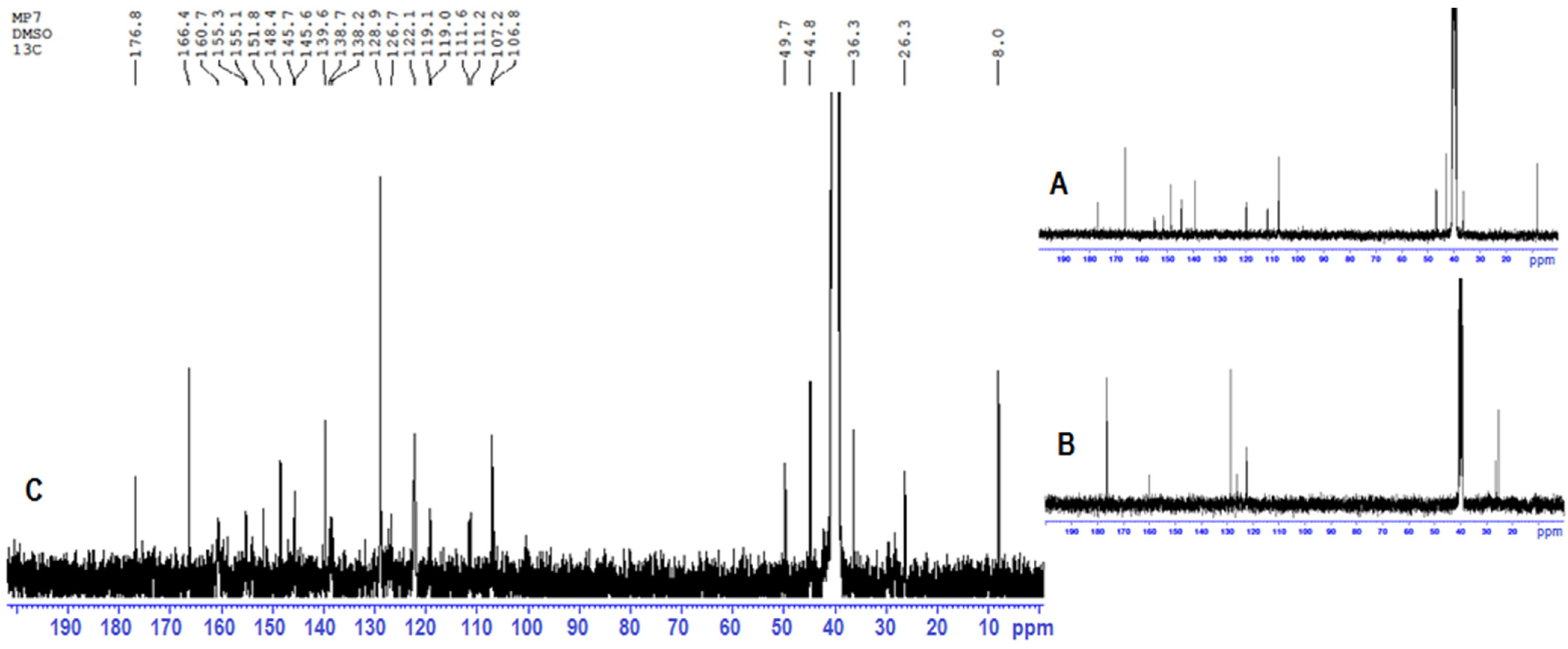
2.3. 1H-NMR and 13C-NMR Analyses
- Differences in the multiplicity of the band;
- Differences in the band intensity (mainly signal reduction);
- Band broadening;
- Significant band shifts;
- Bands disappearance.
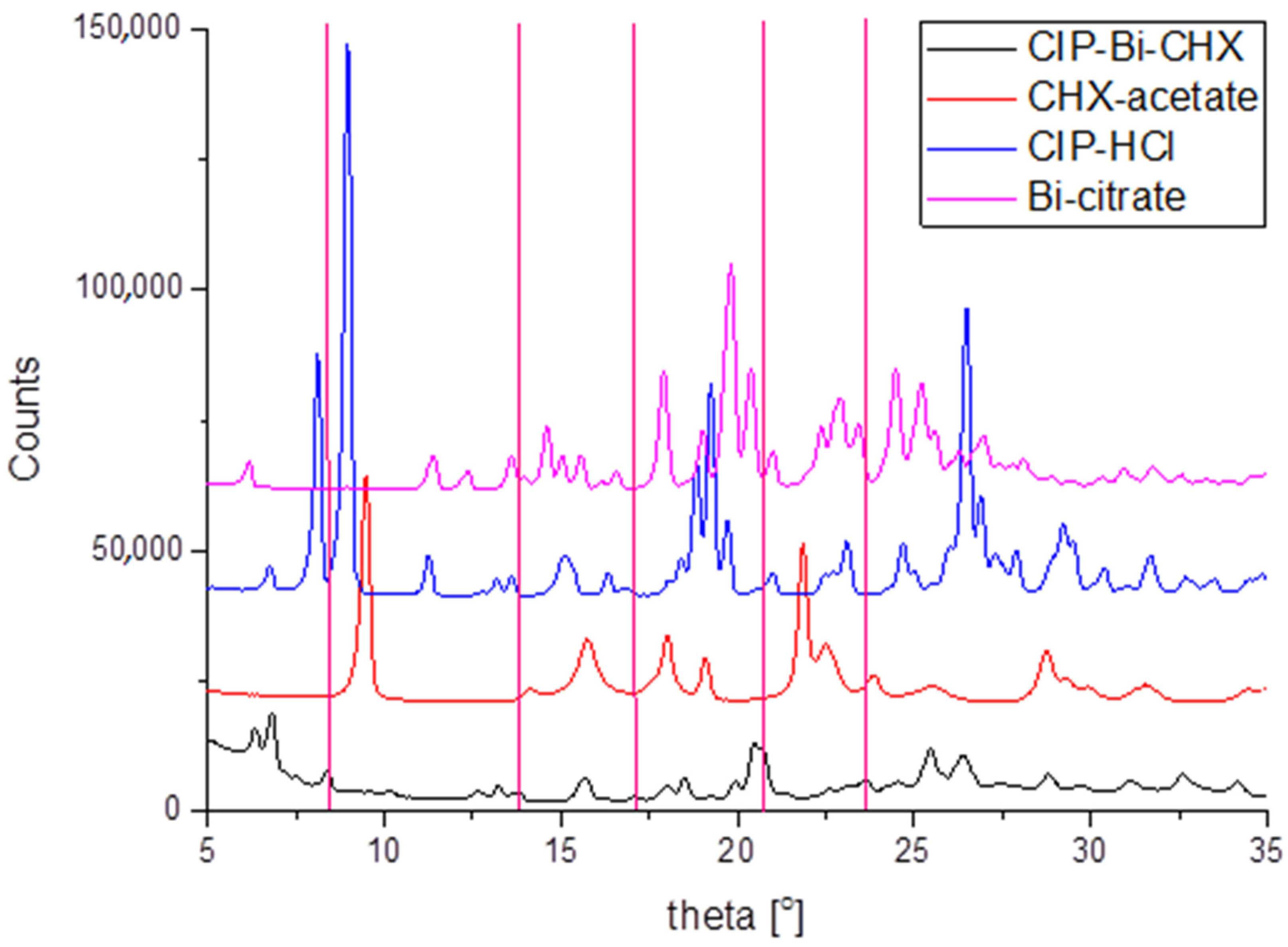
2.4. X-ray Diffraction
3. Materials and Methods
3.1. General Methods
3.2. Preparation of the CIP-Bi-CHX Composite
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Lee Ventola, C. The Antibiotic Resistance Crisis, Part 1: Causes and Threats. P&T 2015, 40, 277–283. [Google Scholar]
- Burke, V.; Richter, D.; Greskowiak, J.; Mehrtens, A.; Schulz, L.; Massmann, G. Occurrence of Antibiotics in Surface and Groundwater of a Drinking Water Catchment Area in Germany. Water Environ. Res. 2016, 88, 652–659. [Google Scholar] [CrossRef]
- Haddad, T.; Baginska, E.; Kümmerer, K. Transformation products of antibiotic and cytostatic drugs in the aquatic cycle that result from effluent treatment and abiotic/biotic reactions in the environment: An increasing challenge calling for higher emphasis on measures at the beginning of the pipe. Water Res. 2015, 72, 75–126. [Google Scholar] [CrossRef] [PubMed]
- Domalaon, R.; Idowu, T.; Zhanel, G.G.; Schweizer, F. Antibiotic Hybrids: The Next Generation of Agents and Adjuvants against Gram-Negative Pathogens? Clin. Microbiol. Rev. 2018, 31, 1–45. [Google Scholar] [CrossRef]
- Keter, F.K.; Darkwa, J. Perspective: The potential of pyrazole-based compounds in medicine. Biometals 2012, 25, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium(III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Singh, B.K.; Bhojak, N.; Adhikari, D. Synthesis and characterization of bioactive zinc(II) and cadmium(II) complexes with new Schiff bases derived from 4-nitrobenzaldehyde and acetophenone with ethylenediamine. Spectrochim. Acta A 2010, 76, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskaya, V.; Baasov, T. Dual-acting hybrid antibiotics: A promising strategy to combat bacterial resistance. Expert Opin. Drug Discov. 2010, 5, 883–902. [Google Scholar] [CrossRef] [PubMed]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of tannic acid/chitosan/pullulan composite nanofibersfrom aqueous solution for potential applications as wound dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, W. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Gordeev, M.F.; Hackbarth, C.; Barbachyn, M.R.; Banitt, S.; Gage, J.R.; Luehr, G.W.; Gomez, M.; Trias, J.; Morin, S.E.; Zurenko, G.E.; et al. Novel Oxazolidinone–Quinolone Hybrid Antimicrobials. Bioorg. Med. Chem. Lett. 2003, 13, 4213–4216. [Google Scholar] [CrossRef]
- Singh, S.; Roy, R. The Application of Absolute Quantitative 1H-NMR Spectroscopy in Drug Discovery and Development. Expert Opin. Drug Discov. 2016, 11, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Tian, Y.; Jiang, H.; Zhang, X.; Ju, X. A NMR-based drug screening strategy for discovering active substances from herbal medicines: Using Radix Polygoni Multiflori as example. J. Ethnopharmacol. 2020, 254, 112712. [Google Scholar] [CrossRef]
- Fiori-Duarte, A.T.; de Paiva, R.; Manzano, C.M.; Lustri, W.R.; Corbi, P.P. Silver(I) and gold(I) complexes with sulfasalazine: Spectroscopic characterization, theoretical studiem and antiproliferative activities over Gram-positive and Gram-negative bacterial strains. J. Mol. Struct. 2020, 1214, 128158. [Google Scholar] [CrossRef]
- Wallis, S.C.; Gahan, L.R.; Charles, B.G.; Hambley, T.W.; Duckworth, P.A. Cooper(II)Complexes of the Fluoroquinolone Antimicrobial Ciprofloxacin. Synthesis, X-Ray Structural Characterization, and Potentiometric Study. J. Inorg. Biochem. 1996, 62, 1–16. [Google Scholar] [CrossRef]
- Debnath, A.; Hussain, F.; Masram, D.T. Synthesis, Characterization, and Antifungal Studies of Cr(III) Complex of Norfloxacin and Bipiridyl Ligand. Bioinorg. Chem. Appl. 2014, 457478. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.; Olar, R.; Ilis, M.; Georgescu, R.; Calinescu, M. Synthesis, characterization, and thermal decomposition of new copper (II) complex compounds with chlorhexidine. J. Therm. Anal. Calorim. 2013, 111, 1763–1770. [Google Scholar] [CrossRef]
- Abdulghani, A.J.; Jasim, H.H.; Hassan, A.S. Determination of β-lactam Antibiotics in Pharmaceutical Preparations by Uv-visible Spectrophotometry Atomic Absorption and High Performance Liquid Chromatography. Pak. J. Chem. 2012, 2, 150–160. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Giridhar, R.; Megraud, F.; Yadav, M.R. Metalloantibiotics: Synthesis, characterization and antimicrobial evaluation of bismuth-fluoroquinolone complexes against Helicobacter pylori. Acta Pharm. 2009, 59, 259–271. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Xu, C.; Wei, H.; Wang, X.; Yang, X. Spectroscopy and Speciation Studies on the Interactions of Aluminum (III) with Ciprofloxacin and β-Nicotinamide Adenine Dinucleotide Phosphate in Aqueous Solutions. Molecules 2012, 17, 9379–9396. [Google Scholar] [CrossRef] [PubMed]
- Călinescu, M.; Negreanu-Pîrjol, T.; Georgescu, R.; Călinescu, O. Synthesis and characterization of new copper(II) complex compounds with chlorhexidine. Part I Cent. Eur. J. Chem. 2010, 8, 543–549. [Google Scholar] [CrossRef][Green Version]
- Cortes, M.E.; Sinisterra, R.D.; Avilacampos, M.J.; Tortamano, N.; Rocha, R.G. The Chlorhexidine: β-Cyclodextrin Inclusion Compound: Preparation, Characterization and Microbiological Evaluation. J. Incl. Phenom. Macrocycl. Chem. 2001, 40, 297–302. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Attar, M.S.; Abd-El-Hamid, S.M. Synthesis, characterization and antibacterial activity of some new transition metal complexes with ciprofloxacin-imine. Bull. Chem. Soc. Ethiop. 2015, 29, 259–274. [Google Scholar] [CrossRef][Green Version]
- Samlíková, M.; Holešová, S.; Hundáková, M.; Pazdziora, E.; Jankovič, L.; Valášková, M. Preparation of antibacterial chlorhexidine/vermiculite and release study. Int. J. Miner. Process. 2017, 159, 1–6. [Google Scholar] [CrossRef]
- Holesová, S.; Valásková, M.; Hlavác, D.; Madejovác, J.; Samlíková, M.; Tokarsky, J.; Pazdziora, E. Antibacterial kaolinite/urea/chlorhexidine nanocomposites: Experiment and molecular modelling. Appl. Surf. Sci. 2014, 305, 783–791. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, L.; Yanga, P.; Jin, X. Bis(1,1-dimethylbiguanido)copper(II) octahydrate. Acta Cryst. 2002, E58, m217–m219. [Google Scholar] [CrossRef]
- Dinari, M.; Gharahi, F.; Asadi, P. Synthesis, spectroscopic characterization, antimicrobial evaluation and molecular docking study of novel triazine-quinazolinone based hybrids. J. Mol. Struct. 2018, 1156, 43–50. [Google Scholar] [CrossRef]
- Azhara, F.F.; Olad, A.; Salehi, R. Fabrication and characterization of chitosan–gelatin/nanohydroxyapatite–polyaniline composite with potential application in tissue engineering scaffolds. Des. Monom. Polym. 2014, 17, 654–667. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Mohamed, A.A. Biological and Spectroscopic Investigations of New Tenoxicam and 1.10-Phenthroline Metal Complexes. Molecules 2020, 25, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, D.; Miazga-Karska, M.; Gładysz, A.; Warda, P.; Baranska, A.; Drop, B. Characterization of Ciprofloxacin-Bismuth-Loaded Antibacterial Wound Dressing. Molecules 2020, 25, 5096. [Google Scholar] [CrossRef] [PubMed]
- Doroffev, V.L. Infrared spectra and the structure of drugs of the fluoroquinolone group. Pharm. Chem. J. 2004, 38, 693–697. [Google Scholar] [CrossRef]
- Spectral Database for Organic Compounds SDBS. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 6 March 2021).
- Zieba, A.; Maslankiewicz, A.; Sitkowski, J. Spectral Assignments and Reference Data. 1H, 13C and 15N NMR spectra of ciprofloxacin. Magn. Reson. Chem. 2004, 42, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Parwe, S.P.; Chaudhari, P.N.; Mohite, K.K.; Selukar, B.S.; Nande, S.S.; Garnaik, B. Synthesis of ciprofloxacin-conjugated poly(L-lactic acid) polymer for nanofiber fabrication and antibacterial evaluation. Int. J. Nanomed. 2014, 9, 1463–1477. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Islam, R.; Ahmad, M.; Hossion, A.M.L. Synthesis of some NH-Derivatives of ciprofloxacin as antibacterial and antifungal agents. Bangladesh J. Pharmacol. 2011, 6, 8–13. [Google Scholar] [CrossRef]
- Jadrijevic-Mladar, T. Effects of substituents on the NMR features of basic bicyclic ring systems of fluoroquinolone antibiotics and the relationships between NMR chemical shifts, molecular descriptors and drug-likeness parameters. Acta Pharm. 2010, 60, 237–254. [Google Scholar] [CrossRef]
- Rusu, A.; Hancu, G.; Tóth, G.; Toma, F.; Mare, A.D.; Man, A.; Velescu, B.S.; Uivaroși, V. Synthesis, characterization and microbiological activity evaluation of two silver complexes with norfloxacin. Farmacia 2016, 64, 922–932. [Google Scholar]
- Chattah, A.K.; Linck, Y.G.; Monti, G.A.; Levstein, P.R.; Breda, S.A.; Manzo, R.H.; Olivera, M.E. NMR and IR characterization of the aluminium complexes of norfloxacin and ciprofloxacin fluoroquinolones. Magn. Reson. Chem. 2007, 45, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, B.; Kokot, Z.J. Spectroscopic investigations of fluoroquinolones metal ion complexes. Acta Pol. Pharm. 2013, 70, 621–629. [Google Scholar] [PubMed]
- Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules 2013, 18, 11153–11197. [Google Scholar] [CrossRef]
- Pansuriya, P.R.; Patel, M.N. Iron(III) complexes: Preparation, characterization, antibacterial activity and DNA-binding Enzyme. Inhib. Med. Chem. 2008, 23, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Poppler, A.C.; Corlett, E.K.; Pearce, H.; Seymour, M.P.; Reid, M.; Montgomery, M.G.; Brown, S.P. Single-crystal X-ray diffraction and NMR crystallography of a 1:1 cocrystal of dithianon and pyrimethanil. Acta Cryst. 2017, C73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Linck, Y.G.; Chattah, A.K.; Graf, R.; Romanuk, C.B.; Olivera, M.E.; Manzo, R.H.; Monti, G.A.; Spiess, H.W. Multinuclear solid state NMR investigation of two polymorphic forms of Ciprofloxacin-saccharinate. Phys. Chem. Chem. Phys. 2011, 13, 6590–6596. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdali, B.I.; Shakir, I.M.A.; Nafea, H.M. Synthesis and Spectroscopic Studies of Some transition metal Complexes with Mixed ligand of Metformin and Cysteine. Iraqi J. Sci. 2015, 56, 3036–3047. [Google Scholar]
- Kowalczuk, D.; Gładysz, A.; Pietraś, R.; Ginalska, G.; Miazga-Karska, M.; Rajtar, B. Antimicrobial Composite for the Prevention or Treatment, Especially of Local Infections, and the Method of Its Preparation. Poland Patent No. 235823, 17 November 2020. [Google Scholar]
- Kowalczuk, D.; Gładysz, R.; Ginalska, G.; Miazga-Karska, M.; Rajtar, B.; Polz-Dacewicz, M.; Pietraś, R. Medical Use of an Antibacterial Composite in Dressing Materials, Especially for the Prevention or Treatment of Local Infections. Poland Patent No. 235824, 17 November 2020. [Google Scholar]



| Parameter | C | H | N | Cl |
|---|---|---|---|---|
| Found value, % (n = 6) | 41.72 | 5.09 | 15.68 | 13.64 |
| Minimum | 37.16 | 4.56 | 15.12 | 12.67 |
| Maximum | 46.85 | 5.69 | 16.06 | 14.72 |
| Variance | 12.9246 | 0.1763 | 0.1285 | 0.5036 |
| Standard deviation | 3.5951 | 0.4199 | 0.3585 | 0.7096 |
| Relative standard deviation, % | 8.62 | 8.25 | 2.29 | 5.20 |
| Calculated value, % | 41.99 | 4.25 | 16.32 | 12.71 |
| T-Student test, P = 95% | t = −0.07 | 1.85 | −1.65 | 1.21 |
| P = 0.9466 | 0.1232 | 0.1601 | 0.2800 |
| Main Bands of Vibrations of Functional Groups | CIP-HCl | CHX-Acetate | Physical Mixture CIP-HCl + CHX-Acetate |
|---|---|---|---|
| CIP-HCl | |||
| ν as/s (O-H in COOH) | 3528m, 3375w | 3525w | |
| ν as/s (C-H in Ar) | 3085w | about 3044w | |
| ν as/s (C-H in CH2-cyclopropane) | 3013w | 3038w | |
| ν as/s (C-H alk) | 2937w | 2939w | |
| ν as/s (>NH2+) | 2688m | 2693m | |
| ν (C=O; COOH) | 1702s | 1704 m | |
| ν (C=O; ketone) | 1623s | 1623s | |
| ν (C=C in Ar) + δ (NH) | 1552–1445s | about 1495s | |
| ν as/s (C-O) | 1267s | 1273 s | |
| CHX-acetate | |||
| ν (-NH) Alk–NH–Ar/(Alk)2NH | 3324m | 3329m | |
| ν (=NH) | 3178m | about 3180m | |
| ν as/s (>NH2+) | 3115m | 3101m | |
| ν (C=N) | 1611s | 1608s | |
| ν as/s (COO- in acetate) | 1536m | 1538m | |
| δ (NH) | 1515s | 1516s | |
| ν (C=C) in Ar | 1488s | 1492s | |
| ν (C–N) | 1249m | 1250m | |
| Main Bands of Vibrations of Functional Groups | CIP- HCl | CIP-Bi Complex | CHX-Acetate | CHX-Bi Complex | CIP-Bi-CHX Hybrid |
|---|---|---|---|---|---|
| CIP-HCl | |||||
| ν (O-H in COOH) | 3528m, 3375w | No peak | No peak | ||
| ν as/s (>NH) | - | 3362m | 3383m | ||
| ν as/s (C-H in Ar) | 3085w | about 3100w | No detected * | ||
| ν as/s (C-H in CH2) | 3013w | 3026w | No detected * | ||
| ν as/s (C-H in CH3) | 2937w | 2838w | 2936w | ||
| ν as/s (>NH2+) | 2688m | No peak | No peak | ||
| ν (C=O; COOH) | 1702s | No peak | No peak | ||
| ν (C=O; ketone) | 1623s | 1614s | 1621vs (int.) | ||
| ν as/s (COO-) | - | 1575s | 1580s | ||
| ν (C=C in Ar) + δ (NH) | 1552–1445s | 1533–1454s | 1491s | ||
| ν as/s (C-O) | 1267s | 1291s | 1291s | ||
| CHX-acetate | |||||
| ν (-NH) Alk–NH–Ar/(Alk)2NH | 3324m | 3307s | 3307s | ||
| ν (=NH) | 3178m | 3195s | 3196s | ||
| ν as/s (>NH2+) | 3115m | 3120s | 3117m | ||
| ν (C=N) | 1611m | 1625vs | 1621vs (int.) | ||
| ν as/s (COO− in acetate) | 1536m | No peak | No peak | ||
| δ (NH) | 1515s | 1520vs | 1520vs | ||
| ν (C=C) in Ar | 1488s | 1492s | 1491s | ||
| ν (C–N) | 1249m | No detected * | No detected * | ||
| H-Atom | Chemical Shifts for the Hydrogen Atoms (δ, ppm) | Change in Chemical Shift (∆δ, ppm) | ||
|---|---|---|---|---|
| CIP-HCl | CHX-Acetate | CIP-Bi-CHX Composite | CIP-Bi-CHX Composite | |
| 1b (CIP, cis CH2 cyclopropyl) | 1.194 | 1.186 | Δ0.008 (−) | |
| 1.203 | ||||
| 9′ (CHX, CH2 hexane) | 1.263 | 1.287 | Δ0.024 (+) | |
| 1b (CIP, trans CH2 cyclopropyl) | 1.317 | 1.303 | Δ0.014 (−) | |
| 1.337 | 1.326 | |||
| 8′ (CHX, CH2 hexane) | 1.444 | 1.457 | Δ0.013 (+) | |
| 10′ (CHX, CH3 acetate) | 1.715 | No peak | ||
| 7′ (CHX, CH2 hexane) | 3.041 | 3.035 | Δ0.006 (−) | |
| 3.064 | ||||
| 3.086 | 3.084 | |||
| 7 (CIP, CH2 piperazinium) | 3.568 | No detected * | No detected * | |
| 1a (CIP, CH cyclopropyl) | 3.854 | 3.837 | Δ0.017 (−) | |
| 3.866 | 3.849 | |||
| 3.878 | ||||
| 2′ (CHX, CH phenyl) | 7.258 | 7.310 | Δ0.052 (+) | |
| 7.281 | 7.339 | |||
| 7.288 | ||||
| 3′ (CHX, CH phenyl) | 7.390 | 7.405 | Δ0.015 (+) | |
| 7.433 | ||||
| 8 (CIP, CH quinolin-4-one) | 7.600 | 7.548 | Δ0.052 (−) | |
| 7.625 | 7.573 | |||
| 5 (CIP, CH quinolin-4-one) | 7.934 | 7.880 | Δ0.054 (−) | |
| 7.977 | 7.924 | |||
| 2 (CIP, CH quinolin-4-one) | 8.684 | 8.659 | Δ0.025 (−) | |
| >NH2+ (CIP piperazinium) | 9.432 | No peak | ||
| 3a (CIP, COOH) | 15.141 | No peak | ||
| C—Atom | Chemical Shifts for the Carbon Atoms (δ, ppm) | Change in Chemical Shift (∆δ, ppm) | ||
|---|---|---|---|---|
| CIP-HCl | CHX-Acetate | CIP-Bi-CHX Composite | CIP-Bi-CHX Composite | |
| 1b (CIP, CH2 cyclopropyl) | 8.091 | 8.048 | Δ0.043 (−) | |
| 10′ (CHX, CH3 acetate) | 25.286 | No peak | ||
| 9′ (CHX, CH2 hexane) | 26.365 | 26.391 | Δ0.026 (+) | |
| 1a (CIP, CH cyclopropyl) | 36.465 | 36.369 | Δ0.096 (−) | |
| 7c/7e (CIP, CH2 piperazinium) | 42.950 | 44.887 | Δ1.937 (+) | |
| 7b/7f (CIP, CH2 piperazinium) | 46.776 | 49.790 | Δ2.952 (+) | |
| 46.838 | ||||
| 8 (CIP, CH quinolin-4-one) | 107.288 | 106.814 | Δ0.474 (−) | |
| 3 (CIP, =C< quinolin-4-one) | 107.411 | 107.234 | Δ0.177 (−) | |
| 5 (CIP, CH quinolin-4-one) | 111.501 | 111.298 | Δ0.203 (−) | |
| 111.808 | 111.610 | |||
| 9 (CIP, =C< quinolin-4-one) | 119.763 | 119.079 | Δ0.684 (−) | |
| 119.867 | 119.181 | |||
| 3′ (CHX, CH phenyl) | 122.467 | 122.159 | Δ0.308 (−) | |
| 1′ (CHX, C-Cl phenyl) | 126.180 | 126.732 | Δ0.552 (+) | |
| 2′ (CHX, CH phenyl) | 128.689 | 128.928 | Δ0.239 (+) | |
| 10 (CIP, =C< quinolin-4-one) | 139.554 | 138.282 | Δ0.799 (−) | |
| 138.755 | ||||
| 139.661 | ||||
| 7 (CIP, =C< quinolin-4-one) | 144.537 | 145.641 | Δ1.104 (+) | |
| 144.675 | 145.774 | |||
| 2 (CIP, CH quinolin-4-one) | 148.697 | 148.483 | Δ0.214 (−) | |
| 6 (CIP, C-F quinolin-4-one) | 151.697 | 151.815 | Δ0.118 (+) | |
| 155.005 | 155.127 | Δ0.122 (+) | ||
| 155.375 | ||||
| 6′ (CHX, biguanide) | 160.072 | 160.741 | Δ0.669 (+) | |
| 3a (CIP, COOH) | 166.334 | 166.454 | Δ0.120 (+) | |
| 11′ (CHX, COOH acetate) | 176.391 | No peak | ||
| 4 (CIP, C=O) | 176.839 | 176.811 | Δ0.028 (−) | |
| 176.872 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczuk, D.; Gładysz, A.; Pitucha, M.; Kamiński, D.M.; Barańska, A.; Drop, B. Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect. Molecules 2021, 26, 1442. https://doi.org/10.3390/molecules26051442
Kowalczuk D, Gładysz A, Pitucha M, Kamiński DM, Barańska A, Drop B. Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect. Molecules. 2021; 26(5):1442. https://doi.org/10.3390/molecules26051442
Chicago/Turabian StyleKowalczuk, Dorota, Agata Gładysz, Monika Pitucha, Daniel M. Kamiński, Agnieszka Barańska, and Bartłomiej Drop. 2021. "Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect" Molecules 26, no. 5: 1442. https://doi.org/10.3390/molecules26051442
APA StyleKowalczuk, D., Gładysz, A., Pitucha, M., Kamiński, D. M., Barańska, A., & Drop, B. (2021). Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect. Molecules, 26(5), 1442. https://doi.org/10.3390/molecules26051442








