Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions
Abstract
:1. Introduction
2. Results
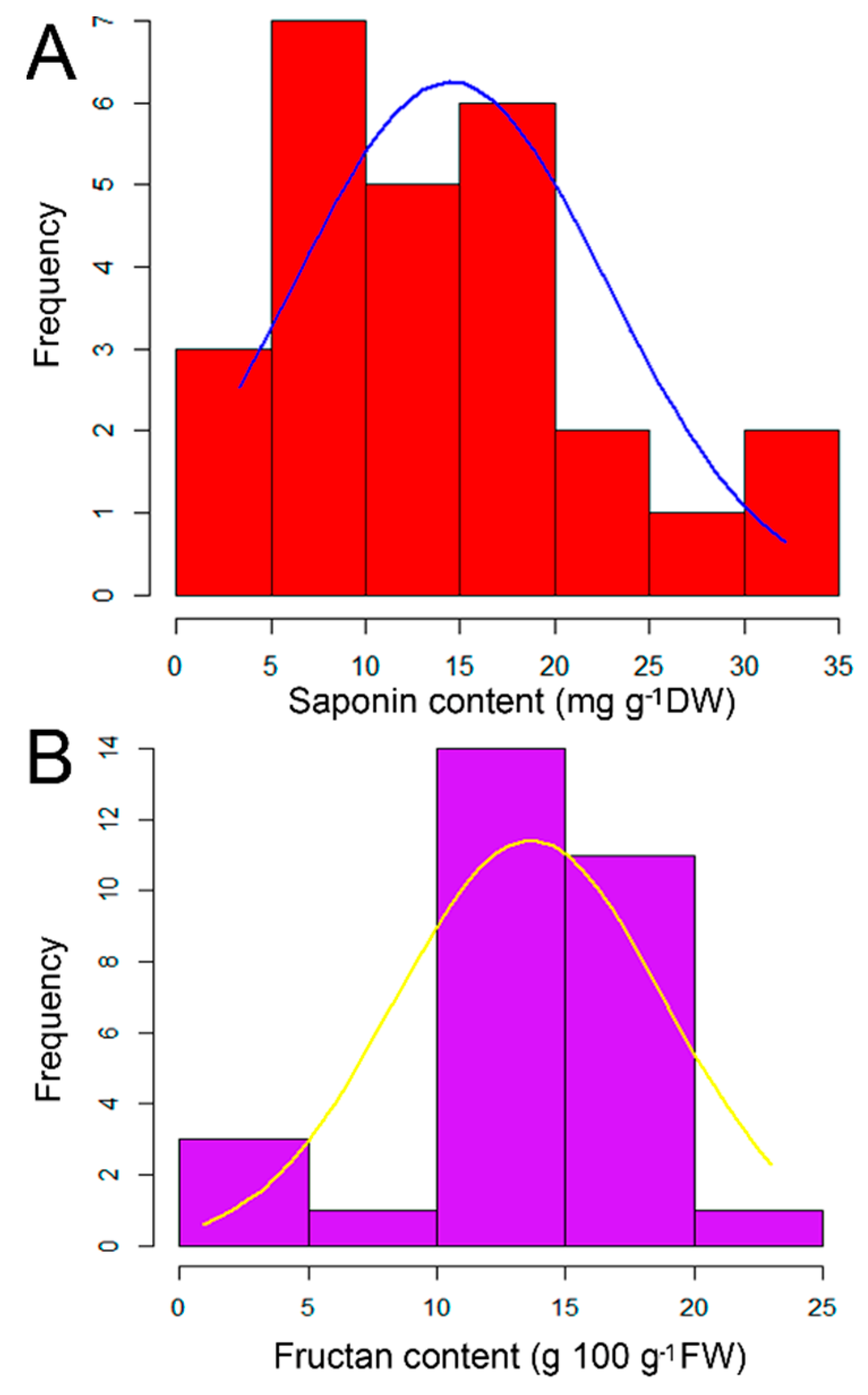
2.1. Quantitative Analysis of Saponin and Fructan Contents in the Examined Garlic Accessions
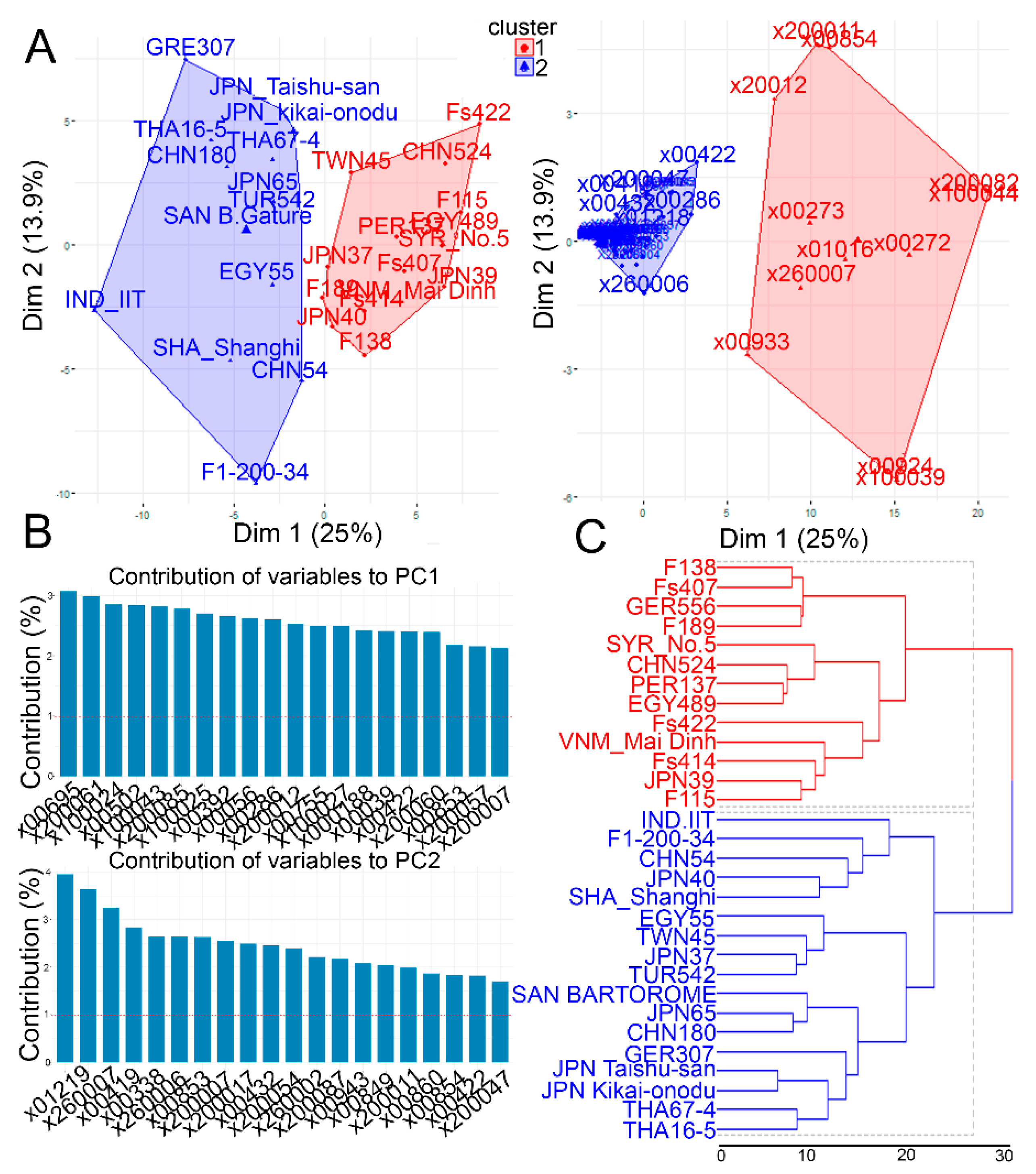
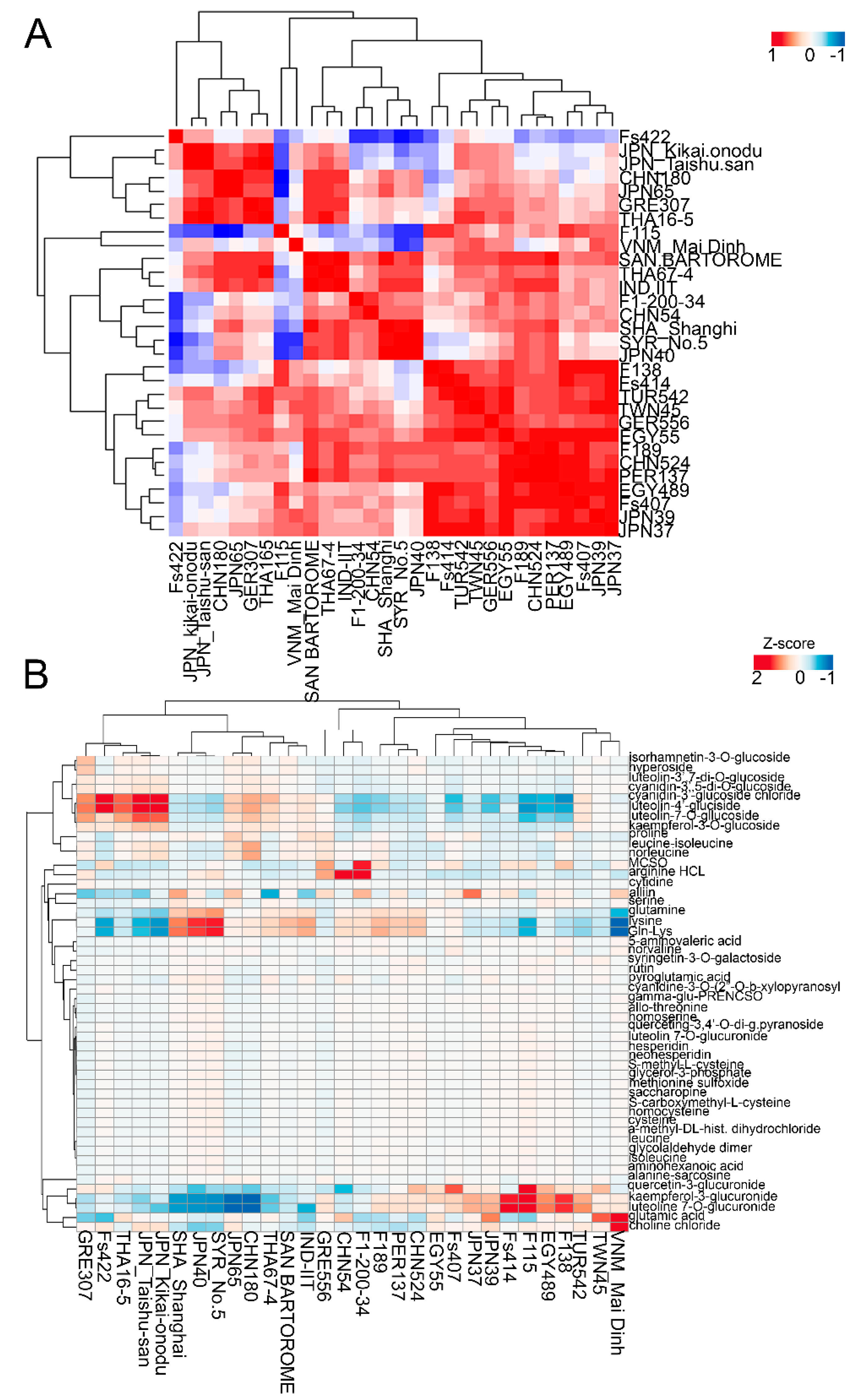
2.2. Metabolic Variations in the Leaf Tissues of the Investigated Garlic Accessions
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of the Total Saponins in the Garlic Accessions
4.3. Determination of the Fructan Contents in the Garlic Cloves
4.4. Targeted Metabolome Profiling of the Garlic Accessions
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirata, S.; Abdelrahman, M.; Yamauchi, N.; Shigyo, M. Characteristics of chemical components in genetic resources of garlic Allium sativum collected from all over the world. Genet. Resour. Crop. Evol. 2015, 63, 35–45. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Abdel-Motaal, F.; El-Sayed, M.; Jogaiah, S.; Shigyo, M.; Ito, S.-C.; Tran, L.-S.P. Dissection of Trichoderma longibrachiatum-induced defense in onion (Allium cepa L.) against Fusarium oxysporum f. sp. cepa by target metabolite profiling. Plant Sci. 2016, 246, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (allicin), a volatile an-timicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 2017, 22, 171. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 2 January 2021).
- Liu, J.; Liu, L.; Guo, W.; Fu, M.; Yang, M.; Huang, S.; Zhang, F.; Liu, Y. A new methodology for sensory quality assessment of garlic based on metabolomics and an artificial neural network. RSC Adv. 2019, 31, 17754–17765. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-Q.; Zhou, H.; Zhou, M.-Y.; Hu, X.-P.; Ou, S.-Y.; Yan, R.-A.; Liao, X.-J.; Huang, X.-S.; Fu, L. Characterization of phenolic constituents inhibiting the formation of sulfur-containing volatiles produced during garlic processing. J. Agric. Food Chem. 2015, 63, 787–794. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Volatile compounds of fresh and processed garlic (Review). Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Etoh, T. Fertility of the garlic clones collected in Soviet Central Asia. J. Jpn. Soc. Hortic. Sci. 1986, 55, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Egea, L.A.; Mérida-García, R.; Kilian, A.; Hernandez, P.; Dorado, G. Assessment of genetic diversity and structure of large garlic (Allium sativum) Germplasm Bank, by Diversity Arrays Technology “Genotyping-by-Sequencing” Platform (DArTseq). Front. Genet. 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamenetsky, R.; Shafir, I.L.; Zemah, H.; Barzilay, A.; Rabinowitch, H.D. Environmental control of garlic growth and Floro-genesis. J. Amer. Soc. Hort. Sci. 2004, 129, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.-H.; Kwag, J.-G.; Zhao, W.; Dixit, A.; Lee, G.-A.; Kim, H.-H.; Chung, I.-M.; Kim, N.-S.; Lee, J.-S.; Ji, J.-J.; et al. Isolation and characteristics of eight novel polymorphic microsatellite loci from the genome of garlic (Allium sativum L.). Sci. Hortic. 2009, 122, 355–361. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Faigenboim, A.; Mayer, E.S.; Ben Michael, T.; Gershberg, C.; Kimhi, S.; Esquira, I.; Shalom, S.R.; Eshel, D.; Rabinowitch, H.D.; et al. Integrated transcriptome catalogue and organ-specific profiling of gene expression in fertile garlic (Allium sativum L.). BMC Genom. 2015, 16, 12. [Google Scholar] [CrossRef] [Green Version]
- Figliuolo, G.; Candido, V.; Logozzo, G.; Miccolis, V.; Zeuli, P.S. Genetic evaluation of cultivated garlic germplasm (Allium sativum L. and A. ampeloprasum L.). Euphytica 2001, 121, 325–334. [Google Scholar] [CrossRef]
- Volk, G.M.; Stern, D. Phenotypic characteristics of ten garlic cultivars grown at different North American locations. HortScience 2009, 44, 1238–1247. [Google Scholar] [CrossRef] [Green Version]
- Etoh, T.; Simon, P.W. Diversity, fertility and seed production of garlic. In Allium Crop Science: Recent Advances; Rabinowitch, H.D., Currah, L., Eds.; CAB International: Wallingford, UK, 2002; pp. 101–117. [Google Scholar]
- Green, E.D. Strategies for the systematic sequencing of complex genomes. Nat. Rev. Genet. 2001, 2, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ovesná, J.; Mitrova, K.; Kučera, L. Garlic (A. sativum L.) alliinase gene family polymorphism reflects bolting types and cysteine sulphoxides content. BMC Genet. 2015, 16, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.G.; Hughes, J.; Tregova, A.; Milne, J.; Tomsett, A.B.; Collin, H.A. Biosynthesis of the flavour precursors of onion and garlic. J. Exp. Bot. 2004, 55, 1903–1918. [Google Scholar] [CrossRef] [Green Version]
- Khar, A.; Banerjee, K.; Jadhav, M.R.; Lawande, K. Evaluation of garlic ecotypes for allicin and other allyl thiosulphinates. Food Chem. 2019, 128, 988–996. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasanthi, A.R.; Mansingh, D.; Dalpati, N.; Sali, V. Alliin the precursor of allicin in garlic extract mitigates proliferation of gastric adenocarcinoma cells by modulating apoptosis. Pharmacogn. Mag. 2018, 14, S84–S91. [Google Scholar] [CrossRef]
- Yoo, M.; Lee, S.; Kim, S.; Hwang, J.-B.; Choe, J.; Shin, D. Composition of organosulfur compounds from cool- and warm-type garlic (Allium sativum L.) in Korea. Food Sci. Biotechnol. 2014, 23, 337–344. [Google Scholar] [CrossRef]
- Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gómez-Gómez, L.; Ahrazem, O. Tissue-specific accumulation of sulfur compounds and saponins in different parts of garlic cloves from purple and white ecotypes. Molecules 2017, 22, 1359. [Google Scholar] [CrossRef]
- Nagella, P.; Thiruvengadam, M.; Ahmad, A.; Yoon, J.-Y.; Chung, I.-M. Composition of polyphenols and antioxidant activity of garlic bulbs collected from different locations of Korea. Asian J. Chem. 2014, 26, 897–902. [Google Scholar] [CrossRef]
- Jo, M.H.; Ham, I.K.; Moe, K.T.; Kwon, S.W.; Lu, F.H.; Park, Y.J.; Kim, W.S.; Won, M.K.; Kim, T.I.; Lee, E.M. Classification of genetic variation in garlic (Allium sativum L.) using SSR markers. Aust. J. Crop Sci. 2012, 6, 625–631. [Google Scholar]
- Bibak, A.; Stürup, S.; Haahr, V.; Gundersen, P.; Gundersen, V. Concentrations of 50 major and trace elements in Danish ag-ricultural crops measured by inductively coupled plasma mass spectrometry. 3. Potato (Solanum tuberosum Folva). J. Agric. Food Chem. 1999, 47, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Hrbek, V.; Rektorisova, M.; Chmelarova, H.; Ovesna, J.; Hajslova, J. Authenticity assessment of garlic using a metabolomic approach based on high resolution mass spectrometry. J. Food Compos. Anal. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Nakabayashi, R.; Mori, T.; Ikeuchi, T.; Mori, M.; Murakami, K.; Ozaki, Y.; Matsumoto, M.; Uragami, A.; Tsujimoto, H.; et al. Comparative metabolome and transcriptome analyses of susceptible asparagus officinalis and resistant wild A. kiusianus reveal insights into stem blight disease resistance. Plant Cell Physiol. 2020, 61, 1464–1476. [Google Scholar] [CrossRef]
- Abdelrahman, M.; El-Sayed, M.; Sato, S.; Hirakawa, H.; Ito, S.-I.; Tanaka, K.; Mine, Y.; Sugiyama, N.; Suzuki, M.; Yamauchi, N.; et al. RNA-sequencing-based transcriptome and biochemical analyses of steroidal saponin pathway in a complete set of Allium fistulosum—A. cepa monosomic addition lines. PLoS ONE 2017, 12, e0181784. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, M.; Hirata, S.; Ito, S.-I.; Yamauchi, N.; Shigyo, M. Compartmentation and localization of bioactive metabolites in different organs of Allium roylei. Biosci. Biotechnol. Biochem. 2014, 78, 1112–1122. [Google Scholar] [CrossRef]
- Mostafa, A.; Sudisha, J.; El-Sayed, M.; Ito, S.-I.; Ikeda, T.; Yamauchi, N.; Shigyo, M. Aginoside saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem. Lett. 2013, 6, 274–280. [Google Scholar] [CrossRef]
- Itakura, Y.; Ichikawa, M.; Mori, Y.; Okino, R.; Udayama, M.; Morita, T. How to distinguish garlic from the other Allium vegetables. J. Nutr. 2001, 131, 963S–967S. [Google Scholar] [CrossRef] [Green Version]
- Lanzotti, V.; Barile, E.; Antignani, V.; Bonanomi, G.; Scala, F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera. Phytochemistry 2012, 78, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Mansell, T.J. A Review of “Garlic and Other Alliums: The Lore and the science”. Food Foodways 2010, 18, 170–172. [Google Scholar] [CrossRef]
- Santhosha, S.G.; Jamuna, P.; Prabhavathi, S.N. Bioactive components of garlic and their physiological role in health mainte-nance: A review. Food Biosci. 2013, 3, 59–74. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Shalom, S.R.; Faigenboim-Doron, A.; Teper-Bamnolker, P.; Salam, B.B.; Daus, A.; Kamenetsky, R.; Eshel, D. Differential carbohydrate gene expression during preplanting temperature treatments controls meristem termination and bulbing in garlic. Environ. Exp. Bot. 2018, 150, 280–291. [Google Scholar] [CrossRef]
- Carrillo-Navarrete, F.; Mercado-Silva, E.; Rivera-Pastrana, D.M.; Nava-Morales, G.M.; Reynoso-Camacho, R.; Casta-ño-Tostado, E.; Vázquez-Barrios, M.E. Extraction and characterization of fructans from non-differentiated garlic (Allium sativum L.) and evaluation of its prebiotic effect. Acta Hortic. 2016, 1194. [Google Scholar] [CrossRef]
- Guevara-Figuero, T.; López-Hernández, L.; Lopez, M.G.; Dufoo, H.M.D.; Vázquez Barrios, M.E.; Guevara-Olvera, L.; Guevara González, R.G.; Rivera-Pastrana, D.M.; Torres-Robles, H.; Mercado-Silva, E.M. Conditioning garlic “seed” cloves at low temperature modifies plant growth, sugar, fructan content, and sucrose fructosyltransferase (1-SST) expression. Sci. Hortic. 2015, 189, 150–158. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Ebskamp, M.J.M.; Paul, M.J.; Jeuken, M.J.W.; Weisbeek, P.J.; Smeekens, S.C.M. Improved perfor-mance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 1995, 107, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic-Malinovska, R.; Kuzmanova, S.; Winkelhausen, E. Oligosaccharide Profile in Fruits and Vegetables as Sources of Prebiotics and Functional Foods. Int. J. Food Prop. 2014, 17, 949–965. [Google Scholar] [CrossRef]
- Ariyanti, N.A.; Torikai, K.; Kirana, R.P.; Hirata, S.; Sulistyaningsih, E.; Ito, S.-I.; Yamauchi, N.; Kobayashi, N.; Shigyo, M. Comparative study on phytochemical variations in Japanese F1 varieties of bulb onions and South-East Asian shallot landraces. Hortic. J. 2018, 87, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, M.; Ariyanti, N.A.; Sawada, Y.; Tsuji, F.; Hirata, S.; Hang, T.T.M.; Okamoto, M.; Yamada, Y.; Tsugawa, H.; Hirai, M.Y.; et al. Metabolome-Based Discrimination analysis of shallot landraces and bulb onion cultivars associated with differences in the amino acid and flavonoid profiles. Molecules 2020, 25, 5300. [Google Scholar] [CrossRef]
- Jo, S.; Song, Y.; Jeong, J.-H.; Hwang, J.; Kim, Y. Geographical discrimination of Allium species (garlic and onion) using 1H NMR spectroscopy with multivariate analysis. Int. J. Food Prop. 2020, 23, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of nitrogen and sulfur fertilization on the alliin content of onions and garlic. J. Plant Nutr. 2005, 27, 1827–1839. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- Hirata, S.; Abdelrahman, M.; Yamauchi, N.; Shigyo, M. Diversity evaluation based on morphological, physiological and iso-zyme variation in genetic resources of garlic (Allium sativum L.) collected worldwide. Genes Genet. Syst. 2016, 91, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Kamenetsky, R.; Shafir, I.L.; Khassanov, F.; Kik, C.; van Heusden, A.W.; Vrielink-van Ginkel, M.; Burger-Meijer, K.; Auger, J.; Arnault, I.; Rabinowitch, H.D. Diversity in fertility potential and organo-sulphur compounds among garlics from Central Asia. Biodivers. Conserv. 2005, 14, 281–295. [Google Scholar] [CrossRef]
- Etoh, T. Studies on the sterility in garlic, Allium sativum L. Mem. Fac. Agric. Kagoshima Univ. 1985, 21, 77–132. [Google Scholar]
- Hirata, S. Evaluation of Genetic Diversity in Worldwide Germplasm Collection of Garlic (Allium sativum L.). Ph.D. Thesis, Tottori University, Tottori, Japan, 2016. [Google Scholar]
- Japan Meteorological Agency. Tables of Monthly Climate Statistics. Climate Data. Available online: https://www.data.jma.go.jp/gmd/risk/obsdl/index.php#!table (accessed on 2 January 2021).
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 131, 716S–725S. [Google Scholar] [CrossRef] [Green Version]
- Percheron, F. Dosage colorimetrique du fructose et des fructofuranosides par l’ acide thiobarbiturique. C. R. Acad. Sci. 1962, 255, 2521–2522. [Google Scholar]
- Sawada, Y.; Tsukaya, H.; Li, Y.; Sato, M.; Kawade, K.; Hirai, M.Y. A novel method for single-grain-based metabolic profiling of Arabidopsis seed. Metabolomics 2017, 13, 75. [Google Scholar] [CrossRef]
| World Collection No. | Sample Name | Region of Origin |
|---|---|---|
| AsWC146 | JPN37 | Japan |
| AsWC147 | JPN39 | Japan |
| AsWC148 | JPN40 | Japan |
| AsWC149 | TWN45 | Taiwan |
| AsWC150 | CHN54 | China |
| AsWC151 | EGY55 | Egypt |
| AsWC154 | JPN65 | Japan |
| AsWC158 | PER137 | Peru |
| AsWC159 | CHN180 | China |
| AsWC162 | GRE307 | Greek |
| AsWC169 | EGY489 | Egypt |
| AsWC173 | CHN524 | China |
| AsWC175 | TUR542 | Turkey |
| AsWC177 | GER556 | German |
| AsWC181 | F115 | Central Asia |
| AsWC183 | F138 | Central Asia |
| AsWC186 | F189 | Central Asia |
| AsWC189 | F1-200-34 | Central Asia |
| AsWC193 | Fs407 | Central Asia |
| AsWC195 | Fs414 | Central Asia |
| AsWC196 | Fs422 | Central Asia |
| AsWC198 | SAN BARTOROME (Gatur) | Turkey |
| AsWC201 | SYR_No.5 | Syria |
| AsWC202 | IND-IIT | India |
| AsWC205 | VNM_Mai Dinh | Vietnam |
| AsWC208 | THA67-4 | Thailand |
| AsWC209 | THA16-5 | Thailand |
| AsWC214 | JPN_Taishu-san | Japan |
| AsWC224 | SHA_Shanghi | China |
| AsWC226 | JPN_Kikai-onodu | Japan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, M.; Hirata, S.; Mukae, T.; Yamada, T.; Sawada, Y.; El-Syaed, M.; Yamada, Y.; Sato, M.; Hirai, M.Y.; Shigyo, M. Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions. Molecules 2021, 26, 1415. https://doi.org/10.3390/molecules26051415
Abdelrahman M, Hirata S, Mukae T, Yamada T, Sawada Y, El-Syaed M, Yamada Y, Sato M, Hirai MY, Shigyo M. Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions. Molecules. 2021; 26(5):1415. https://doi.org/10.3390/molecules26051415
Chicago/Turabian StyleAbdelrahman, Mostafa, Sho Hirata, Takuya Mukae, Tomohiro Yamada, Yuji Sawada, Magdi El-Syaed, Yutaka Yamada, Muneo Sato, Masami Yokota Hirai, and Masayoshi Shigyo. 2021. "Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions" Molecules 26, no. 5: 1415. https://doi.org/10.3390/molecules26051415
APA StyleAbdelrahman, M., Hirata, S., Mukae, T., Yamada, T., Sawada, Y., El-Syaed, M., Yamada, Y., Sato, M., Hirai, M. Y., & Shigyo, M. (2021). Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions. Molecules, 26(5), 1415. https://doi.org/10.3390/molecules26051415







