Structural, Optical and Electrical Properties of Cu0.6CoxZn0.4−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4) Soft Ferrites
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. Fourier-Transformation Infrared Spectroscopy (FTIR)
2.3. UV-Vis Spectroscopy
2.4. Electrical Properties
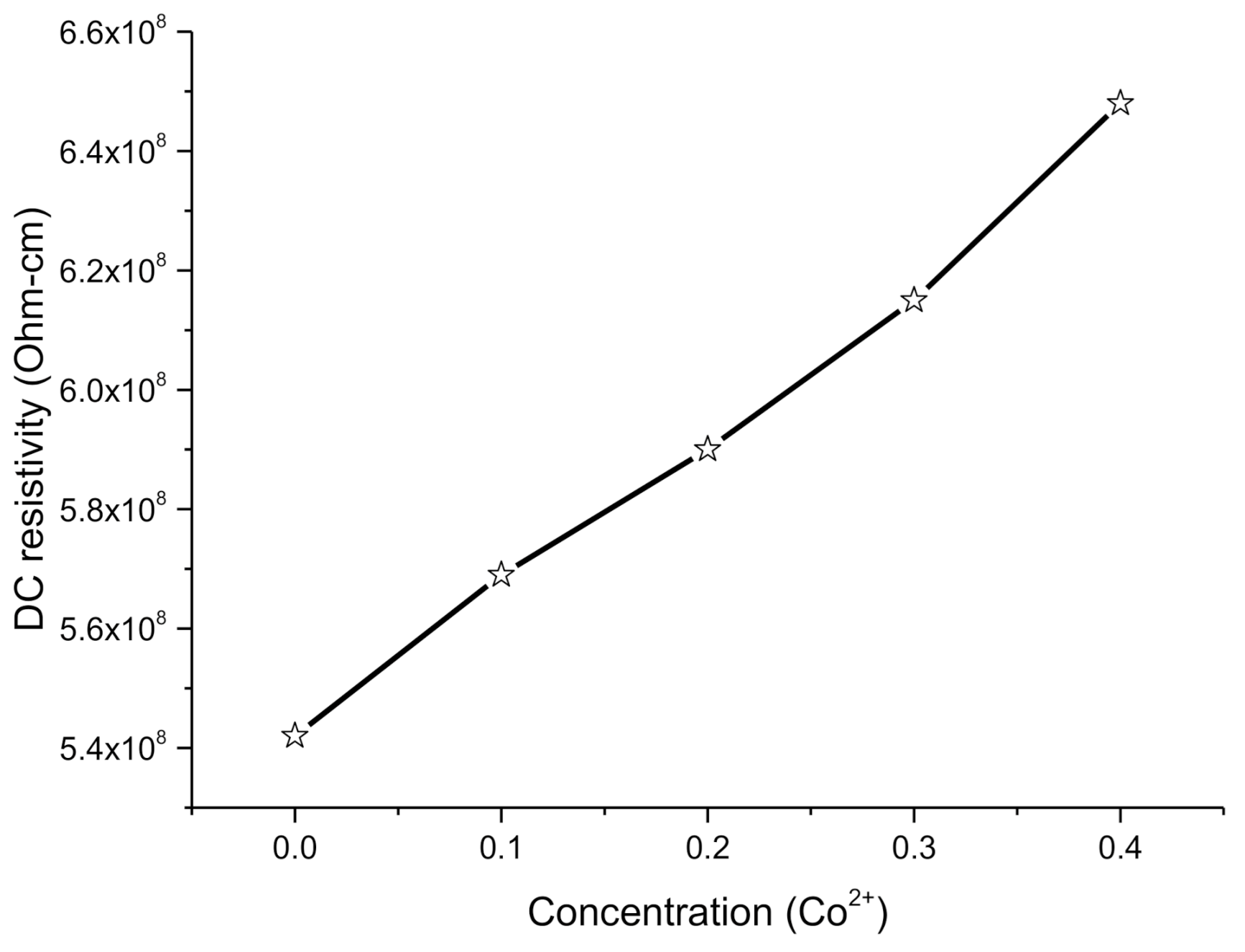
2.4.1. DC Electrical Resistivity
2.4.2. Effect of Co on DC Resistivity
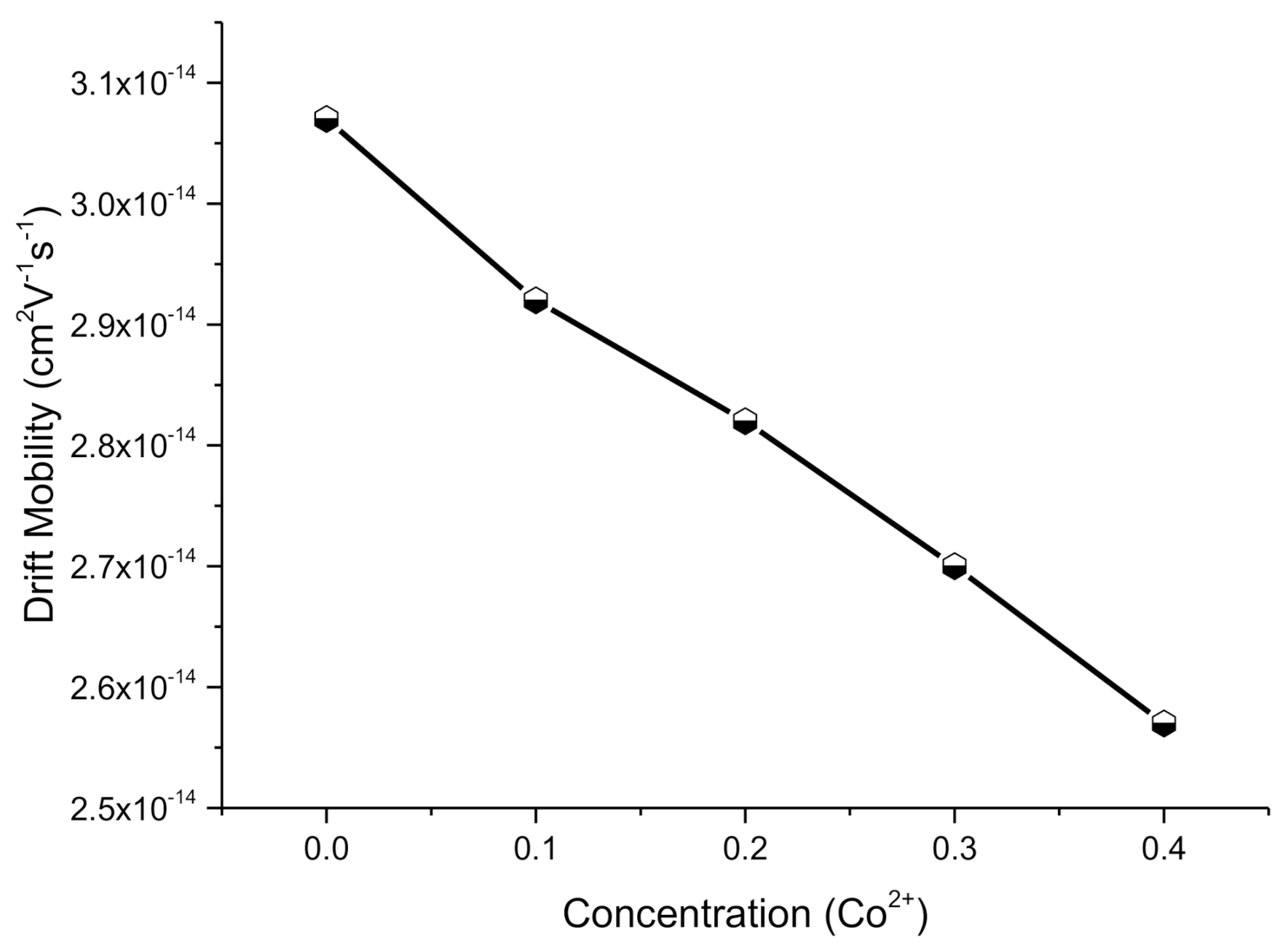
2.4.3. Drift Mobility
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bhukal, S.; Singhal, S. Magnetically separable copper substituted cobalt–zinc nano-ferrite photocatalyst with enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2014, 26, 467–476. [Google Scholar] [CrossRef]
- Anu, K.; Hemalatha, J. Magnetic and electrical conductivity studies of zinc doped cobalt ferrite nanofluids. J. Mol. Liq. 2019, 284, 445–453. [Google Scholar] [CrossRef]
- Prasad, B.V.; Ramesh, K.; Srinivas, A. Co0.5-xCuxZn0.5Fe2O4 (0 ≤ x ≤ 0.25) nano crystalline ferrites: Structural, magnetic and electric properties. Solid State Sci. 2020, 107, 106325. [Google Scholar] [CrossRef]
- Warsi, M.F.; Iftikhar, A.; Yousuf, M.A.; Sarwar, M.I.; Yousaf, S.; Haider, S.; Aboud, M.F.A.; Shakir, I.; Zulfiqar, S. Erbium substituted nickel–cobalt spinel ferrite nanoparticles: Tailoring the structural, magnetic and electrical parameters. Ceram. Int. 2020, 46, 24194–24203. [Google Scholar] [CrossRef]
- Somvanshi, S.B.; Khedkar, M.V.; Kharat, P.B.; Jadhav, K. Influential diamagnetic magnesium (Mg2+) ion substitution in nano-spinel zinc ferrite (ZnFe2O4): Thermal, structural, spectral, optical and physisorption analysis. Ceram. Int. 2020, 46, 8640–8650. [Google Scholar] [CrossRef]
- Hasan, M.; Arshad, M.; Ali, A.; Mahmood, K.; Amin, N.; Ali, S.; Khan, M.; Mustafa, G.; Khan, M.; Saleem, M. Mg and La co-doped ZnNi spinel ferrites for low resistive applications. Mater. Res. Express 2018, 6, 016302. [Google Scholar] [CrossRef]
- Kaiser, M. Influence of V2O5 ion addition on the conductivity and grain growth of Ni–Zn–Cu ferrites. Curr. Appl. Phys. 2010, 10, 975–984. [Google Scholar] [CrossRef]
- Ponhan, W.; Maensiri, S. Fabrication and magnetic properties of electrospun copper ferrite (CuFe2O4) nanofibers. Solid State Sci. 2009, 11, 479–484. [Google Scholar] [CrossRef]
- Amin, N.; Hasan, M.S.U.; Majeed, Z.; Latif, Z.; un Nabi, M.A.; Mahmood, K.; Ali, A.; Mehmood, K.; Fatima, M.; Akhtar, M. Structural, electrical, optical and dielectric properties of yttrium substituted cadmium ferrites prepared by Co-Precipitation method. Ceram. Int. 2020, 46, 20798–20809. [Google Scholar] [CrossRef]
- Khan, M.; Waqas, M.; Naeem, M.; Hasan, M.; Iqbal, M.; Mahmood, A.; Ramay, S.M.; Al-Masry, W.; Abubshait, S.A.; Abubshait, H.A. Magnetic behavior of Ga doped yttrium iron garnet ferrite thin films deposited by sol-gel technique. Ceram. Int. 2020, 46, 27318–27325. [Google Scholar] [CrossRef]
- Rajeshwari, A.; Punithavthy, I.K.; Jeyakumar, S.J.; Lenin, N.; Vigneshwaran, B. Dependance of lanthanum ions on structural, magnetic and electrical of manganese based spinel nanoferrites. Ceram. Int. 2020, 46, 6860–6870. [Google Scholar] [CrossRef]
- Sujatha, C.; Reddy, K.V.; Babu, K.S.; Reddy, A.R.; Suresh, M.B.; Rao, K. Effect of Mg substitution on electromagnetic properties of NiCuZn ferrite. J. Magn. Magn. Mater. 2013, 340, 38–45. [Google Scholar] [CrossRef]
- Iyer, R.; Desai, R.; Upadhyay, R. Low temperature synthesis of nanosized Mn1-xCdxFe2O4 ferrites. NISCAIR Online Period. Repos. 2009, 2, 180–185. [Google Scholar]
- Hemeda, O.M.; Barakat, M.M.; Hemeda, D.M. Structural, electrical and spectral studies on double rare-earth orthoferrites La_ {1-x} Nd_xFeO_3. Turk. J. Phys. 2004, 27, 537–550. [Google Scholar]
- Patil, S.; Mahajan, V.; Ghatage, A.; Lotke, S. Structure and magnetic properties of Cd and Ti/Si substituted cobalt ferrites. Mater. Chem. Phys. 1998, 57, 86–91. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Verwey, E.; De Boer, J. Cation arrangement in a few oxides with crystal structures of the spinel type. Recl. Trav. Chim. Pays Bas 1936, 55, 531–540. [Google Scholar] [CrossRef]
- Raghasudha, M.; Ravinder, D.; Veerasomaiah, P. Electrical resistivity studies of Cr doped Mg nano-ferrites. Mater. Discov. 2015, 2, 50–54. [Google Scholar] [CrossRef]
- Yousuf, M.A.; Baig, M.M.; Waseem, M.; Haider, S.; Shakir, I.; Khan, S.U.-D.; Warsi, M.F. Low cost micro-emulsion route synthesis of Cr-substituted MnFe2O4 nanoparticles. Ceram. Int. 2019, 45, 22316–22323. [Google Scholar] [CrossRef]
- Lakshman, A.; Rao, P.S.; Rao, B.P.; Rao, K. Electrical properties of In3+ and Cr3+ substituted magnesium–manganese ferrites. J. Phys. D Appl. Phys. 2005, 38, 673. [Google Scholar] [CrossRef]
- Kambale, R.; Shaikh, P.; Kamble, S.; Kolekar, Y. Effect of cobalt substitution on structural, magnetic and electric properties of nickel ferrite. J. Alloy. Compd. 2009, 478, 599–603. [Google Scholar] [CrossRef]
- Mallapur, M.; Shaikh, P.; Kambale, R.; Jamadar, H.; Mahamuni, P.; Chougule, B. Structural and electrical properties of nanocrystalline cobalt substituted nickel zinc ferrite. J. Alloy. Compd. 2009, 479, 797–802. [Google Scholar] [CrossRef]
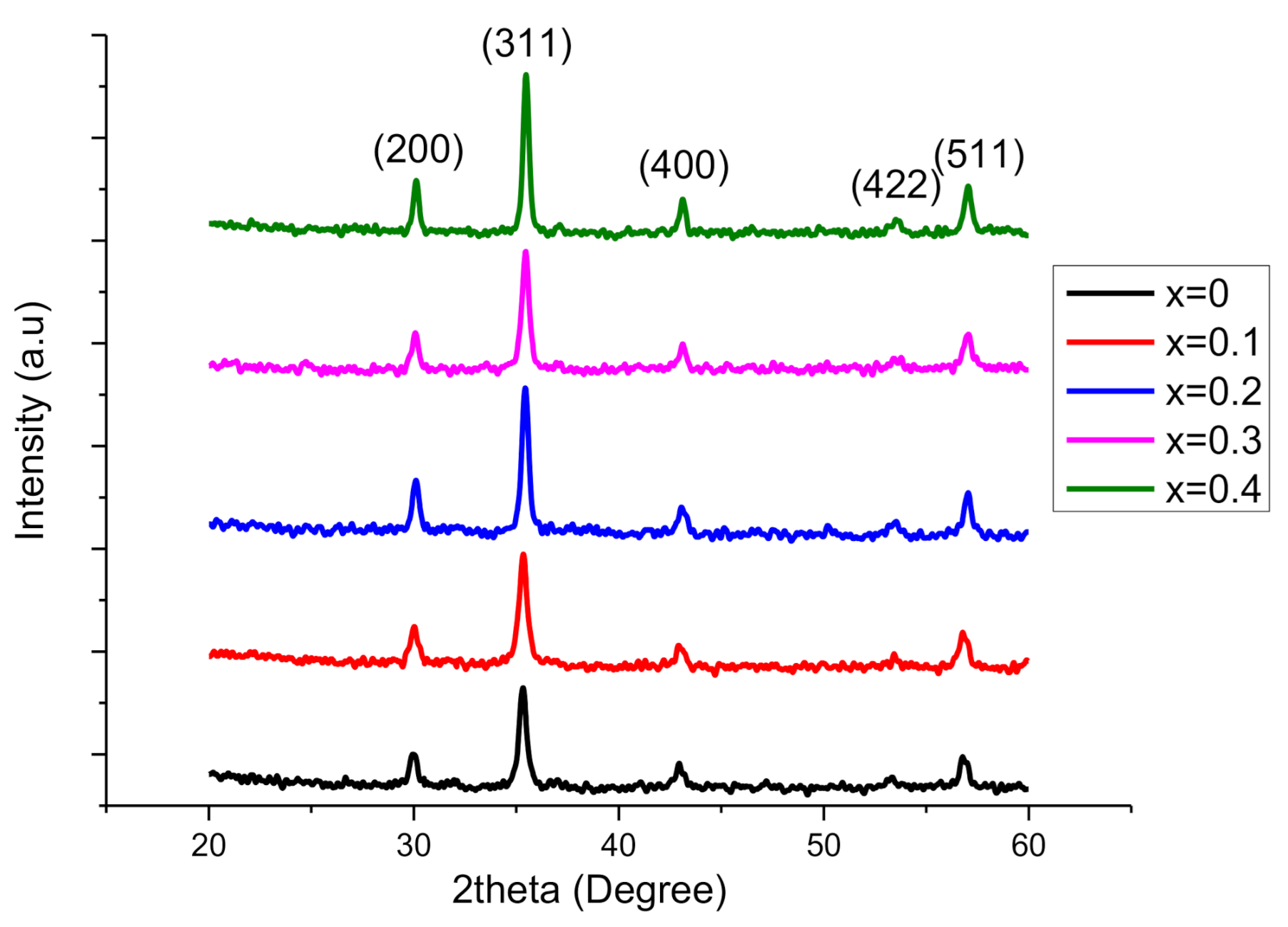
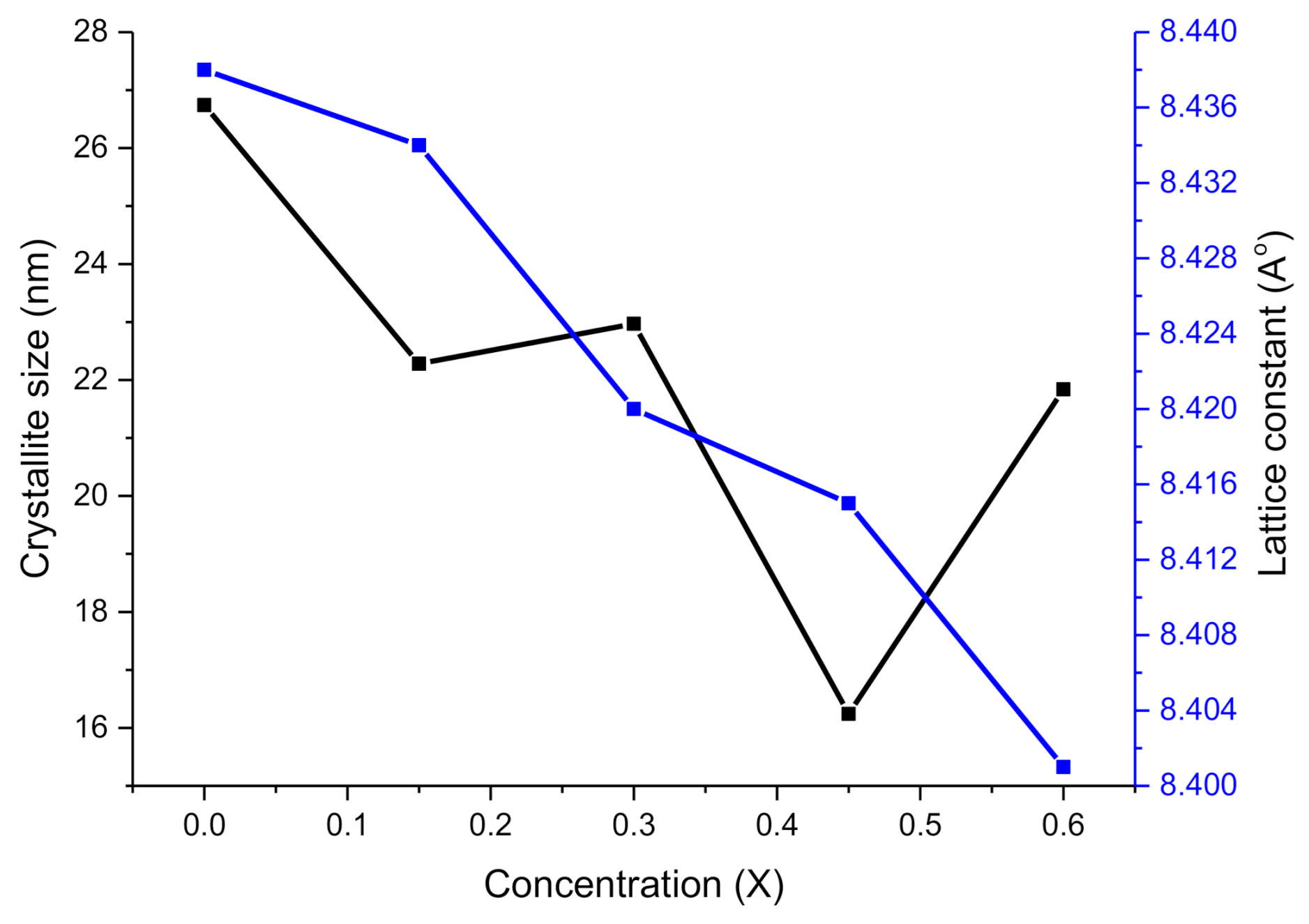
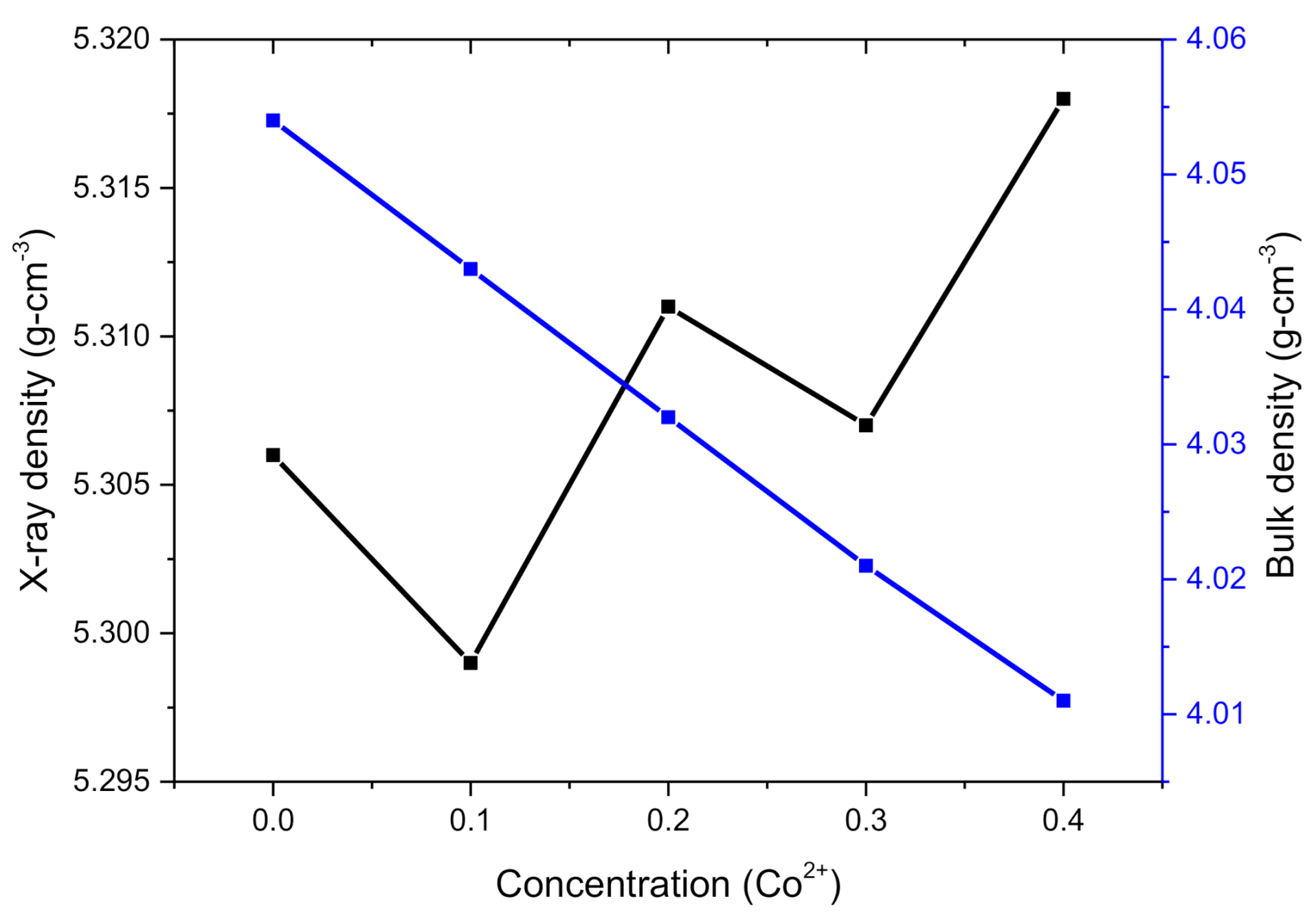

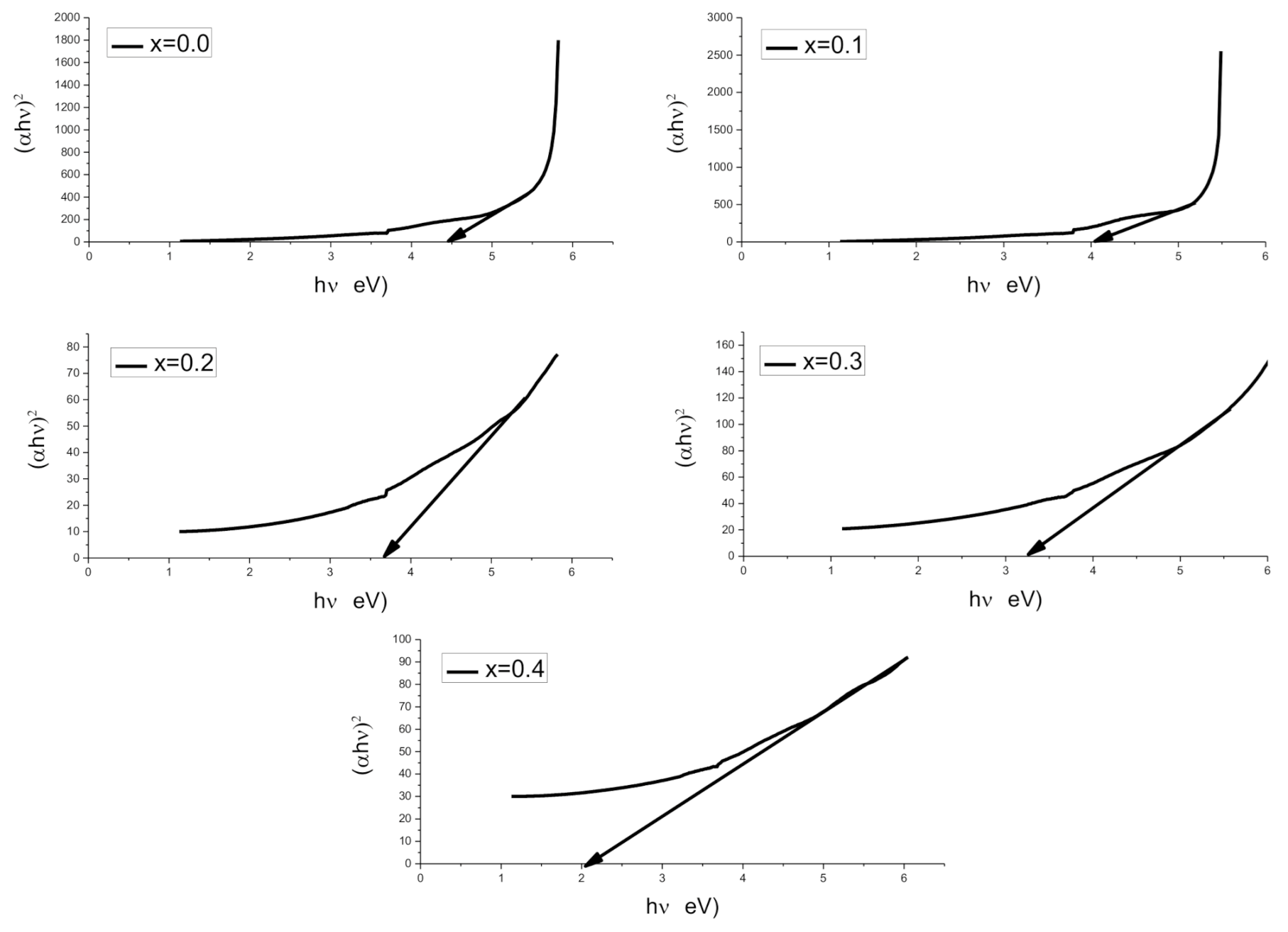

| Parameter | X = 0.00 | 0.1 | 0.2 | 0.3 | 0.4 |
|---|---|---|---|---|---|
| 2θ of (311) peak | 35.32 | 35.34 | 35.40 | 35.43 | 35.48 |
| aexp (Å) | 8.438 | 8.434 | 8.420 | 8.415 | 8.401 |
| D (nm) | 26.74 | 22.28 | 22.97 | 16.24 | 21.84 |
| V (Å)3 | 600.78 | 599.93 | 596.98 | 595.88 | 592.91 |
| d (Å) | 2.544 | 2.542 | 2.538 | 2.535 | 2.533 |
| dx(g-cm−3) | 5.306 | 5.299 | 5.311 | 5.307 | 5.318 |
| db (g-cm−3) | 4.054 | 4.043 | 4.032 | 4.021 | 4.011 |
| P (%) | 23.59 | 23.70 | 24.08 | 24.23 | 24.57 |
| X | V1 (cm−1) | I1 (%) | KT × 105 (Dyne cm−1) | V2 (cm−1) | I2 (%) | Ko × 105 (Dyne cm−1) |
|---|---|---|---|---|---|---|
| 0.0 | 540.97 | 62.74 | 2.15 | 476.62 | 64.05 | 1.66 |
| 0.1 | 538.73 | 59.73 | 2.13 | 473.83 | 67.19 | 1.64 |
| 0.2 | 537.49 | 54.44 | 2.12 | 472.86 | 62.11 | 1.63 |
| 0.3 | 524.77 | 62.31 | 2.03 | 467.87 | 63.14 | 1.60 |
| 0.4 | 524.32 | 47.46 | 2.02 | 462.94 | 47.27 | 1.56 |
| Parameters | Results | ||||
|---|---|---|---|---|---|
| X | 0.0 | 0.1 | 0.2 | 0.3 | 0.4 |
| Eg (eV) | 4.44 | 4.05 | 3.64 | 3.23 | 2.05 |
| ρDC(Ω·cm) | 5.42 × 108 | 5.69 × 108 | 5.90 × 108 | 6.15 × 108 | 6.48 × 108 |
| µd (cm2V−1s−1) | 3.07 × 10−14 | 2.92 × 10−14 | 2.82 × 10−14 | 2.70 × 10−14 | 2.57 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, W.A.; Sajjad Ul Hasan, M.; Khan, M.I.; Ashraf, A.R.; Abdul Qayyum, M.; Yaqub, N.; Almutairi, M.A.; Atif, M.; Hanif, A. Structural, Optical and Electrical Properties of Cu0.6CoxZn0.4−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4) Soft Ferrites. Molecules 2021, 26, 1399. https://doi.org/10.3390/molecules26051399
Farooq WA, Sajjad Ul Hasan M, Khan MI, Ashraf AR, Abdul Qayyum M, Yaqub N, Almutairi MA, Atif M, Hanif A. Structural, Optical and Electrical Properties of Cu0.6CoxZn0.4−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4) Soft Ferrites. Molecules. 2021; 26(5):1399. https://doi.org/10.3390/molecules26051399
Chicago/Turabian StyleFarooq, W. Aslam, Muhammad Sajjad Ul Hasan, Muhammad Iftikhar Khan, Ahmad Raza Ashraf, Muhammad Abdul Qayyum, Nafeesah Yaqub, Mona A. Almutairi, Muhammad Atif, and Atif Hanif. 2021. "Structural, Optical and Electrical Properties of Cu0.6CoxZn0.4−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4) Soft Ferrites" Molecules 26, no. 5: 1399. https://doi.org/10.3390/molecules26051399
APA StyleFarooq, W. A., Sajjad Ul Hasan, M., Khan, M. I., Ashraf, A. R., Abdul Qayyum, M., Yaqub, N., Almutairi, M. A., Atif, M., & Hanif, A. (2021). Structural, Optical and Electrical Properties of Cu0.6CoxZn0.4−xFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4) Soft Ferrites. Molecules, 26(5), 1399. https://doi.org/10.3390/molecules26051399








