1,3,5-Triaza-7-Phosphaadamantane (PTA) as a 31P NMR Probe for Organometallic Transition Metal Complexes in Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. PTA Complexes of Group 8 and 9 Transition Metals
2.2. PTA Complexes of Group 10 Transition Metals
2.3. PTA Complexes of Group 11 Transition Metals
2.4. PTA Complexes of Group 12 Transition Metals
3. Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kukushkin, V.Y.; Pombeiro, A.J.L. Additions to Metal-Activated Organonitriles. Chem. Rev. 2002, 102, 1771–1802. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, G.S.; Bilyachenko, A.N.; Korlyukov, A.A.; Levitsky, M.M.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. High-Cluster (Cu9) Cage Silsesquioxanes: Synthesis, Structure, and Catalytic Activity. Inorg. Chem. 2018, 57, 11524–11529. [Google Scholar] [CrossRef]
- Epstein, L.M.; Shubina, E.S. New types of hydrogen bonding in organometallic chemistry. Coord. Chem. Rev. 2002, 231, 165–181. [Google Scholar] [CrossRef]
- Belkova, N.V.; Shubina, E.S.; Epstein, L.M. Diverse World of Unconventional Hydrogen Bonds. Acc. Chem. Res. 2005, 38, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.A.; Prior, T.J.; Savoie, H.; Boyle, R.W.; Murray, B.S. The Application of Reversible Intramolecular Sulfonamide Ligation to Modulate Reactivity in Organometallic Ruthenium (II) Diamine Complexes. Molecules 2020, 25, 244. [Google Scholar] [CrossRef] [PubMed]
- Solum, M.S.; Altmann, K.L.; Strohmeier, M.; Berges, D.A.; Zhang, Y.; Facelli, J.C.; Pugmire, R.J.; Grant, D.M. 15N Chemical Shift Principal Values in Nitrogen Heterocycles. J. Am. Chem. Soc. 1997, 119, 9804–9809. [Google Scholar] [CrossRef]
- Caulkins, B.G.; Young, R.P.; Kudla, R.A.; Yang, C.; Bittbauer, T.J.; Bastin, B.; Hilario, E.; Fan, L.; Marsella, M.J.; Dunn, M.F.; et al. NMR Crystallography of a Carbanionic Intermediate in Tryptophan Synthase: Chemical Structure, Tautomerization, and Reaction Specificity. J. Am. Chem. Soc. 2016, 138, 15214–15226. [Google Scholar] [CrossRef]
- Kong, S.; Borissova, A.O.; Lesnichin, S.B.; Hartl, M.; Daemen, L.L.; Eckert, J.; Antipin, M.Y.; Shenderovich, I.G. Geometry and Spectral Properties of the Protonated Homodimer of Pyridine in the Liquid and Solid States. A Combined NMR, X-ray Diffraction and Inelastic Neutron Scattering Study. J. Phys. Chem. A 2011, 115, 8041–8048. [Google Scholar] [CrossRef]
- Marek, R.; Lycka, A.; Kolehmainen, E.; Sievanen, E.; Tousek, J. 15N NMR Spectroscopy in Structural Analysis: An Update (2001–2005). Curr. Org. Chem. 2007, 11, 1154. [Google Scholar] [CrossRef]
- Xin, D.; Sader, C.A.; Fischer, U.; Wagner, K.; Jones, P.-J.; Xing, M.; Fandrick, K.R.; Gonnella, N.C. Systematic investigation of DFT-GIAO 15N NMR chemical shift prediction using B3LYP/cc-pVDZ: Application to studies of regioisomers, tautomers, protonation states and N-oxides. Org. Biomol. Chem. 2017, 15, 928–936. [Google Scholar] [CrossRef]
- Limbach, H.-H.; Chan-Huot, M.; Sharif, S.; Tolstoy, P.M.; Shenderovich, I.G.; Denisov, G.S. Critical Hydrogen Bonds and Protonation States of Pyridoxal 5’-phosphate Revealed by NMR. Biochim. Biophys. Acta 2011, 1814, 1426–1437. [Google Scholar] [CrossRef]
- Lesnichin, S.B.; Tolstoy, P.M.; Limbach, H.-H.; Shenderovich, I.G. Counteranion-Dependent Mechanisms of Intramolecular Proton Transfer in Aprotic Solution. Phys. Chem. Chem. Phys. 2010, 12, 10373–10379. [Google Scholar] [CrossRef] [PubMed]
- Buntkowsky, G.; Vogel, M. Small Molecules, Non-Covalent Interactions, and Confinement. Molecules 2020, 25, 3311. [Google Scholar] [CrossRef]
- Zhao, L.; Smolarkiewicz, I.; Limbach, H.-H.; Breitzke, H.; Pogorzelec-Glaser, K.; Pankiewicz, R.; Tritt-Goc, J.; Gutmann, T.; Buntkowsky, G. Imidazole-Doped Cellulose as Membrane for Fuel Cells: Structural and Dynamic Insights from Solid-State NMR. J. Phys. Chem. C 2016, 120, 19574–19585. [Google Scholar] [CrossRef]
- Gurinov, A.A.; Rozhkova, Y.A.; Zukal, A.; Čejka, J.; Shenderovich, I.G. Mutable Lewis and Brønsted Acidity of Aluminated SBA-15 as Revealed by NMR of Adsorbed Pyridine-15N. Langmuir 2011, 27, 12115–12123. [Google Scholar] [CrossRef]
- Pazderski, L. 15N NMR coordination shifts in transition metal complexes and organometallics with heterocycles containing nitrogen—Update for 2012–20. Annu. Rep. NMR Spectrosc. 2020, 101, 151–284. [Google Scholar] [CrossRef]
- Pazderski, L. 15N and 31P NMR Coordination Shifts in Transition Metal Complexes with Nitrogen- and Phosphorus-Containing Heterocycles. Annu. Rep. NMR Spectrosc. 2013, 80, 33–179. [Google Scholar] [CrossRef]
- Mulloyarova, V.V.; Ustimchuk, D.O.; Filarowski, A.; Tolstoy, P.M. H/D Isotope Effects on 1H-NMR Chemical Shifts in Cyclic Heterodimers and Heterotrimers of Phosphinic and Phosphoric Acids. Molecules 2020, 25, 1907. [Google Scholar] [CrossRef]
- Mulloyarova, V.V.; Giba, I.S.; Kostin, M.A.; Denisov, G.S.; Shenderovich, I.G.; Tolstoy, P.M. Cyclic Trimers of Phosphinic Acids in Polar Aprotic Solvent: Symmetry, Chirality and H/D Isotope Effects on NMR Chemical Shifts. Phys. Chem. Chem. Phys. 2018, 20, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Shenderovich, I.G. For Whom a Puddle Is the Sea? Adsorption of Organic Guests on Hydrated MCM-41 Silica. Langmuir 2020, 36, 11383–11392. [Google Scholar] [CrossRef]
- Hubbard, P.J.; Benzie, J.W.; Bakhmutov, V.I.; Blümel, J. Disentangling Different Modes of Mobility of Triphenylphosphine Oxide Adsorbed on Alumina. J. Chem. Phys. 2020, 152, 054718. [Google Scholar] [CrossRef] [PubMed]
- Kharel, S.; Cluff, K.J.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Structures and Dynamics of Secondary and Tertiary Alkylphosphine Oxides Adsorbed on Silica. Chem.—Asian J. 2019, 14, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Machida, S.; Sohmiya, M.; Ide, Y.; Sugahara, Y. Solid-State 31P Nuclear Magnetic Resonance Study of Interlayer Hydroxide Surfaces of Kaolinite Probed with an Interlayer Triethylphosphine Oxide Monolayer. Langmuir 2018, 34, 12694–12701. [Google Scholar] [CrossRef]
- Begimova, G.U.; Tupikina, E.Y.; Yu, V.K.; Denisov, G.S.; Bodensteiner, M.; Shenderovich, I.G. Effect of Hydrogen Bonding to Water on the 31P Chemical Shift Tensor of Phenyl- and Trialkylphosphine Oxides and a-Amino Phosphonates. J. Phys. Chem. C 2016, 120, 8717–8729. [Google Scholar] [CrossRef]
- Shenderovich, I.G. Effect of Noncovalent Interactions on the 31P Chemical Shift Tensor of Phosphine Oxides, Phosphinic, Phosphonic, and Phosphoric Acids, and Their Complexes with Lead(II). J. Phys. Chem. C 2013, 117, 26689–26702. [Google Scholar] [CrossRef]
- Cluff, K.J.; Bhuvanesh, N.; Blümel, J. Monometallic Ni-0 and Heterobimetallic Ni-0/Au-I Complexes of Tripodal Phosphine Ligands: Characterization in Solution and in the Solid State and Catalysis. Chem. Eur. J. 2015, 21, 10138–10148. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.; Fraile, J.M. Study of interactions between Brønsted acids and triethylphosphine oxide in solution by 31P NMR: Evidence for 2:1 species. Phys. Chem. Chem. Phys. 2020, 22, 24351–24358. [Google Scholar] [CrossRef] [PubMed]
- Ostras’, A.S.; Ivanov, D.M.; Novikov, A.S.; Tolstoy, P.M. Phosphine Oxides as Spectroscopic Halogen Bond Descriptors: IR and NMR Correlations with Interatomic Distances and Complexation Energy. Molecules 2020, 25, 1406. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Bodensteiner, M.; Tolstoy, P.M.; Denisov, G.S.; Shenderovich, I.G. P=O Moiety as an Ambidextrous Hydrogen Bond Acceptor. J. Phys. Chem. C 2018, 122, 1711–1720. [Google Scholar] [CrossRef]
- Arp, F.F.; Bhuvanesh, N.; Blümel, J. Hydrogen peroxide adducts of triarylphosphine oxides. Dalton Trans. 2019, 48, 14312–14325. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lindhardt, D.; Bhuvanesh, N.; Blümel, J. Di(hydroperoxy)cycloalkanes Stabilized via Hydrogen Bonding by Phosphine Oxides: Safe and Efficient Baeyer−Villiger Oxidants. ACS Sustainable Chem. Eng. 2018, 6, 6829–6840. [Google Scholar] [CrossRef]
- Chernyshov, I.Y.; Vener, M.V.; Shenderovich, I.G. Local-structure effects on 31P NMR chemical shift tensors in solid state. J. Chem. Phys. 2019, 150, 144706. [Google Scholar] [CrossRef] [PubMed]
- Golubev, N.S.; Melikova, S.M.; Shchepkin, D.N.; Shenderovich, I.G.; Tolstoy, P.M.; Denisov, G.S. Interpretation of H/D Isotope Effects on NMR Chemical Shifts of [FHF]- Ion Based on Calculations of Nuclear Magnetic Shielding Tensor Surface. Z. Phys. Chem. 2003, 217, 1549–1563. [Google Scholar] [CrossRef]
- Shenderovich, I.G. Experimentally Established Benchmark Calculations of 31P NMR Quantities. Chem.-Methods 2021, 1, 61–70. [Google Scholar] [CrossRef]
- Pilar, K.; Deng, Z.; Preefer, M.B.; Cooley, J.A.; Clément, R.; Seshadri, R.; Cheetham, A.K. Ab initio computation for solid-state 31P NMR of inorganic phosphates: Revisiting X-ray structures. Phys. Chem. Chem. Phys. 2019, 21, 10070–10074. [Google Scholar] [CrossRef] [PubMed]
- Grasa, P.; Baker, A.; Combes, C.; Rey, C.; Sarda, S.; Wright, A.J.; Smith, M.E.; Hanna, J.V.; Gervais, C.; Laurencin, D.; et al. From crystalline to amorphous calcium pyrophosphates: A solid state Nuclear Magnetic Resonance perspective. Acta Biomater. 2016, 31, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.J.; Alyea, E.C.; Shehnazarian, N. A 31P NMR Study of the Water Soluble Derivatives of 1,3,5-triaza-7-phosphaadamantane (PTA). Phosphorus Sulfur Silicon 1990, 48, 37–40. [Google Scholar] [CrossRef]
- Phillips, A.D.; Gonsalvi, L.; Romerosa, A.; Vizza, F.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA) Transition metal complexes and related catalytic, medicinal and photoluminescent applications. Coord. Chem. Rev. 2004, 248, 955–993. [Google Scholar] [CrossRef]
- Shenderovich, I.G. Electric field effect on 31P NMR magnetic shielding. J. Chem. Phys. 2020, 153, 184501. [Google Scholar] [CrossRef] [PubMed]
- Shenderovich, I.G.; Denisov, G.S. Adduct under Field—A Qualitative Approach to Account for Solvent Effect on Hydrogen Bonding. Molecules 2020, 25, 436. [Google Scholar] [CrossRef]
- Shenderovich, I.G.; Denisov, G.S. Solvent effects on acid-base complexes. What is more important: A macroscopic reaction field or solute-solvent interactions? J. Chem. Phys. 2019, 150, 204505. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Joó, F.; Kannisto, M.; Kathó, A.; Reibenspies, J.H.; Daigle, D.J. Water-Soluble Organometallic Compounds. 4. Catalytic Hydrogenation of Aldehydes in an Aqueous Two-Phase Solvent System Using a l,3,5-Triaza-7-phosphaadamantane Complex of Ruthenium. Inorg. Chem. 1994, 33, 200–208. [Google Scholar] [CrossRef]
- Smoleński, P.; Kirillov, A.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Transformations of the Vaska-type complex trans-[RhCl(CO)(PTA)2] (PTA = 1,3,5-triaza-7-phosphaadamantane) during stepwise addition of HCl: Synthesis, characterization and crystal structure of trans-[RhCl2(PTA)(PTAH)]. Inorganica Chim. Acta 2011, 378, 342–346. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Robertson, J.B.; Larkins, D.L.; Reibenspies, J.H. Water-Soluble Organometallic Compounds. 7.1 Further Studies of 1,3,5-Triaza-7-Phosphaadamantane Derivatives of Group 10 Metals, Including Metal Carbonyls and Hydrides. Inorg. Chem. 1999, 38, 2473–2481. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Decuir, T.J.; Stafford, N.W.; Robertson, J.B.; Draper, J.D.; Reibenspies, J.H. Water-Soluble Organometallic Compounds. 6.1 Synthesis, Spectral Properties, and Crystal Structures of Complexes of 1,3,5-Triaza-7-phosphaadamantane with Group 10 Metals. Inorg. Chem. 1997, 36, 4218–4226. [Google Scholar] [CrossRef]
- Alyea, E.C.; Ferguson, G.; Kannan, S. Some water-soluble organometallic complexes of group 10 transition metal(II) ions with 1,3,5-triaza-7-phosphaadamantane (TPA). Syntheses, characterization and reactivity. The crystal and molecular structure of [Ni(CN)2(TPA)3]·4.3H2O. Polyhedron 1998, 17, 2727–2732. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Smoleński, P.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. The First Copper Complexes Bearing the 1,3,5-Triaza-7-phosphaadamantane (PTA) Ligand. Eur. J. Inorg. Chem. 2007, 2007, 2686–2692. [Google Scholar] [CrossRef]
- Pellei, M.; Alidori, S.; Camalli, M.; Campi, G.; Lobbia, G.G.; Mancini, M.; Papini, G.; Spagna, R.; Santini, C. Copper(I)–organophosphine complexes of bis(3,5-dimethylpyrazol-1-yl)dithioacetate ligand. Inorganica Chim. Acta 2008, 361, 1456–1462. [Google Scholar] [CrossRef]
- Assefa, Z.; McBurnett, B.G.; Staples, R.J.; Fackler, J.P., Jr.; Assmann, B.; Angermaier, K.; Schmidbaur, H. Syntheses, Structures, and Spectroscopic Properties of Gold(I) Complexes of 1,3,5-Triaza-7-phosphaadamantane (TPA). Correlation of the Supramolecular Au.cntdot. .cntdot. .cntdot.Au Interaction and Photoluminescence for the Species (TPA)AuCl and [(TPA-HCl)AuCl]. Inorg. Chem. 1995, 34, 75–83. [Google Scholar] [CrossRef]
- Gavara, R.; Pinto, A.; Donamaria, R.; Olmos, M.E.; de Luzuriaga, J.M.L.; Rodriguez, L. Polarized Supramolecular Aggregates Based on Luminescent Perhalogenated Gold Derivatives. Inorg. Chem. 2017, 56, 11946–11955. [Google Scholar] [CrossRef]
- Forward, J.M.; Bohmann, D.; Fackler, J.P., Jr.; Staples, R.J. Luminescence Studies of Gold(I) Thiolate Complexes. Inorg. Chem. 1995, 34, 6330–6336. [Google Scholar] [CrossRef]
- Bertrand, B.; Spreckelmeyer, S.; Bodio, E.; Cocco, F.; Picquet, M.; Richard, P.; Le Gendre, P.; Orvig, C.; Cinellu, M.A.; Casini, A. Exploring the potential of gold (III) cyclometallated compounds as cytotoxic agents: Variations on the C^N theme. Dalton Trans. 2015, 44, 11911–11918. [Google Scholar] [CrossRef]
- Alyea, E.C.; Fisher, K.J.; Johnson, S. Synthesis, solid state 31P CP-MAS NMR, infrared and Raman studies of mercury(II) complexes of 1,3,5-triaza-7-phosphaadamantane (PTA). Can. J. Chem. 1989, 67, 1319–1323. [Google Scholar] [CrossRef]
- Alyea, E.C.; Ferguson, G.; Kannan, S. Intermolecular hydrogen···metal interactions. The crystal structure of {cis-[PdCl2(TPA)2]}2·H2O, a water-soluble palladium (II) tertiary phosphine complex. Chem. Commun. 1998, 345–346. [Google Scholar] [CrossRef]
- Otto, S.; Roodt, A.; Purcell, W. Synthesis and characterisation of water soluble Pt(II) complexes of 1,3,5-triaza-7-phosphaadamantane (PTA). Crystal and molecular structure of {cis-[PtCl2(PTA)2]}2·H2O. Inorg. Chem. Commun. 1998, 1, 415–417. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Acharya, S.; Rath, N.P. Synthesis and characterization of Pt(II) and Pd(II) PTA and DAPTA complexes. Polyhedron 2014, 79, 16–28. [Google Scholar] [CrossRef]
- Shenderovich, I.G. Simplified calculation approaches designed to reproduce the geometry of hydrogen bonds in molecular complexes in aprotic solvents. J. Chem. Phys. 2018, 148, 124313. [Google Scholar] [CrossRef]
- Gurinov, A.A.; Denisov, G.S.; Borissova, A.O.; Goloveshkin, A.S.; Greindl, J.; Limbach, H.-H.; Shenderovich, I.G. NMR Study of Solvation Effect on the Geometry of Proton-Bound Homodimers of Increasing Size. J. Phys. Chem. A 2017, 121, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, UK, 2013. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Dominikowska, J.; Palusiak, M. Tuning Aromaticity of para-Substituted Benzene Derivatives with an External Electric Field. ChemPhysChem 2018, 19, 590–595. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Provasi, P.F.; Pagola, G.I.; Ferraro, M.B. Electric field effects on nuclear magnetic shielding of the 1:1 and 2:1 (homo and heterochiral) complexes of XOOX′ (X, X′ = H, CH3) with lithium cation and their chiral dis-crimination. J. Chem. Phys. 2011, 135, 104116. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between interaction energy, intermolecular distance and elec-tron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Jordan, M.J.T. To What Extent Do External Fields and Vibrational and Isotopic Effects Influence NMR Coupling Constants Across Hydrogen Bonds? Two-Bond Cl-N Spin-Spin Coupling Constants (2hJCl-N) in Model ClH:NH3 Complexes. J. Phys. Chem. A 2002, 106, 5385–5392. [Google Scholar] [CrossRef]
- Ramos, M.; Alkorta, I.; Elguero, J.; Golubev, N.S.; Denisov, G.S.; Benedict, H.; Limbach, H.-H. Theoretical Study of the Influence of Electric Fields on Hydrogen-Bonded Acid−Base Complexes. J. Phys. Chem. A 1997, 101, 9791–9800. [Google Scholar] [CrossRef]
- Sellner, B.; Valiev, M.; Kathmann, S.M. Charge and Electric Field Fluctuations in Aqueous NaCl Electrolytes. J. Phys. Chem. B 2013, 117, 10869–10882. [Google Scholar] [CrossRef]
- Nardo, V.M.; Cassone, G.; Ponterio, R.C.; Saija, F.; Sponer, J.; Tommasini, M.; Trusso, S. Electric-Field-Induced Effects on the Dipole Moment and Vibrational Modes of the Centrosymmetric Indigo Molecule. J. Phys. Chem. A 2020, 124, 10856–10869. [Google Scholar] [CrossRef]
- Cassone, G.; Sponer, J.; Trusso, S.; Saija, F. Ab initio spectroscopy of water under electric fields. Phys. Chem. Chem. Phys. 2019, 21, 21205–21212. [Google Scholar] [CrossRef] [PubMed]
- Cassone, G. Nuclear Quantum Effects Largely Influence Molecular Dissociation and Proton Transfer in Liquid Water under an Electric Field. J. Phys. Chem. Lett. 2020, 11, 8983–8988. [Google Scholar] [CrossRef] [PubMed]
- Cassone, G.; Sofia, A.; Rinaldi, G.; Sponer, J. Catalyst-Free Hydrogen Synthesis from Liquid Ethanol: An ab Initio Molec-ular Dynamics Study. J. Phys. Chem. C 2019, 123, 9202–9208. [Google Scholar] [CrossRef]
- Fluck, E.; Forster, J.-E.; Weidlein, J.; Hadicke, E. 1.3.5-Triaza-7-phosphaadamantan (Monophospha-urotropin)/1,3,5-Triaza-7-phosphaadamantane (Monophospha-urotropine). Z. Naturforsch. B 1977, 32, 499–506. [Google Scholar] [CrossRef]
- Britvin, S.N.; Lotnyk, A. Water-Soluble Phosphine Capable of Dissolving Elemental Gold: The Missing Link between 1,3,5-Triaza-7-phosphaadamantane (PTA) and Verkade’s Ephemeral Ligand. J. Am. Chem. Soc. 2015, 137, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Shenderovich, I.G.; Denisov, G.S. Modeling of solute-solvent interactions using an external electric field—from tautomeric equilibrium in nonpolar solvents to the dissociation of alkali metal halides. Molecules 2021, 26, 1283. [Google Scholar] [CrossRef] [PubMed]
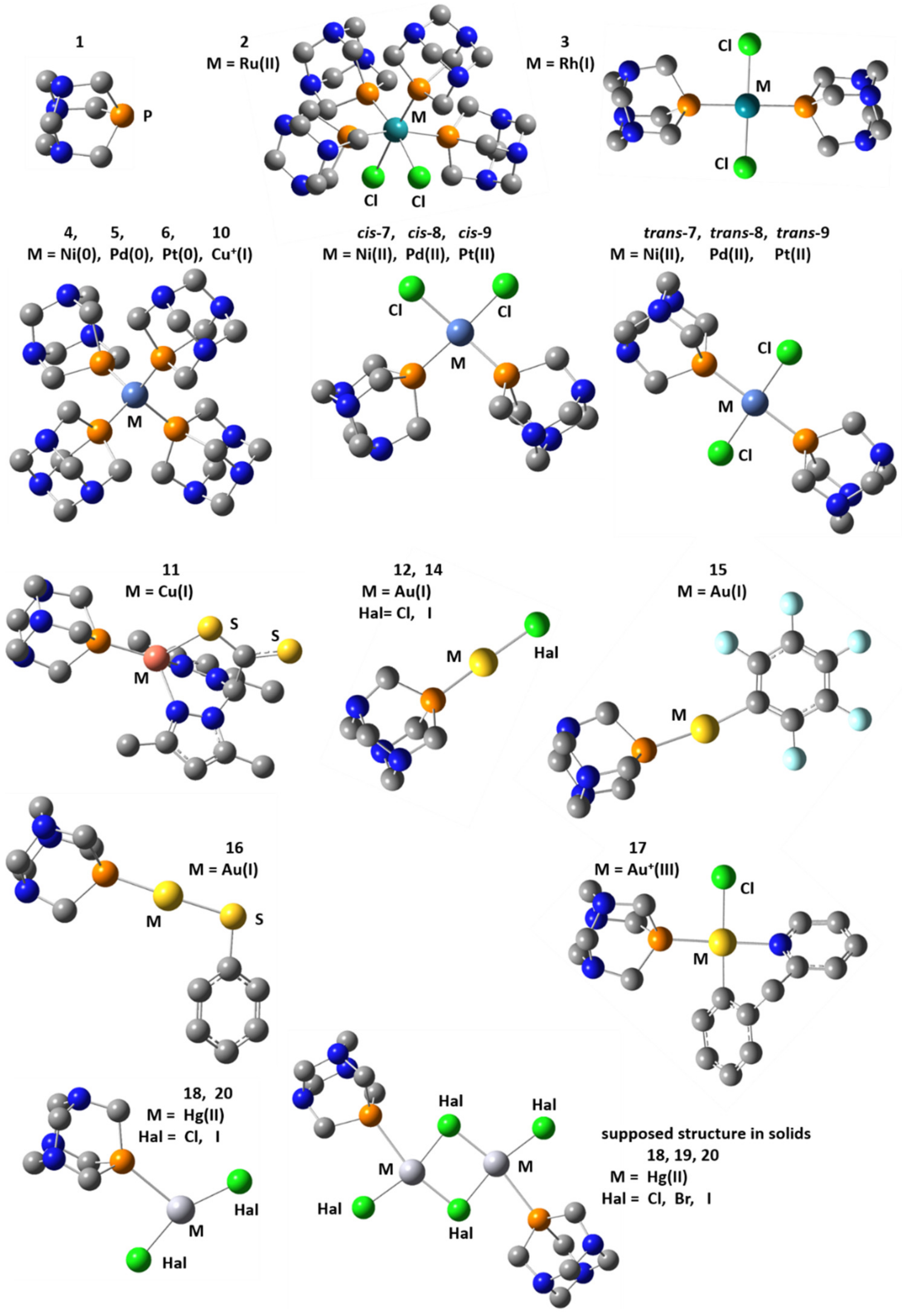
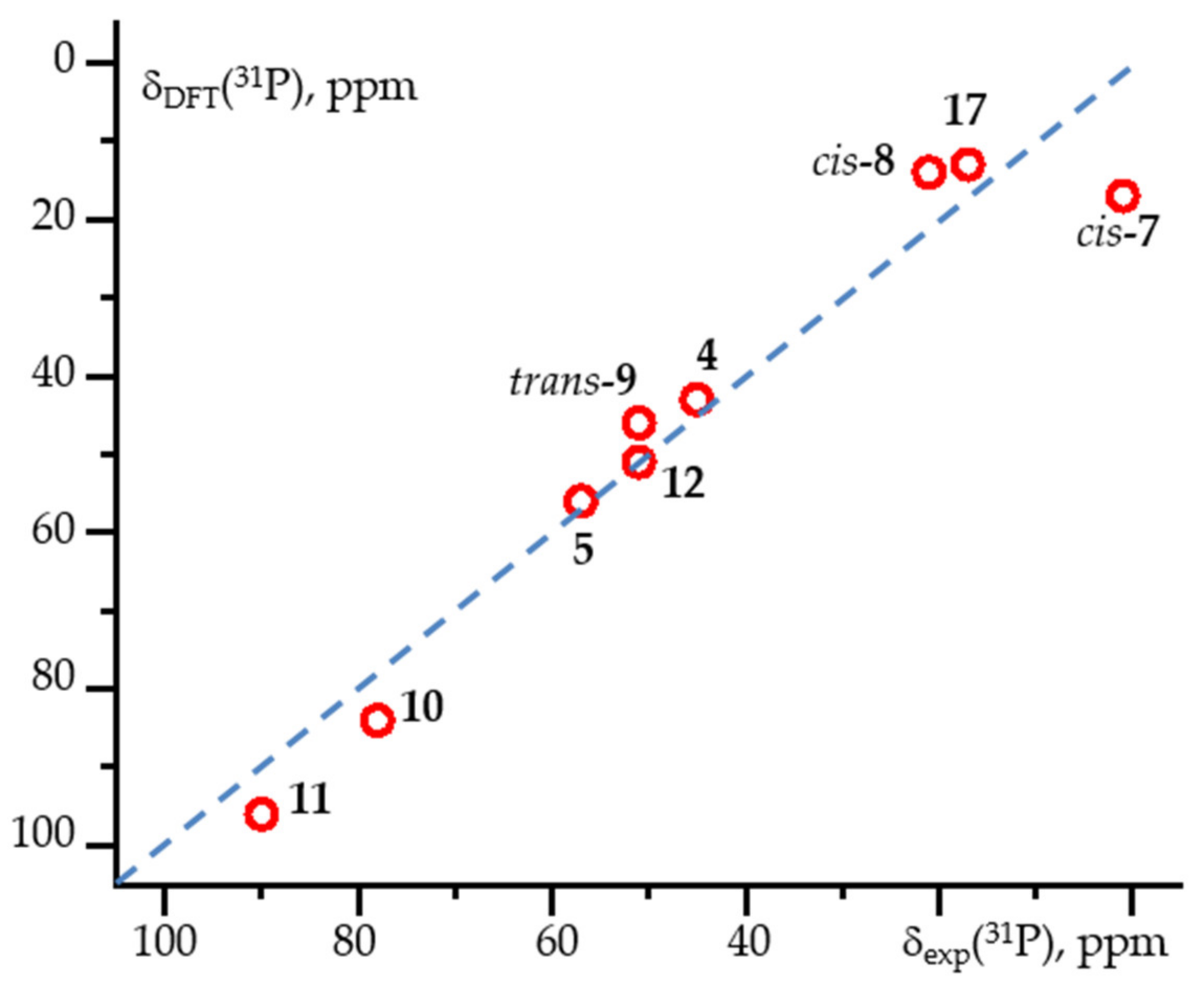
| Complex | Solvent | δiso(31P), ppm | References |
|---|---|---|---|
| PTA (1) | crystalline | −104.3 | [34] |
| cis-Cl2Ru(PTA)4 (2) | water | −47.3 | [42] |
| trans-Cl2Rh(PTA)2H (3) | water | −2.4 | [43] |
| Ni(PTA)4 (4) | water | −44.8, −45.7 | [44,45] |
| Pd(PTA)4 (5) | water | −56.5, −58.7 | [44,45] |
| Pt(PTA)4 (6) | water | −74.5 | [45] |
| Cl2Ni(PTA)2 (7) | water | −1.2 | [46] |
| cis-Cl2Pd(PTA)2 (8) | water | −21 | [45] |
| cis-Cl2Pd(PTA)2 (8) | DMSO | −18 | [46] |
| cis-Cl2Pt(PTA)2 (9) | water | −51 | [45] |
| cis-Cl2Pt(PTA)2 (9) | DMSO | −47.5 | [46] |
| [NO3]- Cu+(PTA)4 (10) | water | −78.2 | [47] |
| [LCS2]Cu(PTA) (11) | CDCl3 | −90.2 | [48] |
| ClAu(PTA) (12) | DMSO | −51.4 | [49] |
| BrAu(PTA) (13) | DMSO | −47.3 | [49] |
| IAu(PTA) (14) | DMSO | −47.6 | [49] |
| F5C6Au(PTA) (15) | acetone | −46.8 | [50] |
| H5C6SAu(PTA) (16) | no data | −49.8 | [51] |
| [(pyb-H)ClAu]+(PTA) (17) | acetone | −16.6 | [52] |
| Cl2Hg(PTA) (18) | solid | −38.3 | [53] |
| Br2Hg(PTA) (19) | solid | −44.1 | [53] |
| I2Hg(PTA) (20) | solid | −61.4 | [53] |
| Structure | Basis | Solvent | δiso(31P), ppm |
|---|---|---|---|
| cis-Cl2Ru(PTA)4; 2XRD | Def2TZVP | water | 30; 29; −18; −23 |
| cis-Cl2Ru(PTA)4; 2opt | Def2QZVP | water | 23; 21; −22; −35 |
| cis-Cl2Ru(PTA)4; 2opt | Def2TZVP | water | 29; 26; −17; −31 |
| Ru2+(PTA)4 | Def2QZVP | water | 70; 70; −28; −28 |
| [trans-Cl2Rh(PTA)2]−; 3XRD- | Def2QZVP | water | −19; −19 |
| [trans-Cl2Rh(PTA)2]−; 3opt- | Def2QZVP | water | −25; −25 |
| Rh+(PTA)2 | Def2QZVP | water | −175; 175 |
| Ni(PTA)4; 4 opt | Def2QZVP | water | −46; −46; −47; −47 |
| Ni(PTA)4; 4 opt | Def2TZVP | water | −42; −42; −43; −43 |
| Pd(PTA)4; 5 opt | Def2QZVP | water | −53; −56; −57; −57 |
| Pd(PTA)4; 5 opt | Def2TZVP | water | −51; −53; −53; −53 |
| Pt(PTA)4; 6opt | Def2QZVP | water | −34; −39; −39; −39 |
| Pt(PTA)4; 6opt | Def2TZVP | water | −30; −35; −35; −35 |
| Cl2Ni(PTA)2; cis-7opt | Def2QZVP | water | −13; −21 |
| Cl2Ni(PTA)2; trans-7opt | Def2QZVP | water | −36; −36 |
| Cl2Pd(PTA)2; cis-8XRD | Def2QZVP | water | −15; −24 |
| Cl2Pd(PTA)2; cis-8opt | Def2QZVP | water | −13; −14 |
| Cl2Pd(PTA)2; trans-8opt | Def2QZVP | water | −37; −37 |
| Cl2Pt(PTA)2; cis-9XRD | Def2QZVP | water | −20; −24 |
| Cl2Pt(PTA)2; cis-9opt | Def2QZVP | water | −17; −18 |
| Cl2Pt(PTA)2; trans-9XRD | Def2QZVP | water | −48; −48 |
| Cl2Pt(PTA)2; trans-9opt | Def2QZVP | water | −46; −46 |
| Cu+(PTA)4; 10opt+ | Def2QZVP | water | −84; −84; −84; −85 |
| [LCS2]Cu(PTA); 11opt | Def2QZVP | CHCl3 | −96 |
| ClAu(PTA); 12XRD | Def2QZVP | DMSO | −62 |
| ClAu(PTA); 12opt | Def2QZVP | DMSO | −62 |
| IAu(PTA); 14opt | Def2QZVP | DMSO | −70 |
| Au+(PTA) | Def2QZVP | DMSO | −26 |
| F5C6Au(PTA); 15XRD | Def2QZVP | CHCl3 | −73 |
| F5C6Au(PTA); 15opt | Def2QZVP | CHCl3 | −76 |
| H5C6SAu(PTA); 16XRD | Def2QZVP | CHCl3 | −78 |
| H5C6SAu(PTA); 16opt | Def2QZVP | CHCl3 | −74 |
| [(pyb-H)ClAu]+(PTA); 17XRD | Def2QZVP | acetone | −16 |
| [(pyb-H)ClAu]+(PTA); 17opt | Def2QZVP | acetone | −13 |
| Cl2Hg(PTA); 18opt(gas) | Def2QZVP | − | −104 |
| Cl2Hg(PTA); 18opt(water) | Def2QZVP | water | −77 |
| I2Hg(PTA); 20opt(gas) | Def2QZVP | − | −103 |
| I2Hg(PTA); 20opt(water) | Def2QZVP | water | −74 |
| Hg2+(PTA) | Def2QZVP | water | −37 |
| Structure | Field, a.u. | Solvent | δiso(31P), ppm |
|---|---|---|---|
| ClAu(PTA); 12opt_25 | 25 | DMSO | −62 |
| ClAu(PTA); 12opt_50 | 50 | DMSO | −60 |
| ClAu(PTA); 12opt_75 | 75 | DMSO | −55 |
| ClAu(PTA); 12opt_100 | 100 | DMSO | −46 |
| F5C6Au(PTA); 15opt_25 | 25 | CHCl3 | −77 |
| F5C6Au(PTA); 15opt_50 | 50 | CHCl3 | −78 |
| F5C6Au(PTA); 15opt_75 | 75 | CHCl3 | −78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenderovich, I.G. 1,3,5-Triaza-7-Phosphaadamantane (PTA) as a 31P NMR Probe for Organometallic Transition Metal Complexes in Solution. Molecules 2021, 26, 1390. https://doi.org/10.3390/molecules26051390
Shenderovich IG. 1,3,5-Triaza-7-Phosphaadamantane (PTA) as a 31P NMR Probe for Organometallic Transition Metal Complexes in Solution. Molecules. 2021; 26(5):1390. https://doi.org/10.3390/molecules26051390
Chicago/Turabian StyleShenderovich, Ilya G. 2021. "1,3,5-Triaza-7-Phosphaadamantane (PTA) as a 31P NMR Probe for Organometallic Transition Metal Complexes in Solution" Molecules 26, no. 5: 1390. https://doi.org/10.3390/molecules26051390
APA StyleShenderovich, I. G. (2021). 1,3,5-Triaza-7-Phosphaadamantane (PTA) as a 31P NMR Probe for Organometallic Transition Metal Complexes in Solution. Molecules, 26(5), 1390. https://doi.org/10.3390/molecules26051390







