Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making
Abstract
1. Introduction
2. Results and Discussion
2.1. Lignocellulose and Cellulose Nanofibers
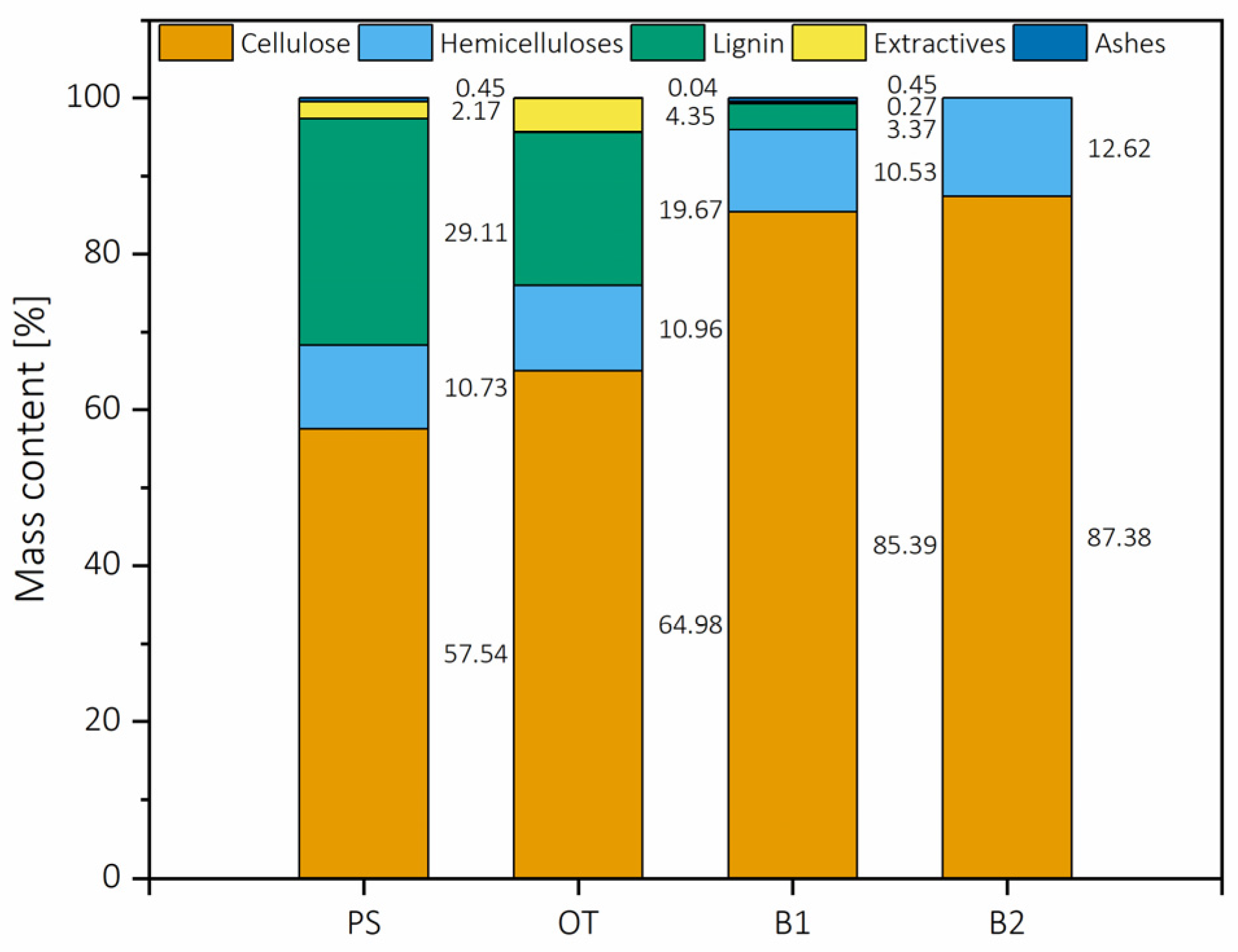
2.1.1. Chemical Composition of the Selected Biomass
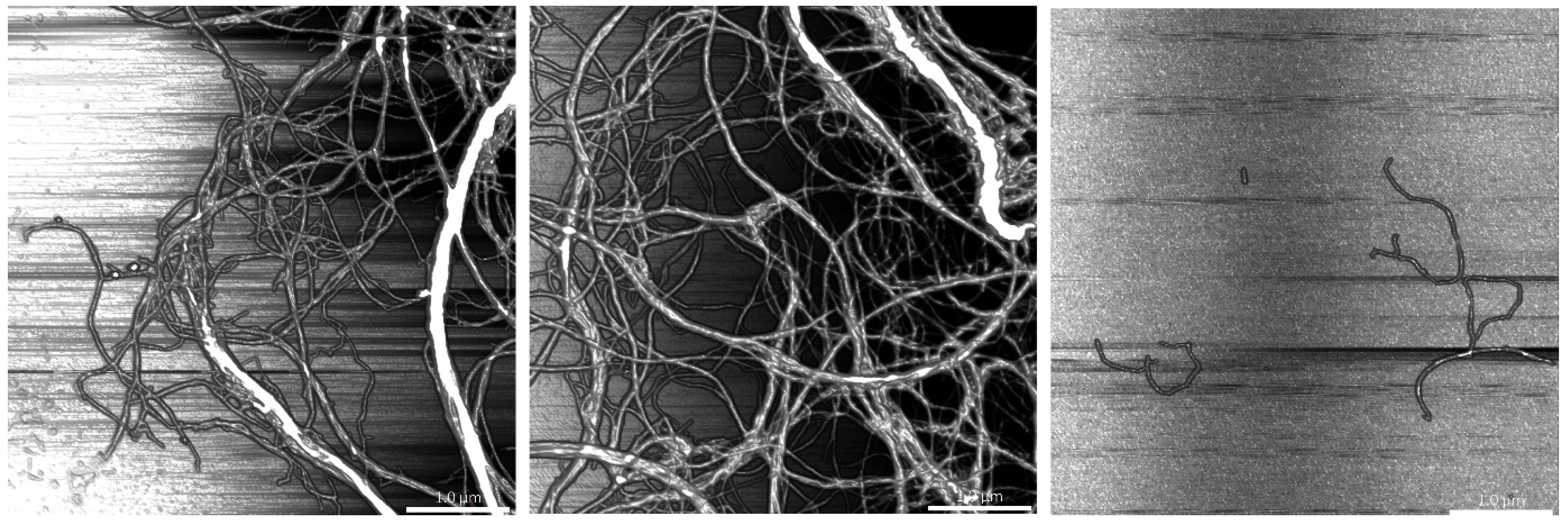
2.1.2. Morphology of the Elaborated Nanofibers
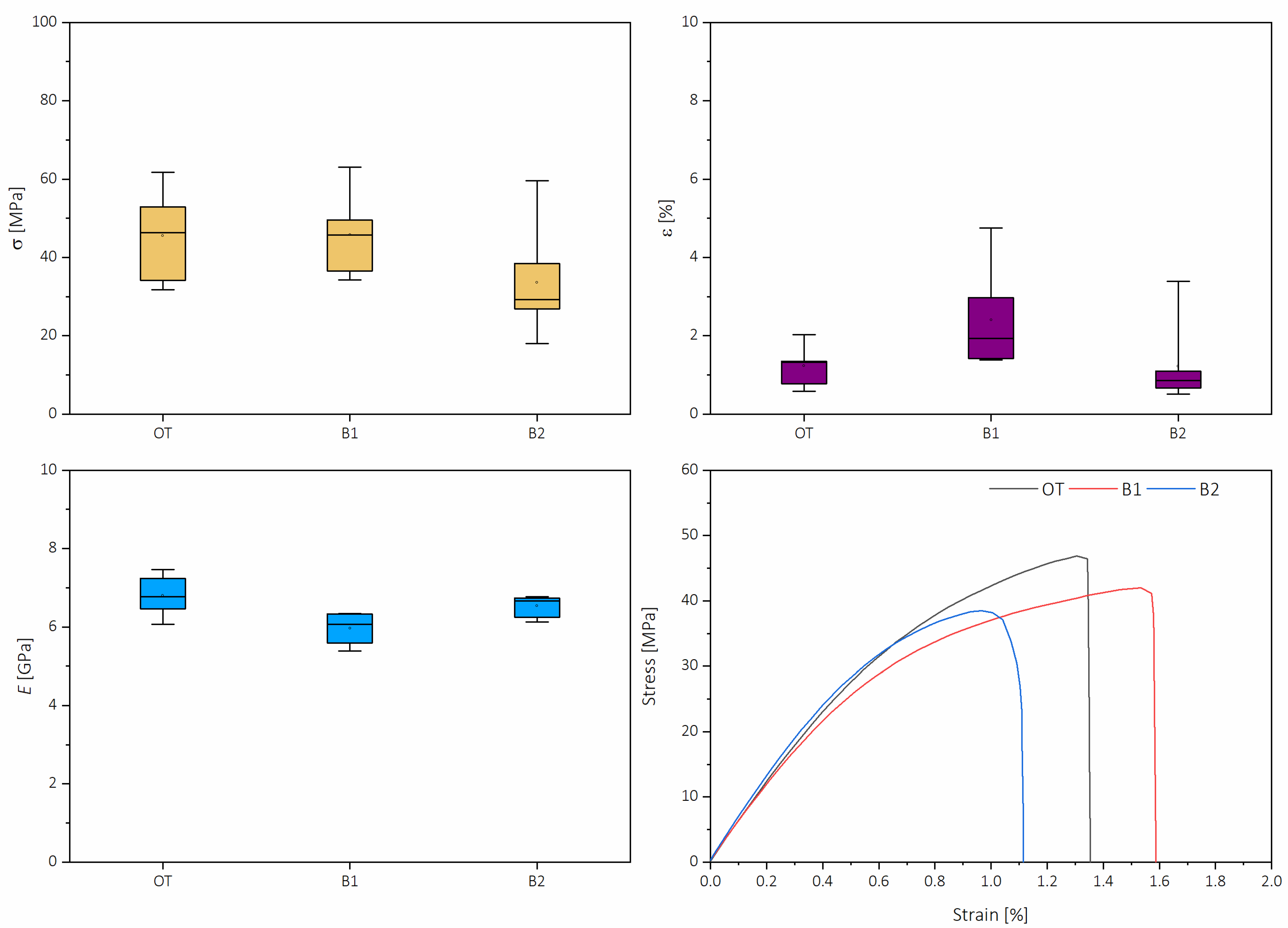
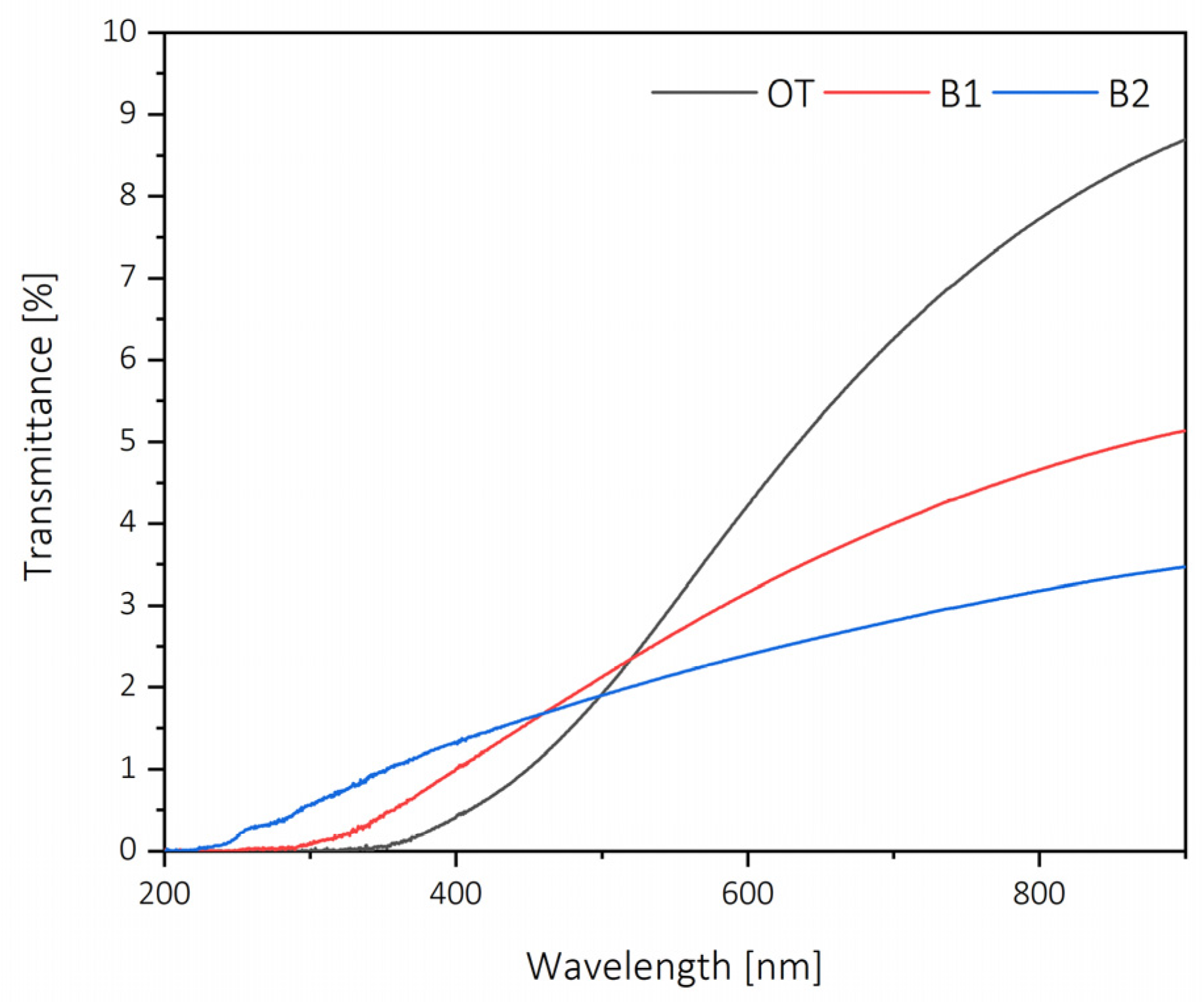
2.2. Lignocellulose and Cellulose Nanopapers
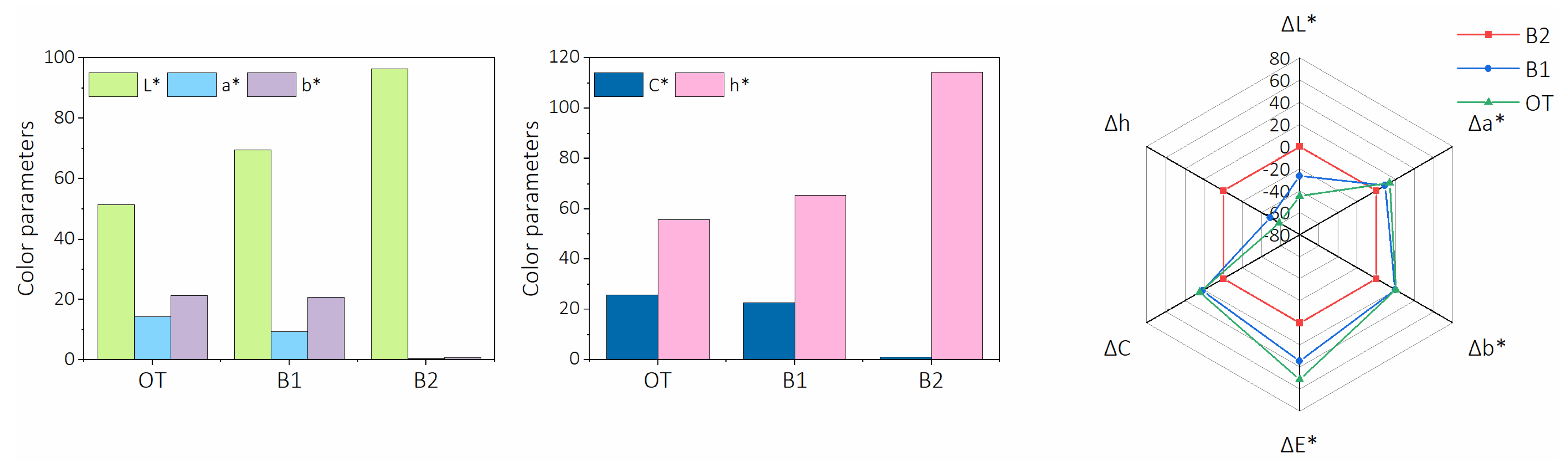
Appearance and Color Properties
3. Materials and Methods
3.1. Obtaining Cellulose and Lignocellulose
3.2. Elaboration of Lignocellulose Nanopaper
3.3. Characterization of the Obtained Nanofibers
3.4. Characterization of the Elaborated Nanopapers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Orlando, E. Legal Basis European Union Environmental Policy EU Law EU Institutions. In The Evolution of EU Policy and Law in the Environmental Field: Achievements and Current Challenges; Istituto Affari Internazionali: Roma, Italy, 2013; ISSN 2281-5252. [Google Scholar]
- Appels, L.; Dewil, R. Biomass valorization to energy and value added chemicals: The future of chemical industry. Resour. Conserv. Recycl. 2012, 59, 1–3. [Google Scholar] [CrossRef]
- Nizami, A.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.; Shahzad, K.; Miandad, R.; Khan, M.; Syamsiro, M.; Ismail, I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Açıkalın, K.; Karaca, F.; Bolat, E. Pyrolysis of pistachio shell: Effects of pyrolysis conditions and analysis of products. Fuel 2012, 95, 169–177. [Google Scholar] [CrossRef]
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Marett, J.; Aning, A.; Foster, E.J. The isolation of cellulose nanocrystals from pistachio shells via acid hydrolysis. Ind. Crop. Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Özbek, H.N.; Yanık, D.K.; Fadıloğlu, S.; Göğüş, F. Effect of microwave-assisted alkali pre-treatment on fractionation of pistachio shell and enzymatic hydrolysis of cellulose-rich residues. J. Chem. Technol. Biotechnol. 2021, 96, 521–531. [Google Scholar] [CrossRef]
- Okutucu, C.; Duman, G.; Ucar, S.; Yasa, I.; Yanik, J. Production of fungicidal oil and activated carbon from pistachio shell. J. Anal. Appl. Pyrolysis 2011, 91, 140–146. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and com-mercial potential. In Proceedings of the Ninth Cellulose Conference, Applied Polymer Symposia; Sarko, A., Ed.; Wiley: New York, NY, USA, 1983; Volume 37, pp. 815–827. [Google Scholar]
- Järvinen, J.; Ojala, J.; Melander, A.; Lamberg, J.A. The Evolution of Pulp and Paper Industries in Finland, Sweden, and Norway, 1800–2005; Springer: Dordrecht, The Netherlands, 2012; pp. 19–47. [Google Scholar]
- Berg, P.; Nordstrôm, P.O. A steel boom for paper? Pulp Pap. Int. 2008, 50, 48. [Google Scholar]
- Espe, C. Solid Waste Issue Creates Boom in Recycled Fiber Capacity Growth. Pulp Pap. 1990, 64, 78–80. [Google Scholar]
- Kamel, R.; Wakil, E.N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable phar-maceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef]
- Rol, F.; Banvillet, G.; Meyer, V.; Conil, P.M.; Bras, J. Combination of twin-screw extruder and homogenizer to produce high-quality nanofibrillated cellulose with low energy consumption. J. Mater. Sci. 2018, 53, 12604–12615. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Gordobil, O.; Robles, E.; Alriols, G.M.; Labidi, J. Lignin valorization from side-streams produced during agricultural waste pulping and total chlorine free bleaching. J. Clean. Prod. 2017, 142, 2609–2617. [Google Scholar] [CrossRef]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from almond (Prunus dulcis) shell. Cellulitis 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Production of lignocellulose nanofibers from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 2019, 239, 8083. [Google Scholar] [CrossRef]
- Kumagai, A.; Lee, S.-H.; Endo, T. Thin Film of Lignocellulosic Nanofibrils with Different Chemical Composition for QCM-D Study. Biomacromolecules 2013, 14, 2420–2426. [Google Scholar] [CrossRef]
- Teramoto, Y.; Tanaka, N.; Lee, S.H.; Endo, T. Pretreatment of eucalyptus wood chips for enzymatic saccharification using combined sulfuric acid-free ethanol cooking and ball milling. Biotechnol. Bioeng. 2007, 99, 75–85. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulitis 2015, 22, 3137–3150. [Google Scholar] [CrossRef]
- Herrera, M.; Thitiwutthisakul, K.; Yang, X.; Rujitanaroj, P.O.; Rojas, R.; Berglund, L. Preparation and evaluation of high-lignin content cellulose nanofibrils from eucalyptus pulp. Cellulitis 2018, 25, 3121–3133. [Google Scholar] [CrossRef]
- Peters, B. Prediction of pyrolysis of pistachio shells based on its components hemicellulose, cellulose and lignin. Fuel Process. Technol. 2011, 92, 1993–1998. [Google Scholar] [CrossRef]
- Abdallah, M.; Ni, D.; Simson, A. Evaluation of biochars derived from food waste for synthesis gas production via pyrolysis and CO2 gasification. Biomass Bioenergy 2020, 143, 5883. [Google Scholar] [CrossRef]
- Gupta, K.A.; Mohanty, S.; Nayak, S.K. Preparation and Characterization of Lignin Nanofibre by Electrospinnig Technique. Int. J. Sci. Eng. Appl. Sci. 2015, 1, 184–190. [Google Scholar]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Robles, E.; Rodríguez, F.J.; Barbosa, A.M.; Gordobil, O.; Carreño, N.L.; Labidi, J. Production of cellulose nanoparticles from blue agave waste treated with environmentally friendly processes. Carbohydr. Polym. 2018, 183, 294–302. [Google Scholar] [CrossRef]
- Oliaei, E.; Lindén, P.A.; Wu, Q.; Berthold, F.; Berglund, L.; Lindström, T. Microfibrillated lignocellulose (MFLC) and nanopaper films from unbleached kraft softwood pulp. Cellulitis 2019, 27, 2325–2341. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Liu, X.; Yang, Q.; Huang, Q.; Wang, L.; Dai, Y.; Qin, C.; Wang, S. Highly Transparent, UV-Shielding, and Water-Resistant Lignocellulose Nanopaper from Agro-Industrial Waste for Green Optoelectronics. ACS Sustain. Chem. Eng. 2020, 8, 17508–17519. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Qian, Y.; Zhang, M.; Zhu, P.; Chen, G. Lignocellulose Enabled Highly Transparent Nanopaper with Tunable Ultraviolet-Blocking Performance and Superior Durability. ACS Sustain. Chem. Eng. 2020, 8, 17033–17041. [Google Scholar] [CrossRef]
- Robles, E.; Kánnár, A.; Labidi, J.; Csoka, L. Assessment of physical properties of self-bonded composites made of cellulose nanofibrils and poly(lactic acid) microfibrils. Cellulitis 2018, 25, 3393–3405. [Google Scholar] [CrossRef]
- Sehaqui, H.; Liu, A.; Zhou, Q.; Berglund, L.A. Fast Preparation Procedure for Large, Flat Cellulose and Cellulose/Inorganic Nanopaper Structures. Biomacromolecules 2010, 11, 2195–2198. [Google Scholar] [CrossRef]
- Wang, Q.; Du, H.; Zhang, F.; Zhang, Y.; Wu, M.; Yu, G.; Liu, C.; Li, B.; Peng, H. Flexible cellulose nanopaper with high wet tensile strength, high toughness and tunable ultraviolet blocking ability fabricated from tobacco stalk via a sustainable method. J. Mater. Chem. A 2018, 6, 13021–13030. [Google Scholar] [CrossRef]
- Izaguirre, N.; Gordobil, O.; Robles, E.; Labidi, J. Enhancement of UV absorbance and mechanical properties of chitosan films by the incorporation of solvolytically fractionated lignins. Int. J. Biol. Macromol. 2020, 155, 447–455. [Google Scholar] [CrossRef]
- Gordobil, O.; Olaizola, P.; Prieto, J.; Labidi, J. Lignins from Agroindustrial by-Products as Natural Ingredients for Cosmetics: Chemical Structure and In Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef]
- ASTM D1746. 15 Standard Test Method for Transparency of Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Chen, F.; Xiang, W.; Sawada, D.; Bai, L.; Hummel, M.; Sixta, H.; Budtova, T. Exploring Large Ductility in Cellulose Nanopaper Combining High Toughness and Strength. ACS Nano 2020, 14, 11150–11159. [Google Scholar] [CrossRef]
- Yousefi, H.; Faezipour, M.; Hedjazi, S.; Mousavi, M.M.; Azusa, Y.; Heidari, A.H. Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibers and fibers/ground cellulose nanofibers of canola straw. Ind. Crop. Prod. 2013, 43, 732–737. [Google Scholar] [CrossRef]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr. Polym. 2016, 144, 41–49. [Google Scholar] [CrossRef]
- Sethi, J.; Visanko, M.; Österberg, M.; Sirviö, J.A. A fast method to prepare mechanically strong and water resistant lignocellulosic nanopapers. Carbohydr. Polym. 2019, 203, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.E.; Murphy, M.; Addieco, D.A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 1946, 122, 35–43. [Google Scholar]
- TAPPI T 222 om-11. Acid-Insoluble Lignin in Wood and Pulp; OSTI.GOV: Peachtree Corners, GA, USA, 2011.
- TAPPI T 211 om-16. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 Degrees; OSTI.GOV: Peachtree Corners, GA, USA, 2016.
- TAPPI T 204 cm-07. Solvent Extractives of Wood and Pulp; OSTI.GOV: Peachtree Corners, GA, USA, 2007.
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der Benetzungstheorie und ihre Anwendung auf die Untersuchung und Veränderung der Ober-flächeneigenschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles, E.; Izaguirre, N.; Martin, A.; Moschou, D.; Labidi, J. Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making. Molecules 2021, 26, 1371. https://doi.org/10.3390/molecules26051371
Robles E, Izaguirre N, Martin A, Moschou D, Labidi J. Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making. Molecules. 2021; 26(5):1371. https://doi.org/10.3390/molecules26051371
Chicago/Turabian StyleRobles, Eduardo, Nagore Izaguirre, Ander Martin, Dimitra Moschou, and Jalel Labidi. 2021. "Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making" Molecules 26, no. 5: 1371. https://doi.org/10.3390/molecules26051371
APA StyleRobles, E., Izaguirre, N., Martin, A., Moschou, D., & Labidi, J. (2021). Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making. Molecules, 26(5), 1371. https://doi.org/10.3390/molecules26051371







