Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study—Ethical Statement
2.2. Study Design—Sample Size
2.3. Housing, Maintenance, Handling, and Animal Welfare
2.4. Experimental Groups
- -
- Apatos group—100% porcine cortical bone granules (600–1000 µm particles) without preservation of collagen (Apatos, Osteobiol®, Tecnoss, Torino, Italy)
- -
- Gen-Os group—100% porcine cortico-cancellous bone granulated mix (250–1000 µm particles) with preserved collagen (Gen-Os, Osteobiol®, Tecnoss, Torino, Italy)
- -
- mp3 group—90% porcine cortico-cancellous bone granulated mix (600–1000µm prehydrated particles) with preserved collagen with 10% collagen gel (mp3, Osteobiol®, Tecnoss, Torino, Italy)
- -
- Putty group—80% porcine cortico-cancellous bone granulated mix (<300 µm micronized particles) with preserved collagen and 20% collagen gel (Putty, Osteobiol®, Tecnoss, Torino, Italy)
- -
- Gel 40 group—60% porcine cortico-cancellous bone granulated mix with preserved collagen (<300 µm micronized particles) and 40% type I and III collagen gel (Gel 40, Osteobiol®, Tecnoss, Torino, Italy)
2.5. Anesthesia
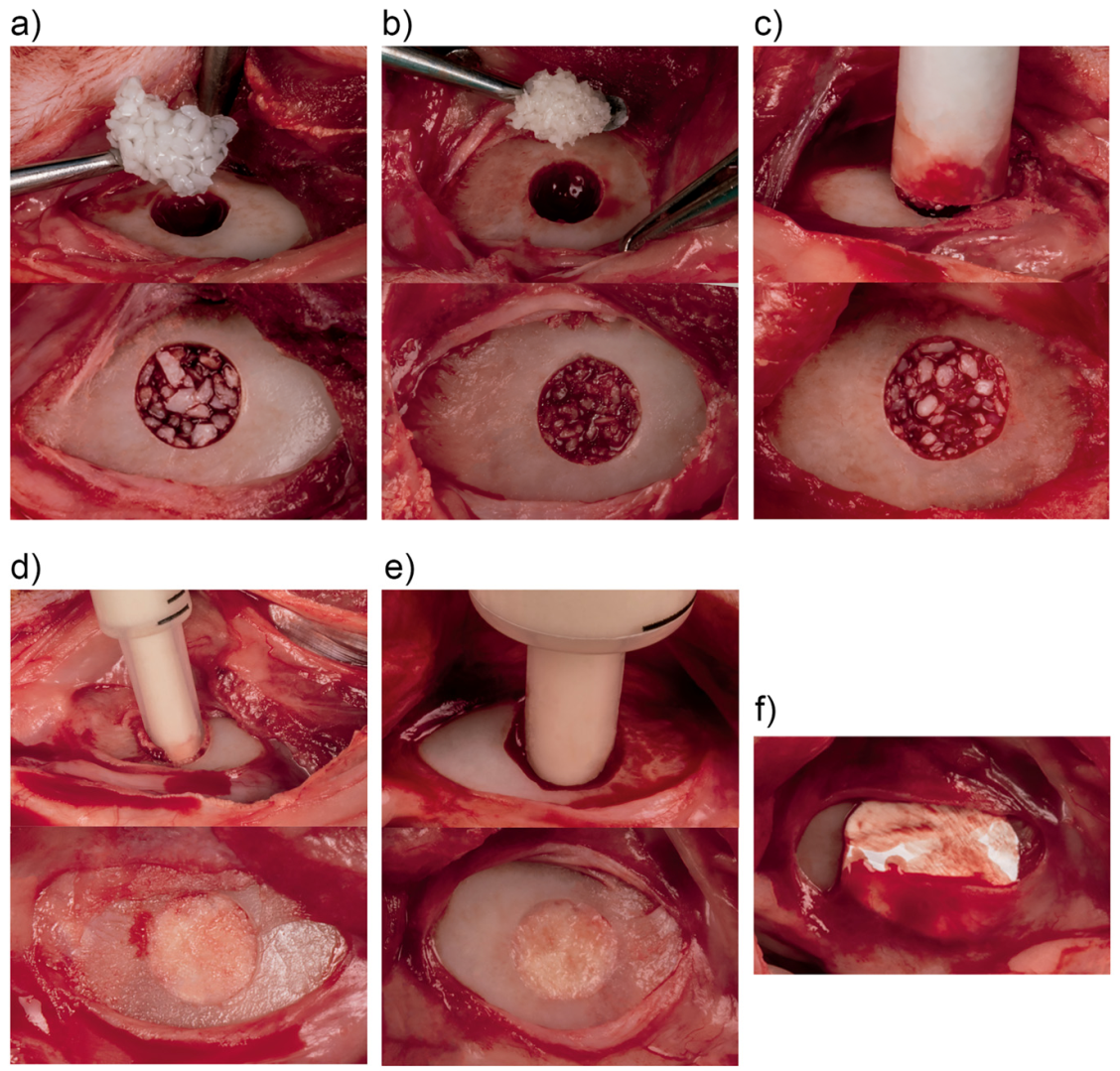
2.6. Surgical Protocol
2.7. Postoperative Care
2.8. Animal Euthanasia and Necropsy
2.9. Study Material Harvesting
2.10. Sample Processing and Analysis
2.11. Statistical Analysis
3. Results
3.1. Clinical Findings
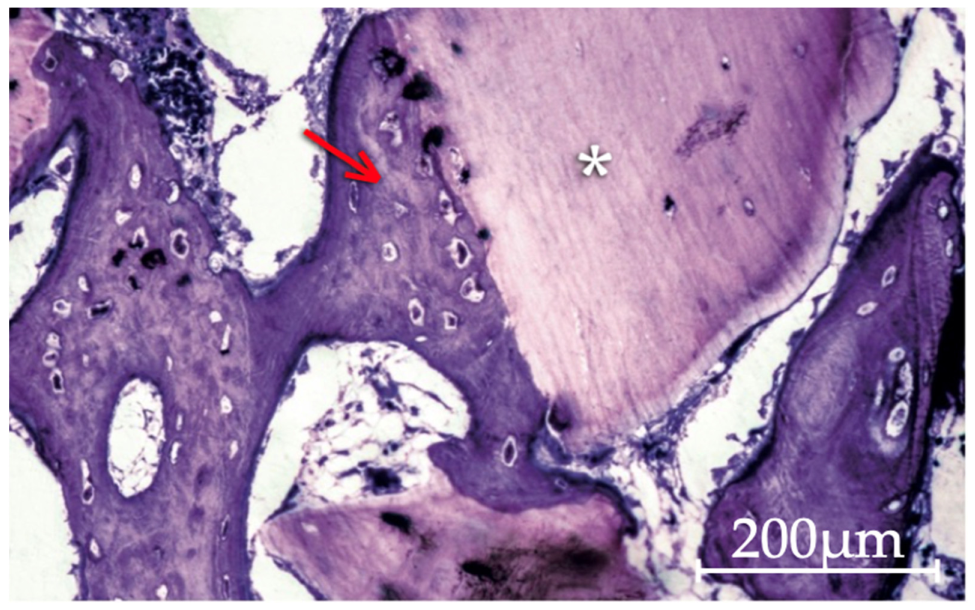
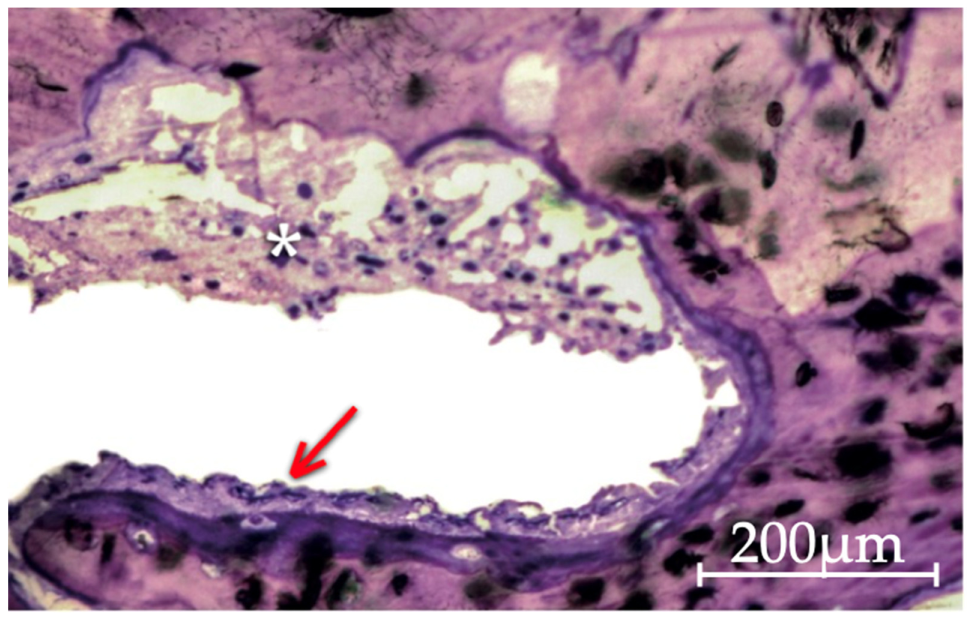
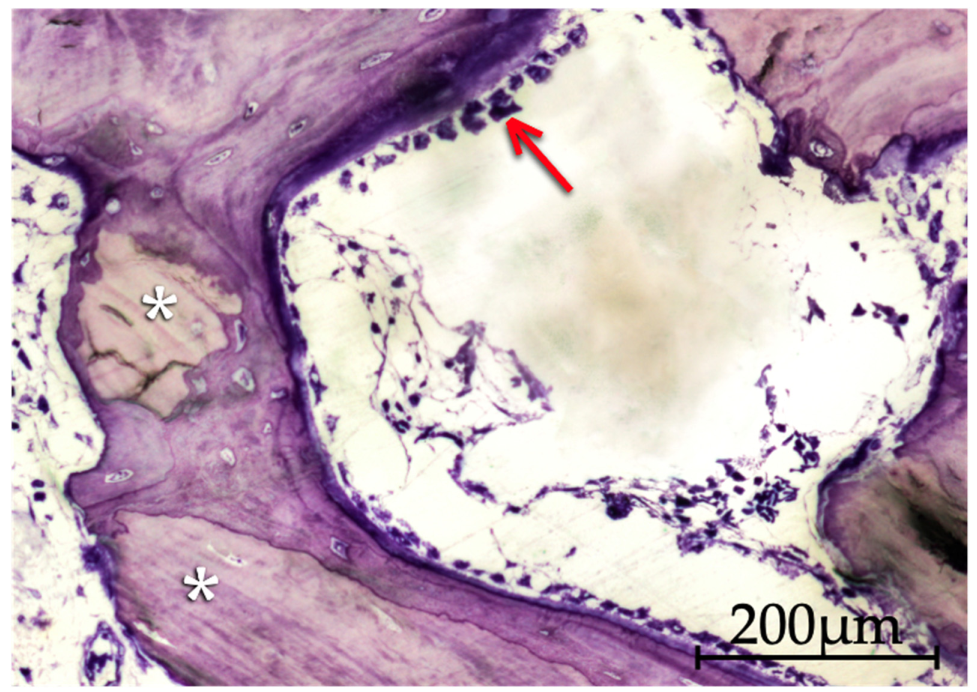
3.2. Qualitative Histological Analysis
3.2.1. Apatos Group
3.2.2. Gen-Os Group
3.2.3. mp3 Group
3.2.4. Putty and Gel 40 Groups
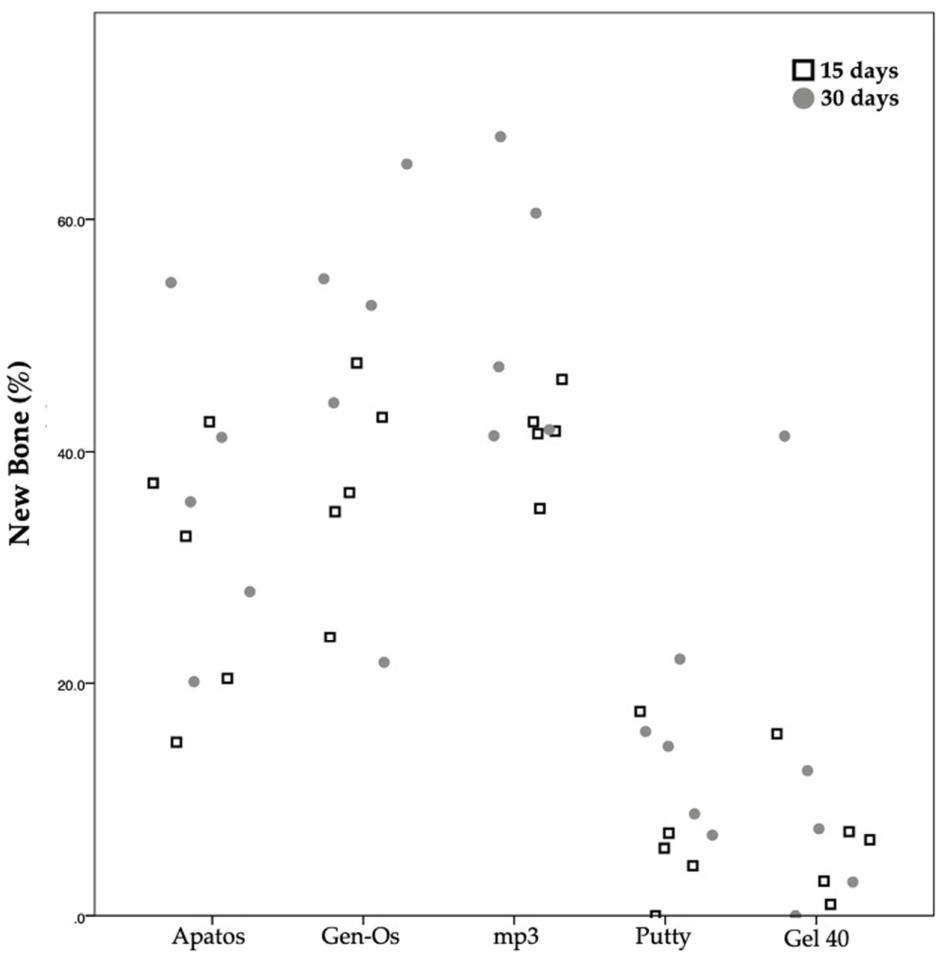
3.3. Quantitative Histomorphometric Analysis
3.3.1. Analysis within Each Group
3.3.2. Analysis between Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, C.; Toti, P.; Martuscelli, R.; Guidetti, F.; Sbordone, L.; Ramaglia, L. A 5-Year Implant Follow-Up in Maxillary and Mandibular Horizontal Osseous Onlay Grafts and Native Bone. J. Oral Implant. 2015, 41, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Domingues, E.P.; Ribeiro, R.F.; Horta, M.C.R.; Zenóbio, E.G.; Côsso, M.G.; Manzi, F.R. Vertical augmentation of the posterior atrophic mandible by interpositional grafts in a split-mouth design: A human tomography evaluation pilot study. Clin. Oral Implant. Res. 2016, 28. [Google Scholar] [CrossRef]
- Marconcini, S.; Covani, U.; Giammarinaro, E.; Ortega, E.V.; De Santis, D.; Alfonsi, F.; Barone, A. Clinical Success of Dental Implants Placed in Posterior Mandible Augmented With Interpositional Block Graft: 3-Year Results From a Prospective Cohort Clinical Study. J. Oral Maxillofac. Surg. 2019, 77, 289–298. [Google Scholar] [CrossRef]
- Karalashvili, L.; Kakabadze, A.; Uhryn, M.; Vyshnevska, H.; Ediberidze, K.; Kakabadze, Z. BONE GRAFTS FOR RECONSTRUCTION OF BONE DEFECTS (REVIEW). Georg. Med News 2018, 44–49. [Google Scholar]
- Ku, J.-K.; Hong, I.; Lee, B.-K.; Yun, P.-Y.; Lee, J.K. Dental alloplastic bone substitutes currently available in Korea. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 51–67. [Google Scholar] [CrossRef]
- Raghuram, A.; Singh, A.; Chang, D.K.; Nunez, M.; Reece, E.M. Bone Grafts, Bone Substitutes, and Orthobiologics: Applications in Plastic Surgery. Semin. Plast. Surg. 2019, 33, 190–199. [Google Scholar] [CrossRef]
- Al-Moraissi, E.; Alkhutari, A.; Abotaleb, B.; Altairi, N.; Del Fabbro, M. Do osteoconductive bone substitutes result in similar bone regeneration for maxillary sinus augmentation when compared to osteogenic and osteoinductive bone grafts? A systematic review and frequentist network meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 107–120. [Google Scholar] [CrossRef]
- Costantino, P.D.; Hiltzik, D.; Govindaraj, S.; Moche, J. Bone Healing and Bone Substitutes. Facial Plast. Surg. 2002, 18, 013–026. [Google Scholar] [CrossRef]
- Cutter, C.S.; Mehrara, B.J. Bone Grafts and Substitutes. J. Autom. Inf. Sci. 2006, 16, 249–260. [Google Scholar] [CrossRef]
- De Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef]
- Schwartz, C.; Lecestre, P.; Fraysinet, P.; Liss, P. Bone substitutes. Eur. J. Orthop. Surg. Traumatol. 1999, 9, 161–165. [Google Scholar] [CrossRef]
- Agner, C.; McConathy, D.; Dujovny, M. Evaluation of autogenic, xenogeneic and alloplastic materials used for cranioplasty. Crit. Rev. Neurosurg. 1997, 7, 365–372. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Cranio-Maxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- De Sousa, C.A.; Lemos, C.A.A.; Santiago-Júnior, J.F.; Faverani, L.P.; Pellizzer, E.P. Bone augmentation using autogenous bone versus biomaterial in the posterior region of atrophic mandibles: A systematic review and meta-analysis. J. Dent. 2018, 76, 1–8. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Van Der Stok, J.; Van Lieshout, E.; El-Massoudi, Y.; Van Kralingen, G.; Patka, P. Bone substitutes in the Netherlands—A systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Idrontino, G.; Valente, N.A. Intraoral and extraoral autologous bone block graft techniques: A review of the recent literature. Int. J. Contemp. Dent. Med. Rev. 2016. [Google Scholar] [CrossRef]
- Kao, S.T.; Scott, D.D. A Review of Bone Substitutes. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes-Comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Messias, A.; Dias, R.; Judas, F.; Salvoni, A.; Guerra, F. Horizontal Resorption of Fresh-Frozen Corticocancellous Bone Blocks in the Reconstruction of the Atrophic Maxilla at 5 Months. Clin. Implant. Dent. Relat. Res. 2014, 17, e444–e458. [Google Scholar] [CrossRef]
- Salamanca, E.; Hsu, C.-C.; Huang, H.-M.; Teng, N.-C.; Lin, C.-T.; Pan, Y.-H.; Chang, W.-J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Guirado, J.L.C.; Fernández, M.P.R.; Negri, B.; Ruiz, R.A.D.; De-Val, J.E.M.S.; Gómez-Moreno, G. Retracted: Experimental Model of Bone Response to Collagenized Xenografts of Porcine Origin (OsteoBiol® mp3): A Radiological and Histomorphometric Study. Clin. Implant. Dent. Relat. Res. 2013, 15, 143–151. [Google Scholar] [CrossRef]
- Ramírez-Fernández, M.P.; Calvo-Guirado, J.L.; Delgado-Ruíz, R.A.; Negri, B.; Barona-Dorado, C.; Del Val, J.E.M.-S. Ultrastructural study by backscattered electron imaging and elemental microanalysis of bone-to-biomaterial interface and mineral degradation of porcine xenografts used in maxillary sinus floor elevation. Clin. Oral Implant. Res. 2012, 24, 523–530. [Google Scholar] [CrossRef]
- Nannmark, U.; Sennerby, L. The Bone Tissue Responses to Prehydrated and Collagenated Cortico-Cancellous Porcine Bone Grafts: A Study in Rabbit Maxillary Defects. Clin. Implant. Dent. Relat. Res. 2008, 10, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.; Kimmel, D.; Dempster, D.; Weinstein, R.; Wronski, T.; Burr, D. Issues in modern bone histomorphometry. Bone 2011, 49, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.; Jolette, J. Bone Toolbox: Biomarkers, Imaging Tools, Biomechanics, and Histomorphometry. Toxicol. Pathol. 2018, 46, 511–529. [Google Scholar] [CrossRef]
- Erben, R.G.; Jolette, J.; Chouinard, L.; Boyce, R.; Smith, S.Y.; Varela, A.; Samadfam, R. Application of Histopathology and Bone Histomorphometry for Understanding Test Article-Related Bone Changes and Assessing Potential Bone Liabilities; Springer International Publishing: Berlin, Germany, 2017; pp. 253–278. [Google Scholar]
- Palma, P.J.; Matos, S.; Ramos, J.; Guerra, F.; Figueiredo, M.H.; Kauser, J. New formulations for space provision and bone regeneration. Biodental Eng. I 2010, 71–76. [Google Scholar]
- Yamamoto, N.; Takahashi, H.; Shimakura, T. Bone histomorphometry; A role of evaluation for bone quality and mechanical strength. Clin. Calcium 2016, 26, 9–15. [Google Scholar] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]
- Neyt, J.G.; Buckwalter, J.A.; Carroll, N.C. Use of animal models in musculoskeletal research. Iowa Orthop. J. 1998, 18, 118–123. [Google Scholar]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Thomas, B.S.; Bhat, K.M.; Mapara, M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111–118. [Google Scholar] [CrossRef]
- Palma, P.J. Estudo de novas formulações para regeneração óssea em defeitos de dimensão crítica. Master’s Thesis, University of Coimbra, Coimbra, Portugal, September 2009. [Google Scholar]
- Lindley, E.M.; Guerra, F.A.; Krauser, J.T.; Matos, S.M.; Burger, E.L.; Patel, V.V. Small peptide (P-15) bone substitute efficacy in a rabbit cancellous bone model. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2010, 94, 463–468. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group NCRRGW. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Dominguez, V.M.; Agnew, A.M. The use of ROI overlays and a semi-automated method for measuring cortical area in ImageJ for histological analysis. Am. J. Phys. Anthr. 2019, 168, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Kuo, S.M.; Lin, Y.T.; Yang, S.-W. The Biological Effects of Sex Hormones on Rabbit Articular Chondrocytes from Different Genders. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oonishi, H.; Kushitani, S.; Yasukawa, E.; Kawakami, H.; Nakata, A.; Koh, S.; Hench, L.; Wilson, J.; Tsuji, E.; Sugihara, T. Bone Growth into Spaces Between 45S5 Bioglass Granules. Bioceramics 1994, 139–144. [Google Scholar]
- Chan, C.; Thompson, I.; Robinson, P.; Wilson, J.; Hench, L. Evaluation of Bioglass/dextran composite as a bone graft substitute. Int. J. Oral Maxillofac. Surg. 2002, 31, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Matos, S.; Guerra, F.; Krauser, J.T.; Figueiredo, H.; Marcelino, J.P.; Sanz, M. Evaluation of an anorganic bovine-derived mineral with P-15 hydrogel bone graft: Preliminary study in a rabbit cranial bone model. Clin. Oral Implant. Res. 2011, 23, 698–705. [Google Scholar] [CrossRef]
- Caiazza, S.; Colangelo, P.; Bedini, R.; Formisano, G.; De Angelis, G.; Barrucci, S. Evaluation of Guided Bone Regeneration in Rabbit Femur Using Collagen Membranes. Implant. Dent. 2000, 9, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Francetti, L.; Weinstein, R.; Del Fabbro, M. Histomorphometric Results After Postextraction Socket Healing with Different Biomaterials: A Systematic Review of the Literature and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Kuboki, Y. Osteoblast-Related Gene Expression of Bone Marrow Cells during the Osteoblastic Differentiation Induced by Type I Collagen. J. Biochem. 2001, 129, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Ricci, M.; Covani, U.; Nannmark, U.; Azarmehr, I.; Calvo-Guirado, J.L. Maxillary Sinus Augmentation Using Prehydrated Corticocancellous Porcine Bone: Hystomorphometric Evaluation after 6 Months. Clin. Implant. Dent. Relat. Res. 2010, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- AbdelGawad, M.E.; Søe, K.; Andersen, T.L.; Merrild, D.M.; Christiansen, P.; Kjærsgaard-Andersen, P.; Delaisse, J.-M. Does collagen trigger the recruitment of osteoblasts into vacated bone resorption lacunae during bone remodeling? Bone 2014, 67, 181–188. [Google Scholar] [CrossRef]
- Petrochenko, P.; Narayan, R.J. Novel approaches to bone grafting: Porosity, bone morphogenetic proteins, stem cells, and the periosteum. J. Autom. Inf. Sci. 2010, 20, 303–315. [Google Scholar] [CrossRef]
- Iezzi, G.; Piattelli, A.; Giuliani, A.; Mangano, C.; Barone, A.; Manzon, L.; Degidi, M.; Scarano, A.; Filippone, A.; Perrotti, V. Molecular, Cellular and Pharmaceutical Aspects of Bone Grafting Materials and Membranes During Maxillary Sinus-lift Procedures. Part 2: Detailed Characteristics of the Materials. Curr. Pharm. Biotechnol. 2017, 18, 33–44. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Calvo-Guirado, J.L.; Negri, B.; Di Felice, R.; Covani, U. Volumetric analysis of remodelling pattern after ridge preservation comparing use of two types of xenografts. A multicentre randomized clinical trial. Clin. Oral Implant. Res. 2015, 27, e105–e115. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Negri, B.; Di Felice, R.; Marchionni, S.; Calvo-Guirado, J.L.; Covani, U.; et al. Clinical and Histological changes after ridge preservation with two xenografts: Preliminary results from a multicentre randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Menchini-Fabris, G.-B.; Derchi, G.; Marconcini, S.; Covani, U. Extra oral digital scanning and imaging superimposition for volume analysis of bone remodeling after tooth extraction with and without 2 types of particulate porcine mineral insertion: A randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, S.; Giammarinaro, E.; Derchi, G.; Alfonsi, F.; Covani, U.; Barone, A. Clinical outcomes of implants placed in ridge-preserved versus nonpreserved sites: A 4-year randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, A.; Perrotti, V.; Manzon, L.; Iezzi, G. Maxillary Sinus Augmentation in Humans Using Cortical Porcine Bone: A Histological and Histomorphometrical Evaluation After 4 and 6 Months. Clin. Implant. Dent. Relat. Res. 2011, 13, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Scarano, A.; Piattelli, M.; Piccirilli, M.; Caputi, S.; Piattelli, A. Histologic and Ultrastructural Analysis of Regenerated Bone in Maxillary Sinus Augmentation Using a Porcine Bone–Derived Biomaterial. J. Periodontol. 2006, 77, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Takahashi, N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Doll, J.; Tanner, M.; Bruckner, T.; Zimmermann, G.; Helbig, L.; Biglari, B.; Schmidmaier, G.; Moghaddam, A.; Information, P.E.K.F.C. Quantification of TGF-ß1, PDGF and IGF-1 cytokine expression after fracture treatment vs. non-union therapy via masquelet. Injury 2016, 47, 342–349. [Google Scholar] [CrossRef]
- Gardner, C.R. Comparison of morphological effects of PGE2 and TGFβ on osteoclastogenesis induced by RANKL in mouse bone marrow cell cultures. Cell Tissue Res. 2007, 330, 111–121. [Google Scholar] [CrossRef]
- Iida, T.; Neto, E.C.M.; Botticelli, D.; Alccayhuaman, K.A.A.; Lang, N.P.; Xavier, S.P. Influence of a collagen membrane positioned subjacent the sinus mucosa following the elevation of the maxillary sinus. A histomorphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 1567–1576. [Google Scholar] [CrossRef]
- Iida, T.; Silva, E.R.; Lang, N.P.; Alccayhuaman, K.A.A.; Botticelli, D.; Xavier, S.P. Histological and micro-computed tomography evaluations of newly formed bone after maxillary sinus augmentation using a xenograft with similar density and mineral content of bone: An experimental study in rabbits. Clin. Exp. Dent. Res. 2018, 4, 284–290. [Google Scholar] [CrossRef]
- Rombouts, C.; Jeanneau, C.; Camilleri, J.; Laurent, P.; About, I. Characterization and angiogenic potential of xenogeneic bone grafting materials: Role of periodontal ligament cells. Dent. Mater. J. 2016, 35, 900–907. [Google Scholar] [CrossRef][Green Version]
- Scarano, A.; Lorusso, F.; Ravera, L.; Mortellaro, C.; Piattelli, A. Bone Regeneration in Iliac Crestal Defects: An Experimental Study on Sheep. BioMed Res. Int. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Ricci, M.; Grassi, R.F.; Nannmark, U.; Quaranta, A.; Covani, U.; Grassi, F.R. A 6-month histological analysis on maxillary sinus augmentation with and without use of collagen membranes over the osteotomy window: Randomized clinical trial. Clin. Oral Implant. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Iezzi, G.; Mazzoni, S.; Piattelli, A.; Perrotti, V.; Barone, A. Regenerative properties of collagenated porcine bone grafts in human maxilla: Demonstrative study of the kinetics by synchrotron radiation microtomography and light microscopy. Clin. Oral Investig. 2017, 22, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, M.; Martegani, P.; D’Avenia, F.; Farneti, M.; Capri, D.; Paolantoni, G.; Landi, L. Simultaneous sinus augmentation with implant placement: Histomorphometric comparison of two different grafting materials. A multicenter double-blind prospective randomized controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2013, 28, 543–549. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Gómez-Moreno, G.; Guardia, J.; Ortiz-Ruiz, A.; Piatelli, A.; Barone, A.; Martínez-González, J.M.; Meseguer-Olmo, L.; López-Marí, L.; Dorado, C.B. Biological Response to Porcine Xenograft Implants. Implant. Dent. 2012, 21, 112–117. [Google Scholar] [CrossRef]
- Develioglu, H.; Ozcan, G.; Gultekin, S.E.; Senguven, B.; Yildirim, A. The short-term effects of various xenografts on bone healing in rats cranial defects. Biomed. Res. 2018, 29. [Google Scholar] [CrossRef]
- Develioğlu, H.; Saraydin, S.U.; Akkus, Z.; Sahin, Z.D.; Bakar, O. Long-term assessment of bone formation in response to Gen Os and Gel 40 xenografts in an experimental rat model. Biomed. Res. 2015, 26, 666–671. [Google Scholar]



| Material | Timepoint | |
|---|---|---|
| 15 Days | 30 Days | |
| Apatos | 5 defects | 5 defects |
| Gen-Os | 5 defects | 5 defects |
| mp3 | 5 defects | 5 defects |
| Putty | 5 defects | 5 defects |
| Gel 40 | 5 defects | 5 defects |
| Control | 1 defect | 1 defect |
| Total | 26 defects | 26 defects |
| 15 Days | ||||
|---|---|---|---|---|
| Material | Apatos | Gen-Os | mp3 | Gel 40 |
| Putty | 0.505 | 0.106 | 0.016 * | 1.000 |
| Apatos | 1.000 | 1.000 | 0.457 | |
| Gen-Os | 1.000 | 0.093 | ||
| mp3 | 0.014 * | |||
| 30 Days | ||||
| Material | Apatos | Gen-Os | mp3 | Gel 40 |
| Putty | 1.000 | 0.099 | 0.035* | 1.000 |
| Apatos | 1.000 | 1.000 | 0.587 | |
| Gen-Os | 1.000 | 0.046 * | ||
| mp3 | 0.015 * | |||
| 15 Days | ||||
|---|---|---|---|---|
| Material | Apatos | Gen-Os | mp3 | Gel 40 |
| Putty | 0.240 | 0.135 | 0.003 * | 1.000 |
| Apatos | 1.000 | 1.000 | 0.371 | |
| Gen-Os | 1.000 | 0.215 | ||
| mp3 | 0.006 * | |||
| 30 Days | ||||
| Material | Apatos | Gen-Os | mp3 | Gel 40 |
| Putty | 0.014 * | 1.000 | 0.022 * | 1.000 |
| Apatos | 0.932 | 1.000 | 0.007 * | |
| Gen-Os | 1.000 | 0.851 | ||
| mp3 | 0.011 * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falacho, R.I.; Palma, P.J.; Marques, J.A.; Figueiredo, M.H.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. https://doi.org/10.3390/molecules26051339
Falacho RI, Palma PJ, Marques JA, Figueiredo MH, Caramelo F, Dias I, Viegas C, Guerra F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules. 2021; 26(5):1339. https://doi.org/10.3390/molecules26051339
Chicago/Turabian StyleFalacho, Rui I., Paulo J. Palma, Joana A. Marques, Maria H. Figueiredo, Francisco Caramelo, Isabel Dias, Carlos Viegas, and Fernando Guerra. 2021. "Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model" Molecules 26, no. 5: 1339. https://doi.org/10.3390/molecules26051339
APA StyleFalacho, R. I., Palma, P. J., Marques, J. A., Figueiredo, M. H., Caramelo, F., Dias, I., Viegas, C., & Guerra, F. (2021). Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules, 26(5), 1339. https://doi.org/10.3390/molecules26051339









