The Localization Behavior of Different CNTs in PC/SAN Blends Containing a Reactive Component
Abstract
1. Introduction
2. Results
2.1. Localization Behavior of Nanocyl™ MWCNTs
2.2. Localization Behavior of Baytubes® MWCNTs
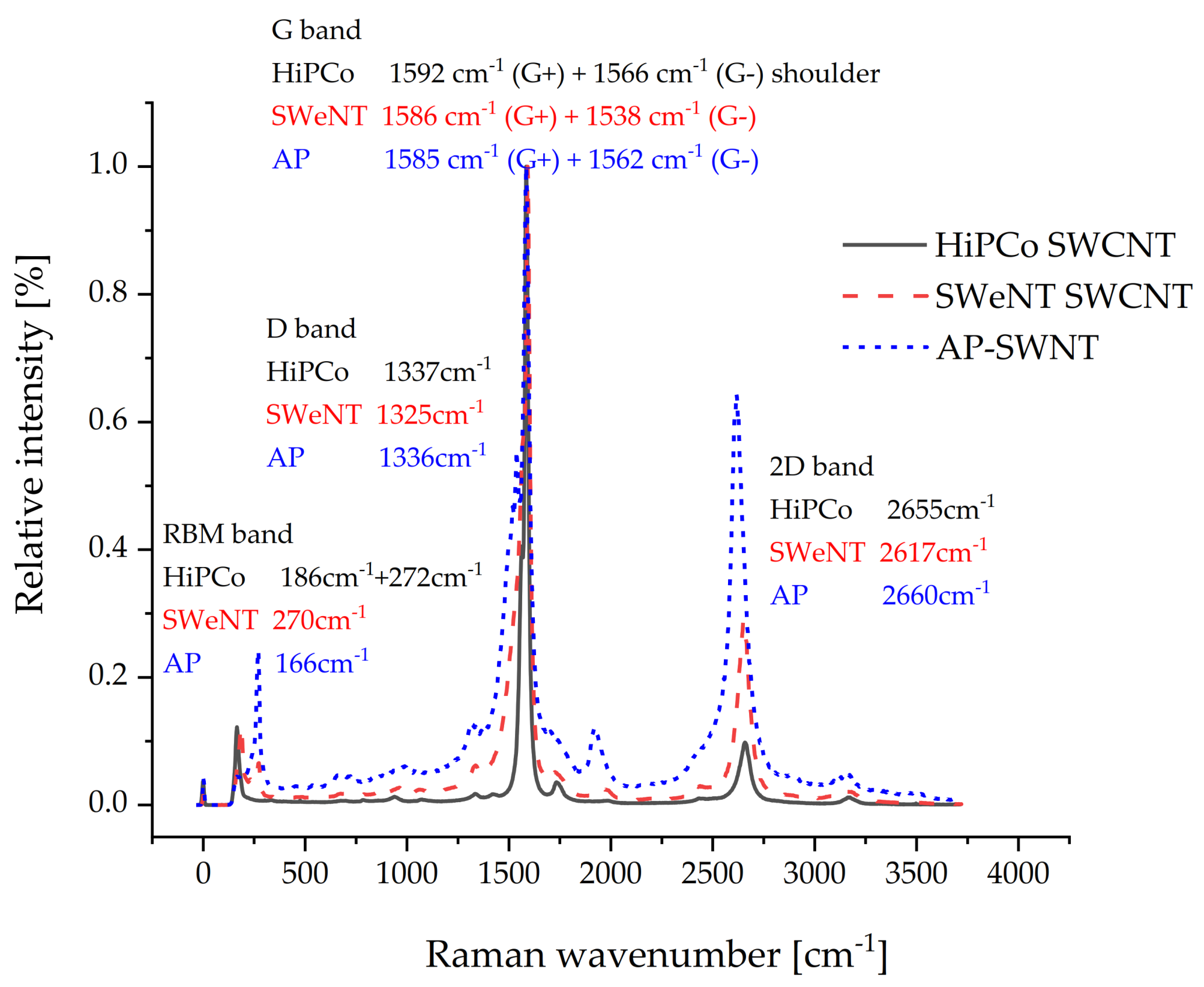
2.3. Localization Behavior of SWCNTs
3. Discussion
3.1. Reasons for Localization Changes
3.2. Differences Between the Behavior of Different CNTs
4. Materials and Methods
4.1. Materials
4.2. Preparation and Compositions
4.3. Morphological Characterization
4.4. Characterization of CNTs
4.5. Other Characterization Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Paul, D.R.; Newman, S. Polymer Blends; Academic Press Inc.: New York, NY, USA, 1978; Volume 1, p. 501. [Google Scholar]
- Utracki, L.A.; Favis, B.D. Polymer alloys and blends. In Handbook of Polymer Science and Technology; National Research Council of Canada: Boucherville, QC, Canada, 1989; Volume 4, pp. 121–185. [Google Scholar]
- Pötschke, P.; Paul, D.R. Formation of Co-continuous structures in melt-mixed immiscible polymer blends. J. Macromol. Sci. Polym. Rev. 2003, C43, 87–141. [Google Scholar] [CrossRef]
- Niebergall, U.; Bohse, J.; Schürmann, B.L.; Seidler, S.; Grellmann, W. Relationship of fracture behavior and morphology in polyolefin blends. Polym. Eng. Sci. 1999, 39, 1109–1118. [Google Scholar] [CrossRef]
- Willemse, R.C.; Speijer, A.; Langeraar, A.E.; Posthuma de Boer, A. Tensile moduli of co-continuous polymer blends. Polymer 1999, 40, 6645–6650. [Google Scholar] [CrossRef]
- Veenstra, H.; Verkooijen, P.C.J.; van Lent, B.J.J.; van Dam, J.; de Boer, A.P.; Nijhof, A.P.H.J. On the mechanical properties of co-continuous polymer blends: Experimental and modelling. Polymer 2000, 41, 1817–1826. [Google Scholar] [CrossRef]
- Kiran, M.D.; Govindaraju, H.K.; Jayaraju, T.; Kumar, N. Review-effect of fillers on mechanical properties of polymer matrix composites. Mater. Today 2018, 5, 22421–22424. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Breuer, O.; Sundararaj, U. Big returns from small fibers: A review of polymer/carbon nanotube composites. Polym. Compos. 2004, 25, 630–645. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-nanotube-based thermoelectric materials and devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Nagy, J.B.; Coleman, J.N.; Fonseca, A.; Destrée, A.; Mekhalif, Z.; Moreau, N.; Vast, L.; Delhalle, J. Carbon Nanotubes and Nanocomposites: Electrical, mechanical and flame-retardant aspects. Nanopages 2006, 1, 121–163. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Review of applications of polymer/carbon nanotubes and epoxy/CNT composites. Polym. Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Pötschke, P.; Bhattacharyya, A.R.; Janke, A. Morphology and electrical resistivity of melt mixed blends of polyethylene and carbon nanotube filled polycarbonate. Polymer 2003, 44, 8061–8069. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.; Kulkarni, A.; Pötschke, P. Electrical, rheological and morphological studies in co-continuous blends of polyamide 6 and acrylonitrile-butadiene-styrene with multiwall carbon nanotubes prepared by melt blending. Compos. Sci. Technol. 2009, 69, 365–372. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Zhang, M.; Yu, W. Selective localization of multiwalled carbon nanotubes in poly(ε-caprolactone)/polylactide blend. Biomacromolecules 2009, 10, 417–424. [Google Scholar] [CrossRef]
- Otero-Navas, I.; Arjmand, M.; Sundararaj, U. Carbon nanotube induced double percolation in polymer blends: Morphology, rheology and broadband dielectric properties. Polymer 2017, 114, 122–134. [Google Scholar] [CrossRef]
- Göldel, A.; Kasaliwal, G.; Pötschke, P. Selective localization and migration of multiwalled carbon nanotubes in blends of polycarbonate and poly(styrene-acrylonitrile). Macromol. Rapid Commun. 2009, 30, 423–429. [Google Scholar] [CrossRef]
- Wu, D.; Lin, D.; Zhang, J.; Zhou, W.; Zhang, M.; Zhang, Y.; Wang, D.; Lin, B. Selective localization of nanofillers: Effect on morphology and crystallization of PLA/PCL blends. Macromol. Chem. Phys. 2011, 212, 613–626. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z.-X.; Yu, J. Effect of ABS rubber content on the localization of MWCNTs in PC/ABS blends and electrical resistivity of the composites. Macromol. Mater. Eng. 2010, 295, 263–268. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, C.; Zhang, Y. Investigation on the multiwalled carbon nanotubes reinforced polyamide 6/polypropylene composites. Polym. Eng. Sci. 2009, 49, 1909–1917. [Google Scholar] [CrossRef]
- Wu, M.; Shaw, L. On the improved properties of injection-molded, carbon nanotube-filled PET/PVDF blends. J. Power Sources 2004, 136, 37–44. [Google Scholar] [CrossRef]
- Pötschke, P.; Pegel, S.; Claes, M.; Bonduel, D. A novel strategy to incorporate carbon nanotubes into thermoplastic matrices. Macromol. Rapid Commun. 2008, 29, 244–251. [Google Scholar] [CrossRef]
- Baudouin, A.-C.; Devaux, J.; Bailly, C. Localization of carbon nanotubes at the interface in blends of polyamide and ethylene-acrylate copolymer. Polymer 2010, 51, 1341–1354. [Google Scholar] [CrossRef]
- Cayla, A.; Campagne, C.; Rochery, M.; Devaux, E. Electrical, rheological properties and morphologies of biphasic blends filled with carbon nanotubes in one of the two phases. Lancet 2011, 161, 1034–1042. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Wu, J.; Huang, T.; Chen, C.; Peng, Y.; Wang, Y. Toughening of poly(L-lactide)/multiwalled carbon nanotubes nanocomposite with ethylene-co-vinyl acetate. J. Polym. Sci. Part B 2011, 49. [Google Scholar] [CrossRef]
- Lee, C.J.; Salehiyan, R.; Ham, D.S.; Cho, S.K.; Lee, S.-J.; Kim, K.J.; Yoo, Y.; Hyun, K.; Lee, J.H.; Choi, W.J. Influence of carbon nanotubes localization and transfer on electrical conductivity in PA66/(PS/PPE)/CNTs nanocomposites. Polymer 2016, 84, 198–208. [Google Scholar] [CrossRef]
- Fenouillot, F.; Cassagnau, P.; Majesté, J.-C. Uneven distribution of nanoparticles in immiscible fluids: Morphology development in polymer blends. Polymer 2009, 50. [Google Scholar] [CrossRef]
- Salehiyan, R.; Ray, S.S. Tuning the conductivity of nanocomposites through nanoparticle migration and interface crossing in immiscible polymer blends: A review on fundamental understanding. Macromol. Mater. Eng. 2019, 304, 1800431. [Google Scholar] [CrossRef]
- Mamunya, Y.; Levchenko, V.; Boiteux, G.; Seytre, G.; Zanoaga, M.; Tanasa, F.; Lebedev, E. Controlling morphology, electrical, and mechanical properties of polymer blends by heterogeneous distribution of carbon nanotubes. Polym. Compos. 2016, 37, 2467–2477. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, B.-Y.; Xiong, Z.-Y.; Guo, Z.-X.; Yu, J. Preparation of conductive polyphenylene sulfide/polyamide 6/multiwalled carbon nanotube composites using the slow migration rate of multiwalled carbon nanotubes from polyphenylene sulfide to polyamide 6. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Göldel, A.; Kasaliwal, G.R.; Pötschke, P.; Heinrich, G. The kinetics of CNT transfer between immiscible blend phases during melt mixing. Polymer 2012, 53, 411–421. [Google Scholar] [CrossRef]
- Huang, J.; Mao, C.; Zhu, Y.; Jiang, W.; Yang, X. Control of carbon nanotubes at the interface of a co-continuous immiscible polymer blend to fabricate conductive composites with ultralow percolation thresholds. Carbon 2014, 73, 267–274. [Google Scholar] [CrossRef]
- Huang, Y.; Ellingford, C.; Bowen, C.; McNally, T.; Wu, D.; Wan, C. Tailoring the electrical and thermal conductivity of multi-component and multi-phase polymer composites. Int. Mater. Rev. 2019, 1–35. [Google Scholar] [CrossRef]
- Baudouin, A.-C.; Bailly, C.; Devaux, J. Interface localization of carbon nanotubes in blends of two copolymers. Polym. Degrad. Stab. 2010, 95, 389–398. [Google Scholar] [CrossRef]
- Zonder, L.; Ophir, A.; Kenig, S.; McCarthy, S. The effect of carbon nanotubes on the rheology and electrical resistivity of polyamide 12/high density polyethylene blends. Polymer 2011, 52, 5085–5091. [Google Scholar] [CrossRef]
- Nishikawa, R.; Tamaki, K.; Notoya, O.; Yamaguchi, M. Carbon nanotube localization at interface in cocontinuous blends of polyethylene and polycarbonate. J. Appl. Polym. Sci. 2020, 137, 48676. [Google Scholar] [CrossRef]
- Göldel, A.; Marmur, A.; Kasaliwal, G.R.; Pötschke, P.; Heinrich, G. Shape-dependent localization of carbon nanotubes and carbon black in an immiscible polymer blend during melt mixing. Macromolecules 2011, 44, 6094–6102. [Google Scholar] [CrossRef]
- Pötschke, P.; Kretzschmar, B.; Janke, A. Use of carbon nanotube filled polycarbonate in blends with montmorillonite filled polypropylene. Compos. Sci. Technol. 2007, 67, 855–860. [Google Scholar] [CrossRef]
- Bai, L.; He, S.; Fruehwirth, J.W.; Stein, A.; Macosko, C.W.; Cheng, X. Localizing graphene at the interface of continuous polymer blends: Morphology, rheology, and conductivity of continuous conductive polymer composites. J. Rheol. 2017, 61, 575–587. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.R.; Kodgire, P.V.; Misra, A.; Pötschke, P. Rheology, morphology, and crystallization Behavior of melt-mixed blends of polyamide6 and acrylonitrile-butadiene-styrene: Influence of reactive compatibilizer premixed with multiwall carbon nanotubes. J. Appl. Polym. Sci. 2007, 106, 3394–3408. [Google Scholar] [CrossRef]
- Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P.; Dupin, G.; Claes, M. Masterbatch-based multi-walled carbon nanotube filled polypropylene nanocomposites: Assessment of rheological and mechanical properties. Compos. Sci. Technol. 2009, 69, 1756–1763. [Google Scholar] [CrossRef]
- Lee, G.-W.; Jagannathan, S.; Chae, H.G.; Minus, M.L.; Kumar, S. Carbon nanotube dispersion and exfoliation in polypropylene and structure and properties of the resulting composites. Polymer 2008, 49, 1831–1840. [Google Scholar] [CrossRef]
- Wang, G.; Qu, Z.; Liu, L.; Shi, Q.; Guo, J. Study of SMA graft modified MWNT/PVC composite materials. Mater. Sci. Eng. 2008, 472, 136–139. [Google Scholar] [CrossRef]
- Gültner, M.; Göldel, A.; Pötschke, P. Tuning the localization of functionalized MWCNTs in SAN/PC blends by a reactive component. Compos. Sci. Technol. 2011, 72, 41–48. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, P.; Du, J.; Zhao, L.; Ajayan, P.M.; Cheng, H.-M. Increasing the electrical conductivity of carbon nanotube/polymer composites by using weak nanotube/polymer interactions. Carbon 2010, 48, 3551–3558. [Google Scholar] [CrossRef]
- Itkis, M.E.; Perea, D.E.; Niyogi, S.; Rickard, S.M.; Hamon, M.A.; Hu, H.; Zhao, B.; Haddon, R.C. Purity evaluation of as-prepared single-walled carbon nanotube soot by use of solution-phase near-IR spectroscopy. Nano Lett. 2003, 3, 309–314. [Google Scholar] [CrossRef]
- Liebscher, M.; Tzounis, L.; Pötschke, P.; Heinrich, G. Influence of the viscosity ratio in PC/SAN blends filled with MWCNTs on the morphological, electrical, and melt rheological properties. Polymer 2013, 54, 6801–6808. [Google Scholar] [CrossRef]
- Feng, J.Y.; Chan, C.M.; Li, J.X. A method to control the dispersion of carbon black in an immiscible polymer blend. Polym. Eng. Sci. 2003, 43, 1058–1063. [Google Scholar] [CrossRef]
- Flaris, V.; Fletcher, C.; Gültner, M.; Pötschke, P. Surface energy effects of PC/SAN/MWCNT blends with the addition of a reactive component. In Proceedings of the 70th Annual Technical Conference Exhibition Conference Proceedings, Orlando, FL, USA, 2–4 April 2012; Volume 2. [Google Scholar]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. Static and dynamic wetting measurements of single carbon nanotubes. Phys. Rev. Lett. 2004, 92, 186103. [Google Scholar] [CrossRef]
- Nuriel, S.; Liu, L.; Barber, A.H.; Wagner, H.D. Direct measurement of multiwall nanotube surface tension. Chem. Phys. Lett. 2005, 404, 263–266. [Google Scholar] [CrossRef]
- Rausch, J.; Zhuang, R.-C.; Mäder, E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Compos. Part A 2010, 41, 1038–1046. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Covestro (Bayer), Makrolon 2600 PC Datasheet. Available online: http://materials.tecves.com/en/148/makrolon-2600 (accessed on 28 January 2021).
- INEOS Styrolution Europe GmbH, CAMPUS® Datenblatt Luran® 358N—SAN. 2018. Available online: https://www.campusplastics.com/material/pdf/78486/Luran358N?sLg=en (accessed on 28 January 2021).
- Denki Kagaku Kōgyō, K.K.; Denka, I.P. Maliimide Typi Heat Resistance Modifie Modifier. Available online: http://www.denka.co.kr/pdf/DENKA-IP_En.pdf (accessed on 28 January 2021).
- Nanocyl, S.A. Technical Data Sheet: NC3152™, 25th January 2016, V01. Available online: http://www.nanocyl.com/wp-content/uploads/2016/02/Technical-Data-Sheet-NC3152-V01.pdf (accessed on 28 January 2021).
- Nanocyl, S.A. Technical Data Sheet: NC3150™, 25th January 2016, V05. Available online: http://www.nanocyl.com/wp-content/uploads/2016/02/Technical-Data-Sheet-NC3150-V05.pdf (accessed on 28 January 2021).
- Nanocyl, S.A. Technical Data Sheet: NC7000™, V08. 2016. Available online: https://www.nanocyl.com/wp-content/uploads/2016/07/DM-TI-02-TDS-NC7000-V08.pdf (accessed on 28 January 2021).
- Bayer Material Science. Data Sheet Baytubes®C150P; Bayer Material Science AG: Leverkusen, Germany, 2009. [Google Scholar]
- Bayer Material Science. Data Sheet Baytubes®C150HP; Bayer Material Science AG: Leverkusen, Germany, 2007. [Google Scholar]
- Bronikowski, M.J.; Willis, P.A.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J. Vac. Sci. Technol. A 2001, 19, 1800–1805. [Google Scholar] [CrossRef]
- CoMoCAT® Single-wall Carbon Nanotubes. Available online: https://www.sigmaaldrich.com/technical-documents/articles/materials-science/nanomaterials/comocat-carbon-nanotubes.html (accessed on 30 January 2021).
- Product information AP-SWNT, Carbon Solutions Inc. Available online: https://www.carbonsolution.com/products/ap-swnt, (accessed on 1 February 2021).
- Lafuente, E.; Callejas, M.A.; Sainz, R.; Benito, A.M.; Maser, W.K.; Sanjuán, M.L.; Saurel, D.; de Teresa, J.M.; Martínez, M.T. The influence of single-walled carbon nanotube functionalization on the electronic properties of their polyaniline composites. Carbon 2008, 46, 1909–1917. [Google Scholar] [CrossRef]
- Utech, T.; Pötschke, P.; Simon, F.; Janke, A.; Kettner, H.; Paiva, M.; Zimmerer, C. Bio-inspired deposition of electrochemically exfoliated graphene layers for electrical resistance heating applications. Nano Express 2020, 1, 030032. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
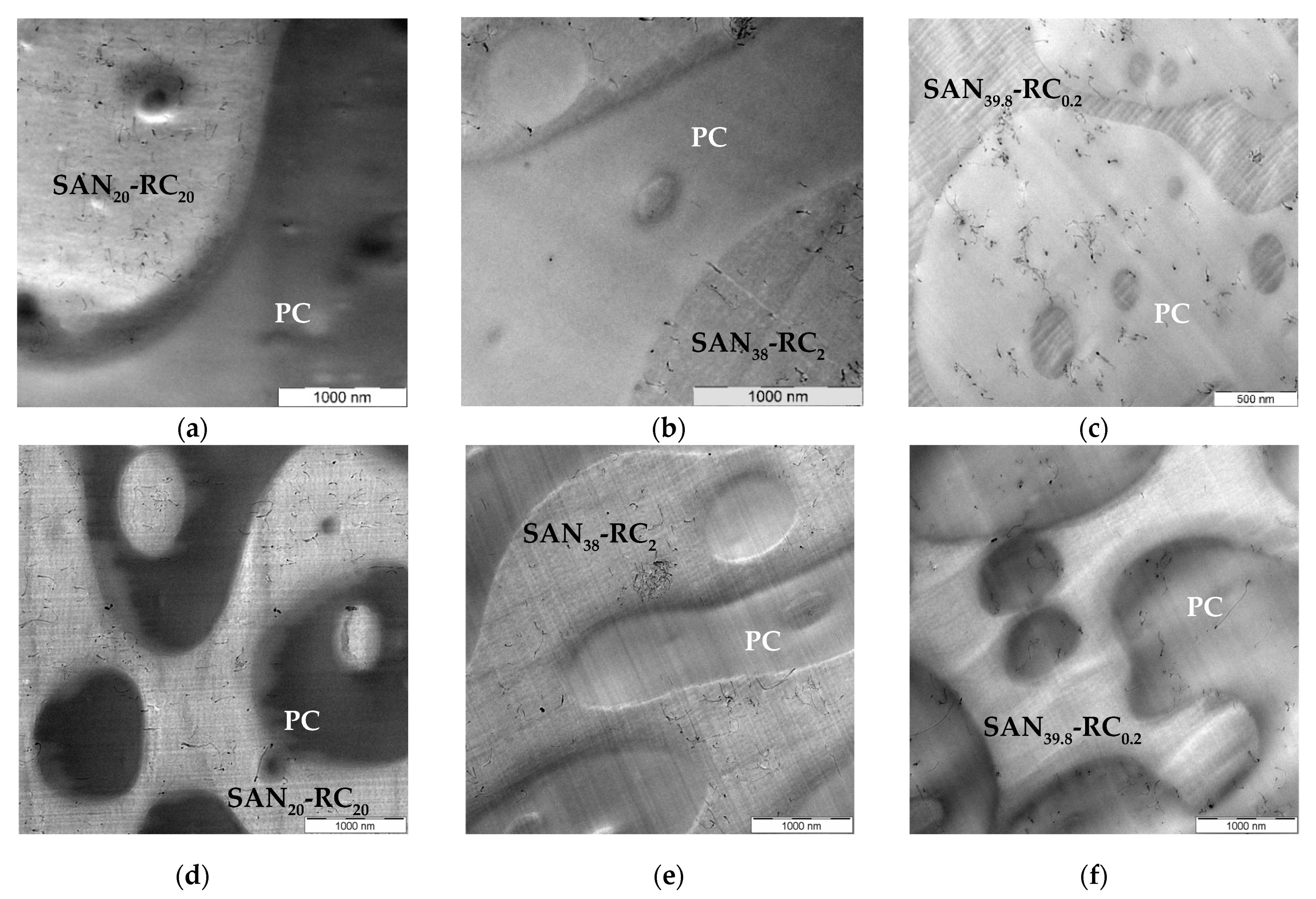
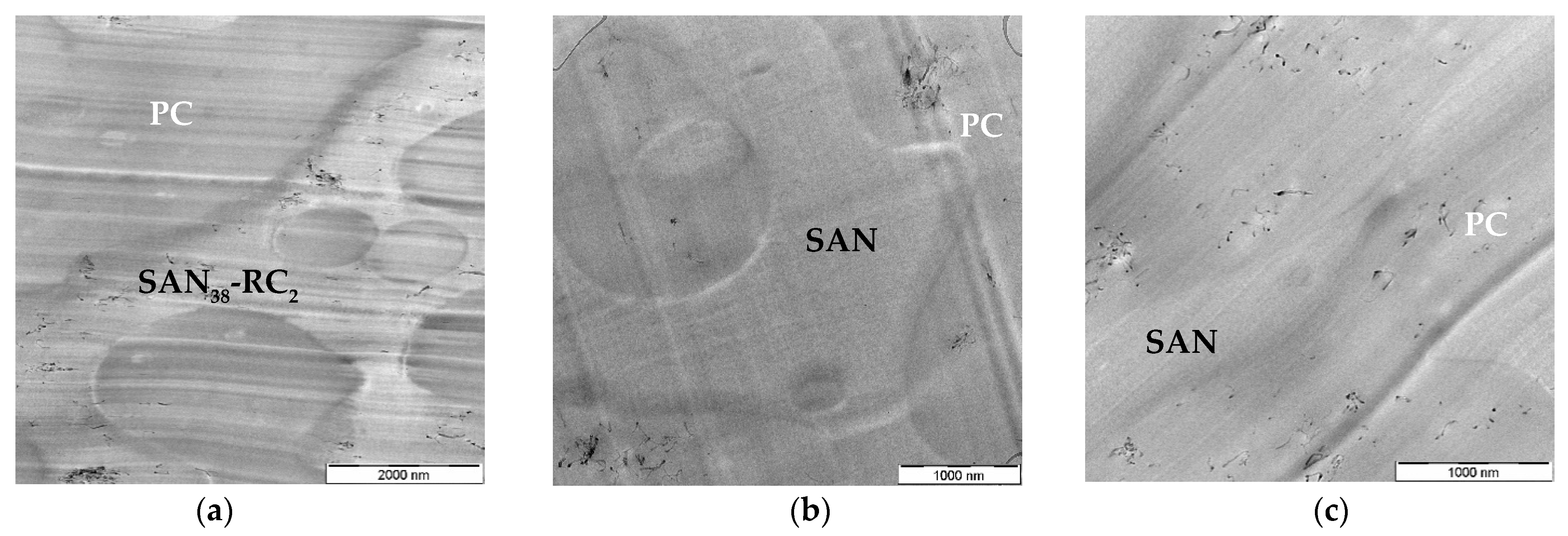
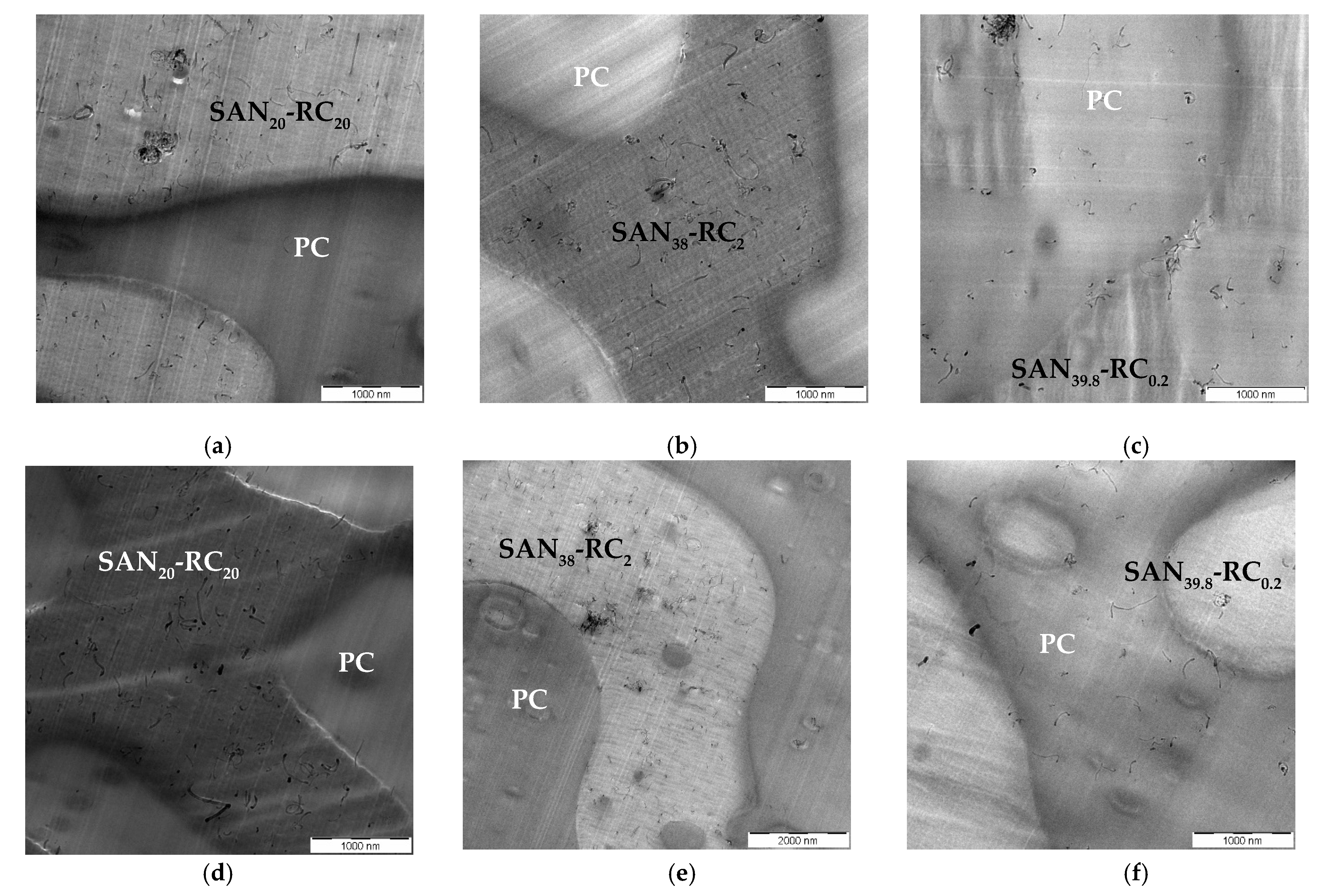
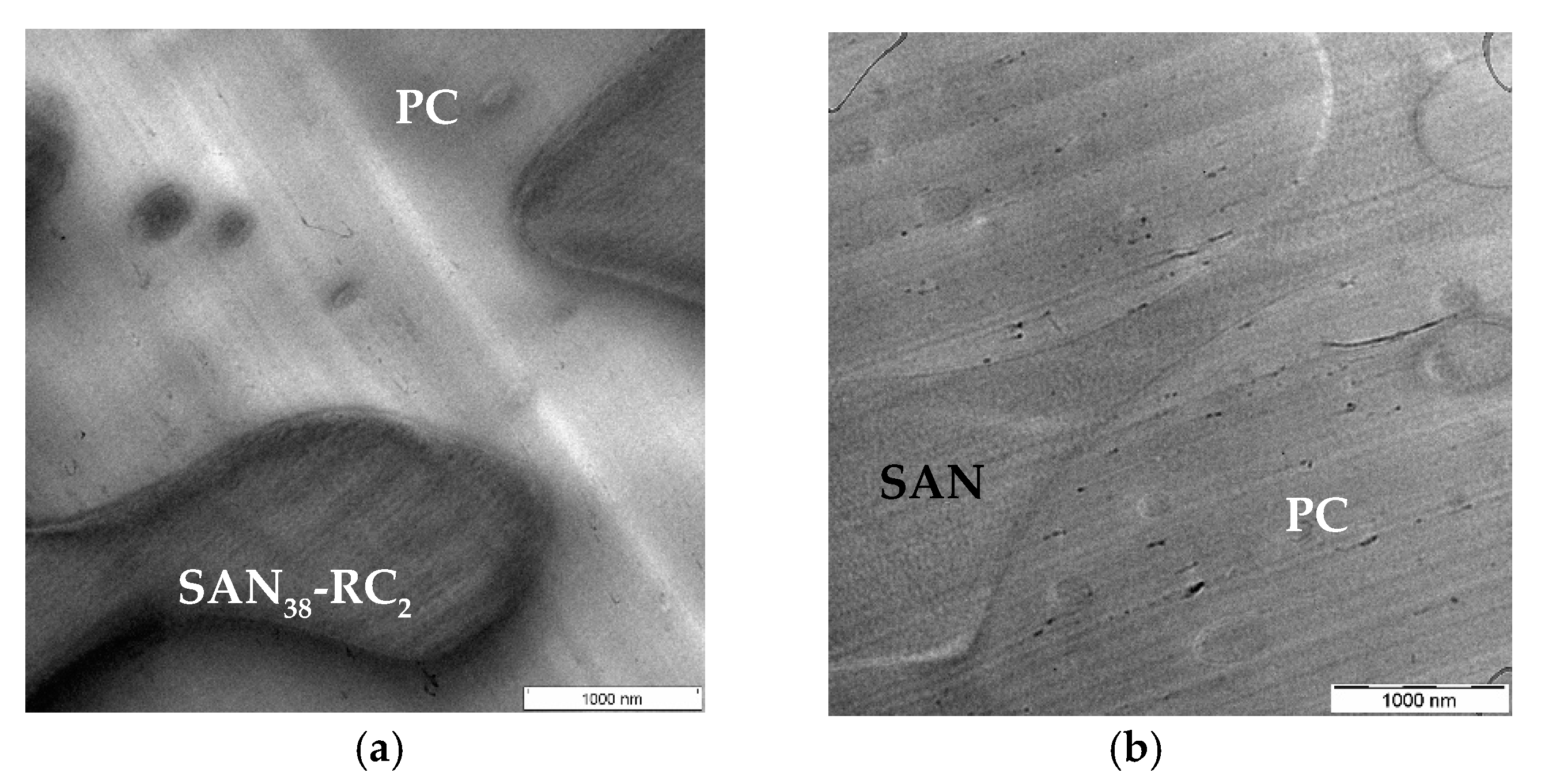
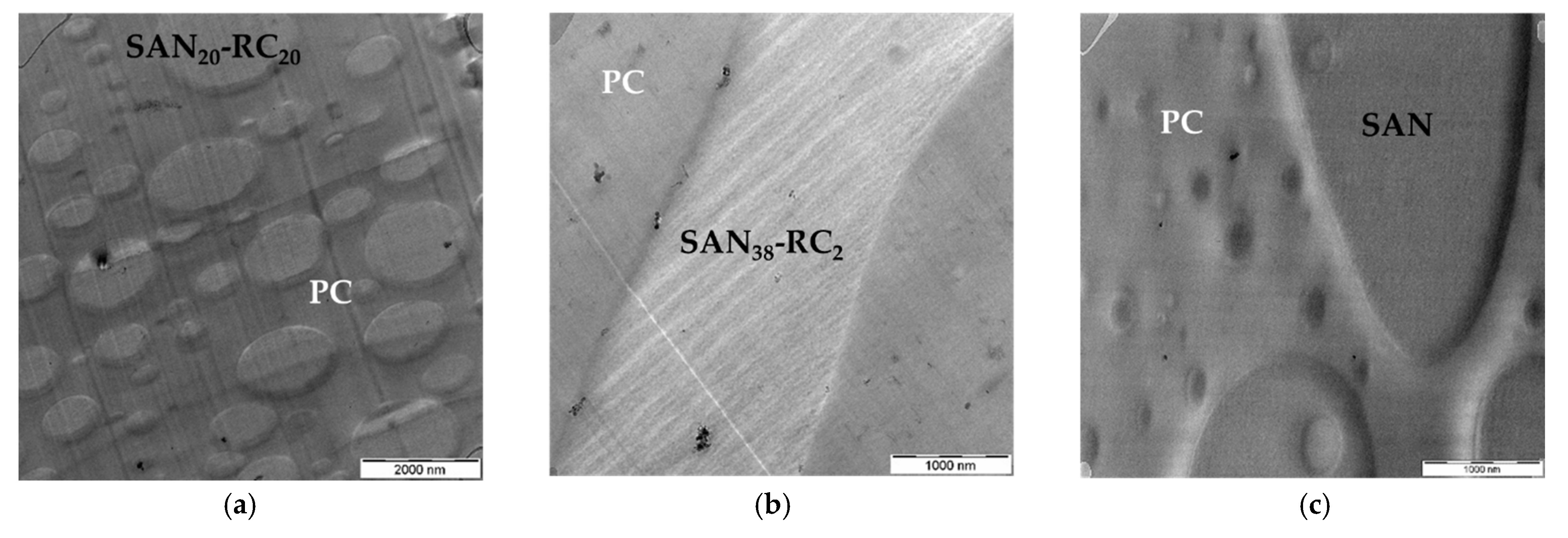
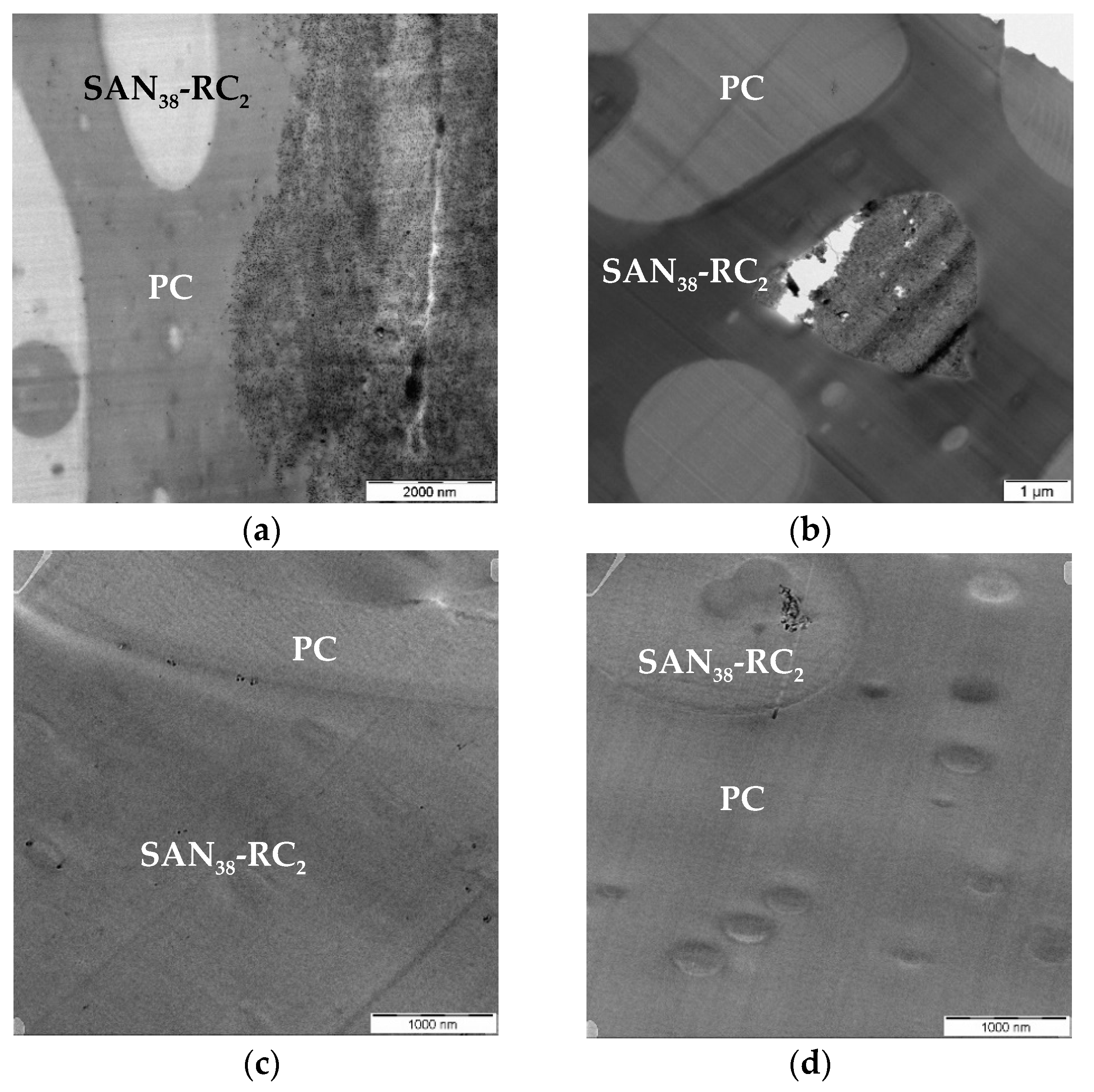
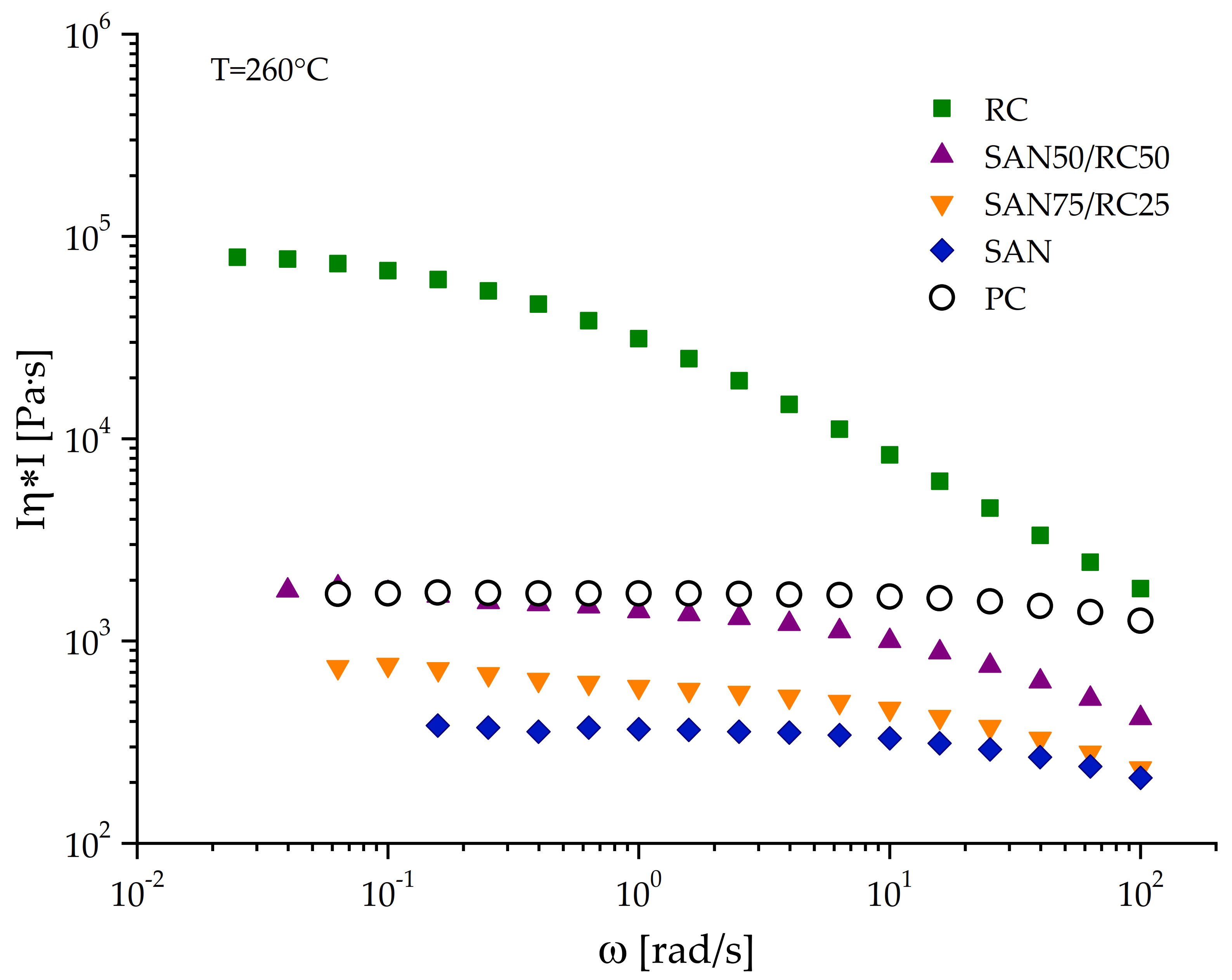
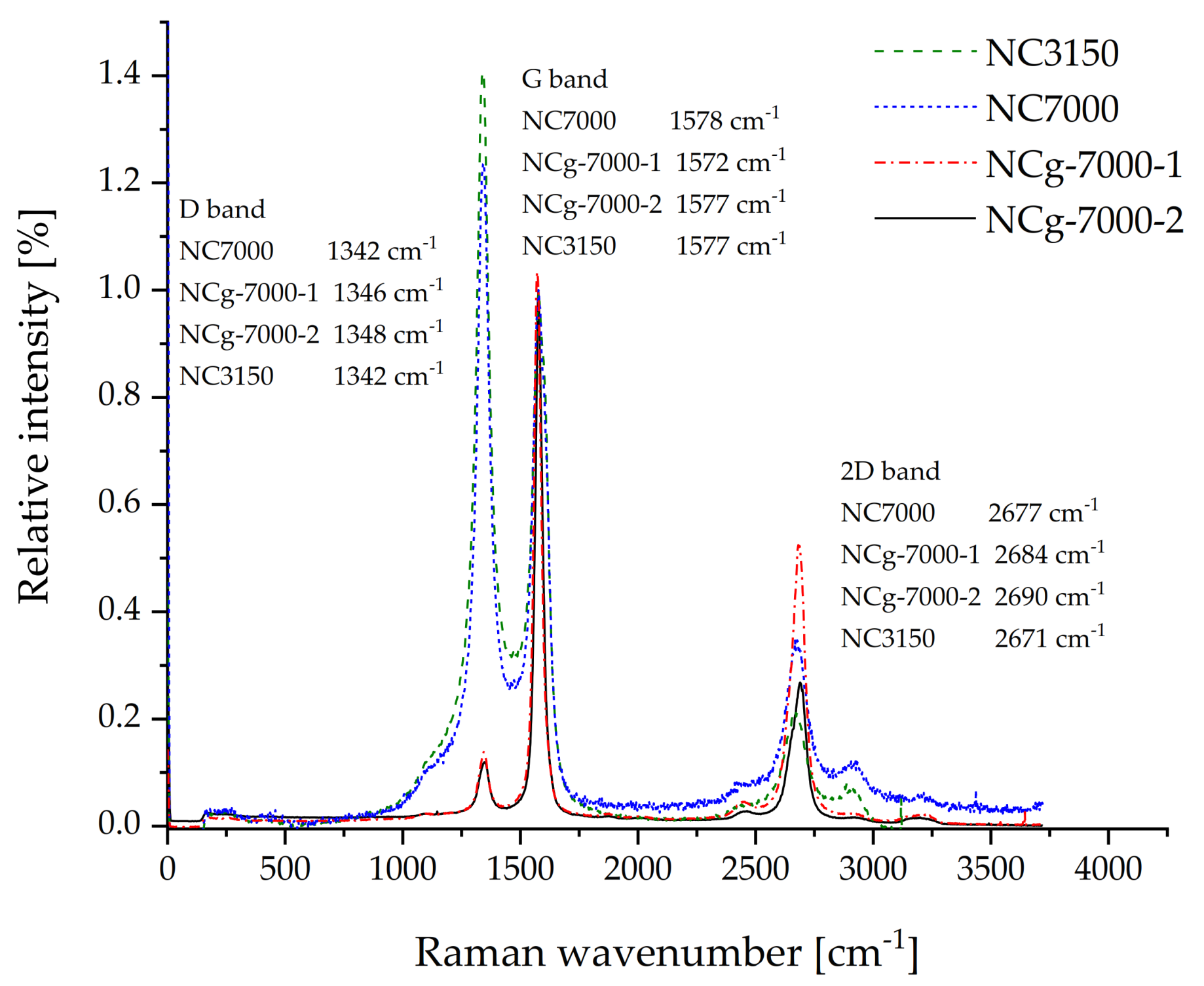

| CNT Type | PC60/SAN20-RC20 | PC60/SAN38-RC2 | PC60/SAN39.8-RC0.2 | PC60/SAN40 |
|---|---|---|---|---|
| NC3152 [47] | SAN-RC | SAN-RC | PC | PC |
| NC3150 | SAN-RC | SAN-RC | PC | PC * |
| NC7000 | SAN-RC | SAN-RC | PC | PC [50] |
| NCg-7000-1 | SAN-RC* | SAN-RC | n. s. | PC |
| NCg-7000-2 | SAN-RC | SAN-RC* | n. s. | PC |
| Baytubes® C150P | SAN-RC | SAN-RC | PC | PC * |
| Baytubes® C150HP | SAN-RC | SAN-RC | PC | PC [20] |
| HiPCo™ | n. s. | PC | n. s. | PC |
| SWeNT® CG100 | SAN-RC and PC | SAN-RC and PC | n. s. | PC |
| AP-SWNT | n. s. | SAN-RC and PC | n. s. | n. s. |
| AP-SWNT-NH2 | n. s. | SAN-RC | n. s. | n. s. |
| CNT type | [O]:[C]│spec | [N]:[C]│spec | [O]:[C]│org a |
|---|---|---|---|
| NC3152 | 0.007 | 0.004 | 0.007 |
| NC3150 | 0.011 | – | 0.011 |
| NC7000 | 0.008 | – | 0.059 |
| NCg-7000-1 | 0.011 | Traces | 0.011 |
| NCg-7000-2 | 0.002 | – | 0.002 |
| Baytubes® C150P | 0.003 | – | 0.002 |
| Baytubes® C150HP | 0.007 | – | – |
| HiPCoTM | 0.024 | Traces | 0.018 |
| SWeNT® CG100 | 0.037 | 0.004 | 0.033 |
| AP-SWNT | 0.059 | 0.067 | 0.053 |
| AP-SWNT-NH2 | 0.057 | 0.025 | 0.054 |
| Property | Polycarbonate (PC) | Poly(styrene-co-acrylonitrile) (SAN) | DENKA IP MS-L2A (RC) |
|---|---|---|---|
| Density, 25 °C [g/cm3] | 1.2 | 1.08 | 1.18 |
| Complex melt viscosity η* (at 100 rad/s, 260 °C) [Pa s] | 1260 | 211 | 1818 |
| Glass transition temperature Tg (DSC)[°C] | 148 | 108 | 196 |
| Name | Producer | Type | Functionality | Synthesis Process |
|---|---|---|---|---|
| NC3152 [61] | Nanocyl™, Sambreville, Belgium | MWCNT | NH2 | CVD, purified, shortened |
| NC3150 [62] | Nanocyl™, Sambreville, Belgium | MWCNT | - | CVD, purified, shortened |
| NC7000 [63] | Nanocyl™, Sambreville, Belgium | MWCNT | as prepared | CVD, as produced |
| NCg-7000-1 | Nanocyl™, Sambreville, Belgium | MWCNT | - | CVD, graphitized at IPF |
| NCg-7000-2 | Nanocyl™, Sambreville, Belgium | MWCNT | - | CVD, graphitized at IPF |
| Baytubes® C150P [64] | Bayer MaterialScience, Leverkusen, Germany | MWCNT | - | CVD |
| Baytubes® C150HP [65] | Bayer MaterialScience, Leverkusen, Germany | MWCNT | - | CVD, high purity |
| HiPCo™ | Carbon Nanotechnologies Inc (CNI), Houston, TX, USA | SWCNT | as prepared | CVD, Fe(CO)5 [66] |
| SWeNT® CG100 [67] | SWeNT, SouthWest NanoTechnologies Inc., Norman, OK, USA | SWCNT | as prepared | CoMoCAT® process, CVD fluidized bed |
| AP-SWNT [50,68] | Carbon Solution Inc., Riverside, CA, USA | SWCNT | as prepared | electric arc, Ni/Y catalyst |
| AP-SWNT-NH2 | Carbon Solution Inc., Riverside, CA, USA modified by CSIC, Spain, acc. to [69] | SWCNT | NH2 | electric arc, Ni/Y catalyst |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gültner, M.; Boldt, R.; Formanek, P.; Fischer, D.; Simon, F.; Pötschke, P. The Localization Behavior of Different CNTs in PC/SAN Blends Containing a Reactive Component. Molecules 2021, 26, 1312. https://doi.org/10.3390/molecules26051312
Gültner M, Boldt R, Formanek P, Fischer D, Simon F, Pötschke P. The Localization Behavior of Different CNTs in PC/SAN Blends Containing a Reactive Component. Molecules. 2021; 26(5):1312. https://doi.org/10.3390/molecules26051312
Chicago/Turabian StyleGültner, Marén, Regine Boldt, Petr Formanek, Dieter Fischer, Frank Simon, and Petra Pötschke. 2021. "The Localization Behavior of Different CNTs in PC/SAN Blends Containing a Reactive Component" Molecules 26, no. 5: 1312. https://doi.org/10.3390/molecules26051312
APA StyleGültner, M., Boldt, R., Formanek, P., Fischer, D., Simon, F., & Pötschke, P. (2021). The Localization Behavior of Different CNTs in PC/SAN Blends Containing a Reactive Component. Molecules, 26(5), 1312. https://doi.org/10.3390/molecules26051312







