A Review of Bacteriochlorophyllides: Chemical Structures and Applications
Abstract
1. Introduction
1.1. A Brief History of Bacteriochlorophylls
1.2. The Chemical Structure of Bacteriochlorophyllides
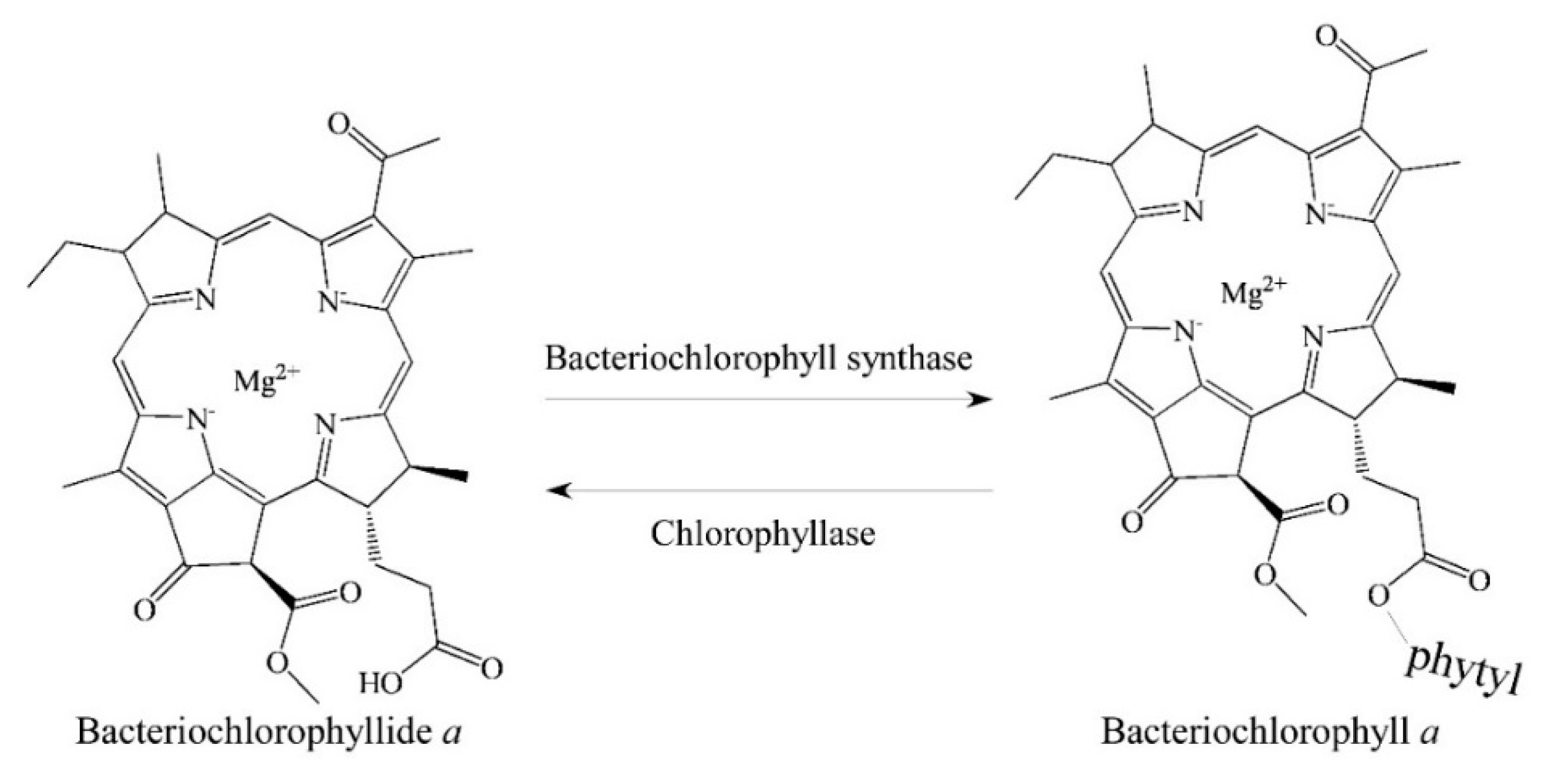
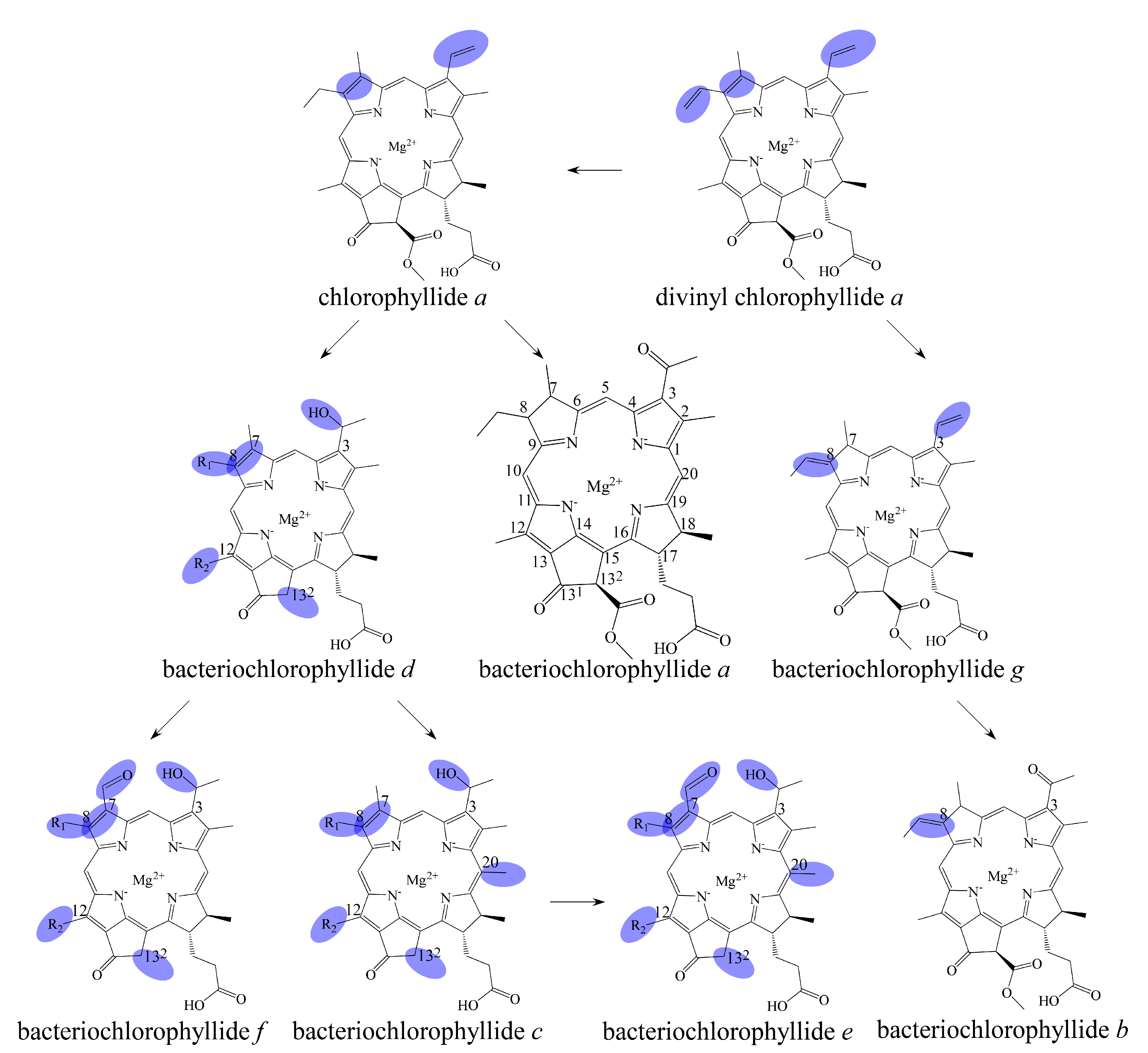
2. Biosynthetic Routes of Bacteriochlorophyllides
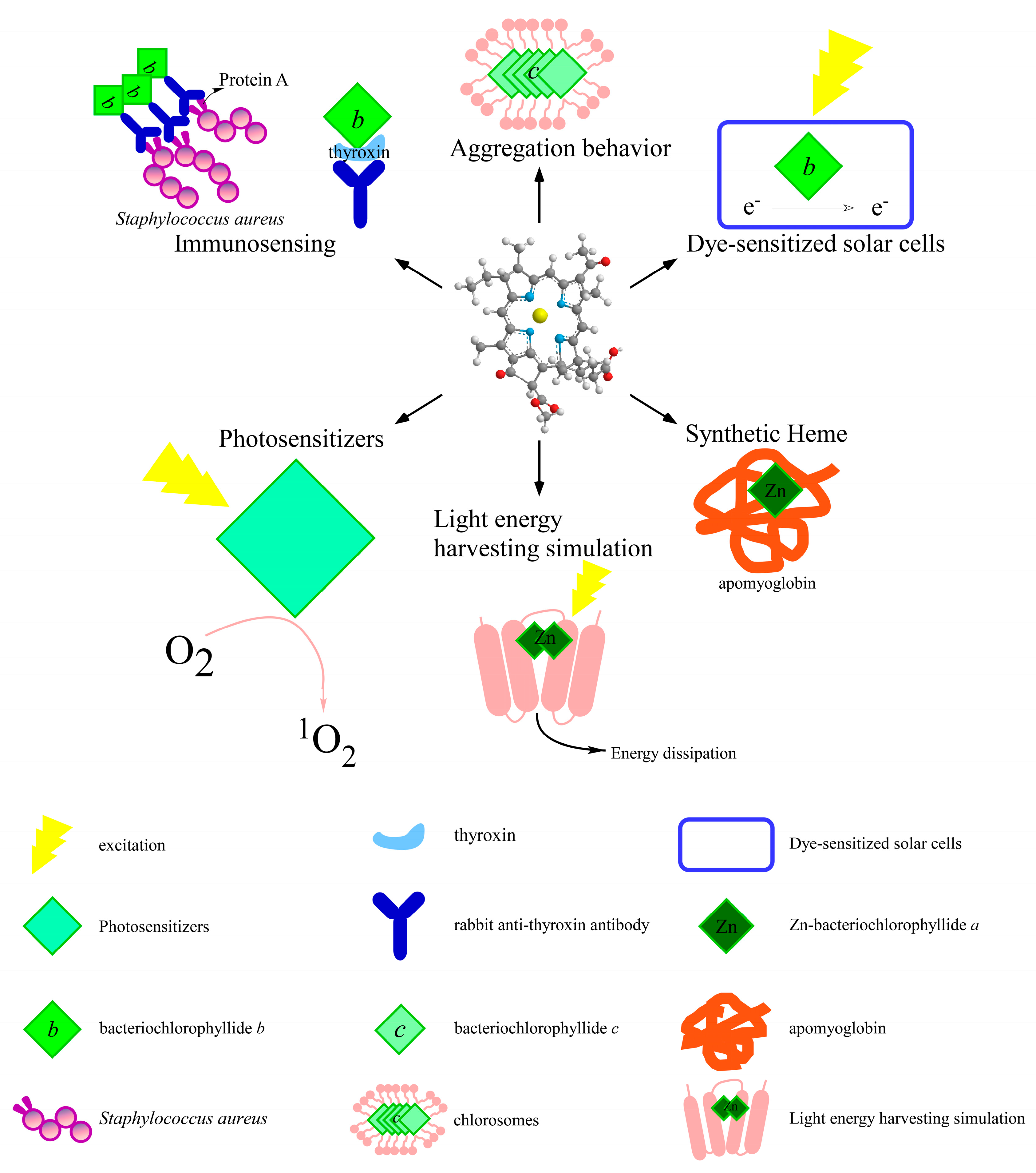
3. Applications
3.1. For Photosensitizers Application
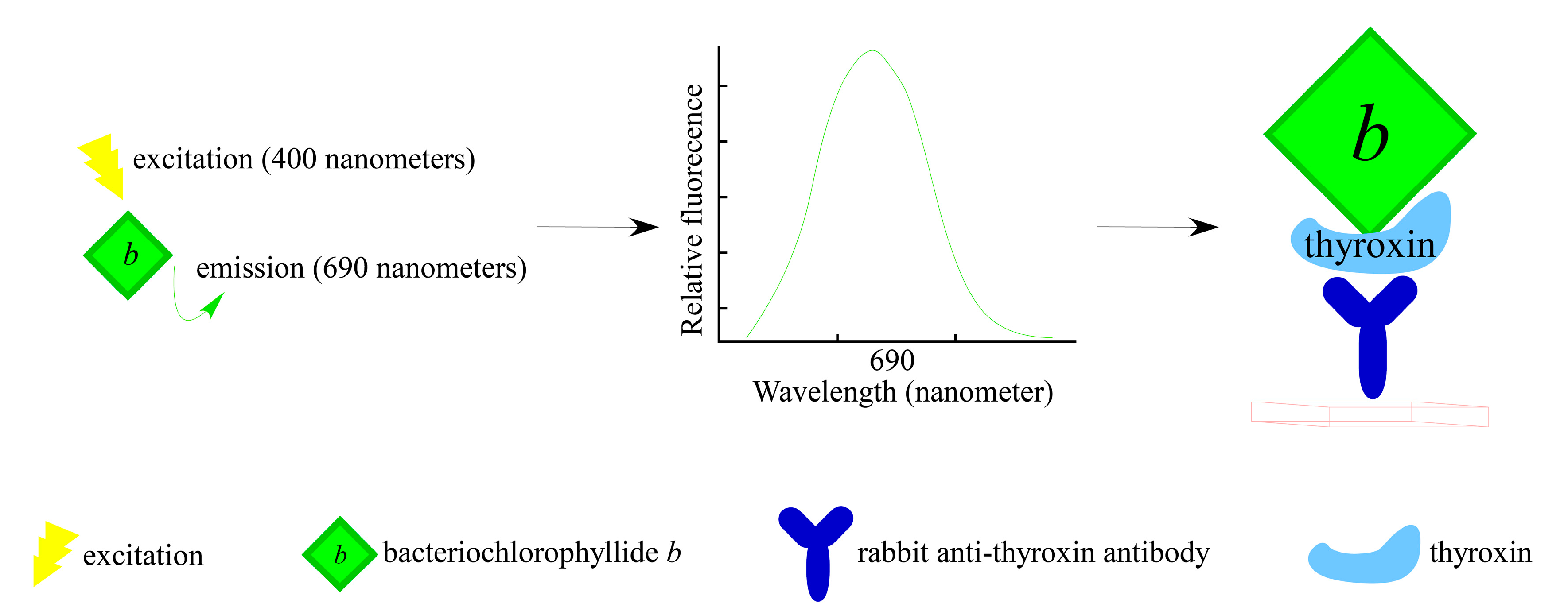
3.2. For Immunosensing Application
3.3. Influence on Bacteriochlorophyll Aggregation
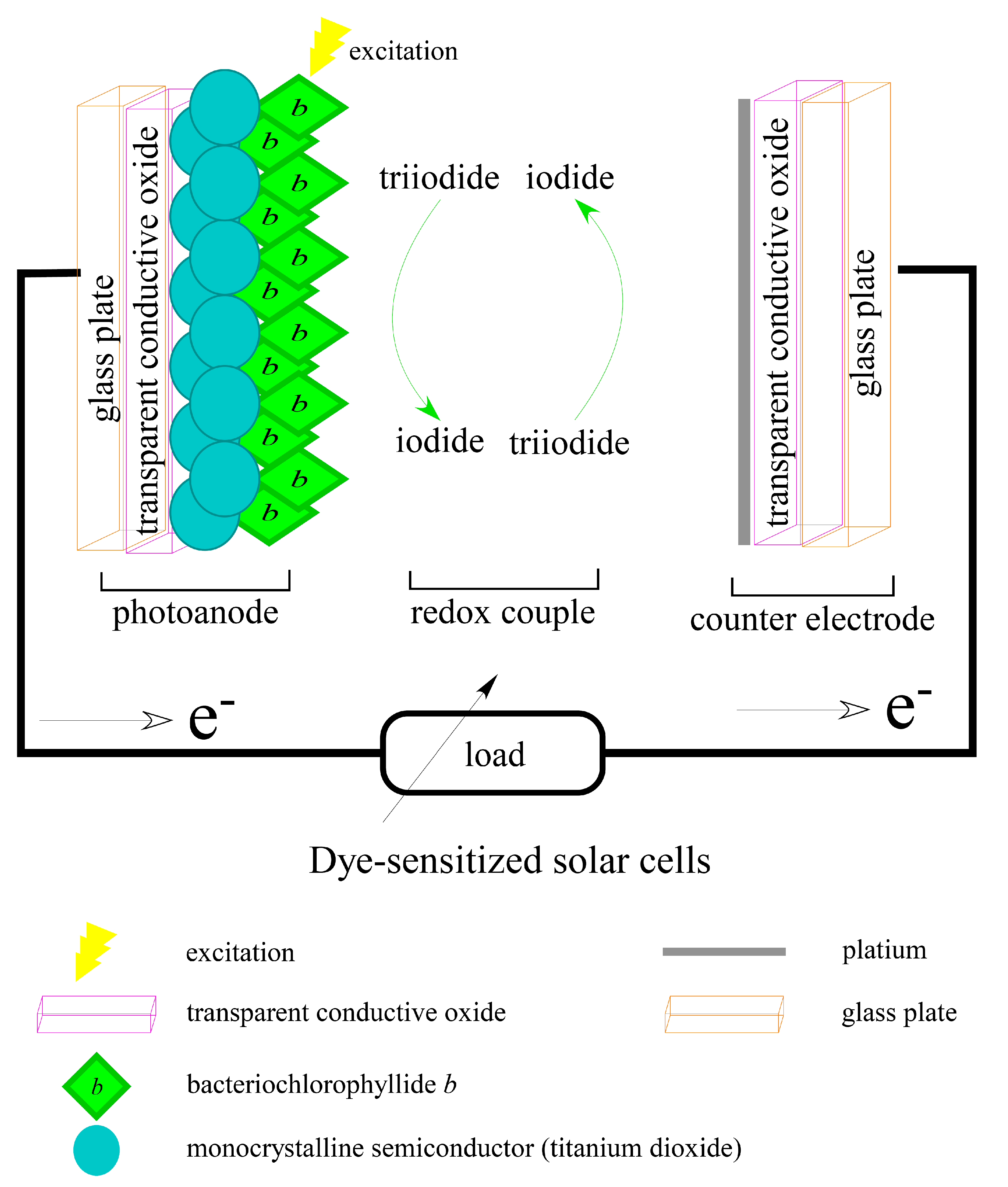
3.4. For Dye-Sensitized Solar Cells Application
3.5. For Heme Synthesis
3.6. For Light Energy Harvesting Simulation
3.7. Challenges and Future Perspectives
- a.
- New recombinant enzymes with high catalytic activity could be developed [10].
- b.
- Extraction, isolation and purification of bacteriochlorophyllides could be optimized.
- c.
- New preservation technology (nanotechnology) for bacteriochlorophyllides could be developed.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Thiel, V.; Tank, M.; Bryant, D.A. Diversity of chlorophototrophic bacteria revealed in the omics era. Annu. Rev. Plant Biol. 2018, 69, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.A.; Hunter, C.N.; Warren, M.J. Biosynthesis of the modified tetrapyrroles-the pigments of life. J. Biol. Chem. 2020, 295, 6888–6925. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef]
- Thweatt, J.L.; Canniffe, D.P.; Bryant, D.A. Chapter Two—Biosynthesis of chlorophylls and bacteriochlorophylls in green bacteria. In Advances in Botanical Research; Grimm, B., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 35–89. [Google Scholar]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef]
- Brown, A.E.; Eiserling, F.A.; Lascelles, J. Bacteriochlorophyll synthesis and the ultrastructure of wild type and mutant strains of Rhodopseudomonas spheroides. Plant Physiol. 1972, 50, 743–746. [Google Scholar] [CrossRef]
- Holt, A.S.; Jacobs, E.E. Spectroscopy of plant pigments. II. Methyl bacteriochlorophyllide and bacteriochlorophyll. Am. J. Bot. 1954, 41, 718–722. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Isaji, M.; Harada, J.; Tsukatani, Y.; Tamiaki, H. The 17-propionate esterifying variants of bacteriochlorophyll-a and bacteriopheophytin-a in purple photosynthetic bacteria. J. Photochem. Photobiol. B 2015, 142, 244–249. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, J.K. Competitive inhibitions of the chlorophyll synthase of Synechocystis sp. strain PCC 6803 by bacteriochlorophyllide a and the bacteriochlorophyll synthase of Rhodobacter sphaeroides by chlorophyllide a. J. Bacteriol. 2010, 192, 198–207. [Google Scholar] [CrossRef][Green Version]
- Chou, Y.L.; Lee, Y.L.; Yen, C.C.; Chen, L.F.; Lee, L.C.; Shaw, J.F. A novel recombinant chlorophyllase from cyanobacterium Cyanothece sp. ATCC 51142 for the production of bacteriochlorophyllide a. Biotechnol. Appl. Biochem. 2016, 63, 371–377. [Google Scholar] [CrossRef]
- Fischer, H.; Hasenkamp, J. Über die Konstitution des Farbstoffes der Purpurbakterien und über 9-Oxy-desoxo-phäoporphyrin a5. Justus Liebigs Ann. Chem. 1935, 515, 148–164. [Google Scholar] [CrossRef]
- Fischer, H.; Lambrecht, R. Über Bacteriochlorophyll a. (Vorläufige Mitteilung.). Biol. Chem. 1937, 249, I. [Google Scholar] [CrossRef]
- Eimhjellen, K.E.; Aasmundrud, O.; Jensen, A. A new bacterial chlorophyll. Biochem. Biophys. Res. Commun. 1963, 10, 232–236. [Google Scholar] [CrossRef]
- Holt, A.S. 4—Recently Characterized Chlorophylls. In The Chlorophylls; Vernon, L.P., Seely, G.R., Eds.; Academic Press: Cambridge, MA, USA, 1966; pp. 111–118. [Google Scholar]
- Gloe, A.; Pfennig, N.; Brockmann, H.; Trowitzsch, W. A new bacteriochlorophyll from brown-colored chlorobiaceae. Arch Microbiol. 1975, 102, 103–109. [Google Scholar] [CrossRef]
- Brockmann, H. Bacteriochlorophyll e: Structure and stereochemistry of a new type of chlorophyll from Chlorobiaceae. Philos. Trans. R. Soc. Lond. B 1976, 273, 277–285. [Google Scholar]
- Smith, K.M. Nomenclature of the bacteriochlorophylls c, d, and e. Photosynth. Res. 1994, 41, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Vogl, K.; Tank, M.; Orf, G.; Blankenship, R.; Bryant, D. Bacteriochlorophyll f: Properties of chlorosomes containing the “forbidden chlorophyll”. Front. Microbiol. 2012, 3, 298. [Google Scholar] [CrossRef]
- Harada, J.; Mizoguchi, T.; Tsukatani, Y.; Noguchi, M.; Tamiaki, H. A seventh bacterial chlorophyll driving a large light-harvesting antenna. Sci. Rep. 2012, 2, 671. [Google Scholar] [CrossRef] [PubMed]
- Orf, G.S.; Tank, M.; Vogl, K.; Niedzwiedzki, D.M.; Bryant, D.A.; Blankenship, R.E. Spectroscopic insights into the decreased efficiency of chlorosomes containing bacteriochlorophyll f. Biochim. Biophys. Acta 2013, 1827, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, H.; Lipinski, A. Bacteriochlorophyll g. A new bacteriochlorophyll from Heliobacterium chlorum. Arch. Microbiol. 1983, 136, 17–19. [Google Scholar] [CrossRef]
- Michalski, T.J.; Hunt, J.E.; Bowman, M.K.; Smith, U.; Bardeen, K.; Gest, H.; Norris, J.R.; Katz, J.J. Bacteriopheophytin g: Properties and some speculations on a possible primary role for bacteriochlorophylls b and g in the biosynthesis of chlorophylls. Proc. Natl. Acad. Sci. USA 1987, 84, 2570–2574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Lindsey, J.S. Total synthesis campaigns toward chlorophylls and related natural hydroporphyrins—Diverse macrocycles, unrealized opportunities. Nat. Prod. Rep. 2018, 35, 879–901. [Google Scholar] [CrossRef]
- Aksu, H.; Maiti, B.; Ptaszek, M.; Dunietz, B.D. Photoinduced charge transfer in Zn(II) and Au(III)-ligated symmetric and asymmetric bacteriochlorin dyads: A computational study. J. Chem. Phys. 2020, 153, 134111. [Google Scholar] [CrossRef]
- Chaudhri, N.; Zeller, M.; Brückner, C. Stepwise reduction of octaethyl-β,β’-dioxochlorin isomers: Access to structurally and electronically diverse hydroporphyrins. J. Org. Chem. 2020, 85, 13951–13964. [Google Scholar] [CrossRef]
- Gazit, E. From green bacteria to human dementia: A novel model for discovering amyloid assembly inhibitors. ACS Chem. Biol. 2006, 1, 417–419. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Synthetic chlorins, possible surrogates for chlorophylls, prepared by derivatization of porphyrins. Chem. Rev. 2017, 117, 344–535. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.G.; Bryant, D.A. Chlorophyll biosynthesis in bacteria: The origins of structural and functional diversity. Annu. Rev. Microbiol. 2007, 61, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, M. Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu. Rev. Biochem. 2014, 83, 317–340. [Google Scholar] [CrossRef]
- Zeng, Y.; Koblížek, M. Phototrophic Gemmatimonadetes: A new “purple” branch on the bacterial tree of life. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Hallenbeck, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 163–192. [Google Scholar]
- Katz, J.J.; Strain, H.H.; Harkness, A.L.; Studier, M.H.; Svec, W.A.; Janson, T.R.; Cope, B.T. Esterifying alcohols in the chlorophylls of purple photosynthetic bacteria. A new chlorophyll, bacteriochlorophyll (gg), all-trans-geranylgeranyl bacteriochlorophyllide a. J. Am. Chem. Soc. 1972, 94, 7938–7939. [Google Scholar] [CrossRef]
- Saga, Y.; Hayashi, K.; Mizoguchi, T.; Tamiaki, H. Biosynthesis of bacteriochlorophyll c derivatives possessing chlorine and bromine atoms at the terminus of esterifying chains in the green sulfur bacterium Chlorobaculum tepidum. J. Biosci. Bioeng. 2014, 118, 82–87. [Google Scholar] [CrossRef]
- Pierson, B.K.; Castenholz, R.W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch. Microbiol. 1974, 100, 5–24. [Google Scholar] [CrossRef]
- Gomez Maqueo Chew, A.; Frigaard, N.U.; Bryant, D.A. Bacteriochlorophyllide c C-8(2) and C-12(1) methyltransferases are essential for adaptation to low light in Chlorobaculum tepidum. J. Bacteriol. 2007, 189, 6176–6184. [Google Scholar] [CrossRef]
- Thweatt, J.L.; Ferlez, B.H.; Golbeck, J.H.; Bryant, D.A. BciD Is a Radical S-adenosyl-l-methionine (SAM) enzyme that completes bacteriochlorophyllide e biosynthesis by oxidizing a methyl group into a formyl group at C-7. J. Biol. Chem. 2017, 292, 1361–1373. [Google Scholar] [CrossRef]
- Tsukatani, Y.; Yamamoto, H.; Harada, J.; Yoshitomi, T.; Nomata, J.; Kasahara, M.; Mizoguchi, T.; Fujita, Y.; Tamiaki, H. An unexpectedly branched biosynthetic pathway for bacteriochlorophyll b capable of absorbing near-infrared light. Sci. Rep. 2013, 3, 1217. [Google Scholar] [CrossRef]
- Maresca, J.A.; Gomez Maqueo Chew, A.; Ponsatí, M.R.; Frigaard, N.U.; Ormerod, J.G.; Bryant, D.A. The bchU gene of Chlorobium tepidum encodes the c-20 methyltransferase in bacteriochlorophyll c biosynthesis. J. Bacteriol. 2004, 186, 2558–2566. [Google Scholar] [CrossRef]
- Wada, K.; Yamaguchi, H.; Harada, J.; Niimi, K.; Osumi, S.; Saga, Y.; Oh-oka, H.; Tamiaki, H.; Fukuyama, K. Crystal structures of BchU, a methyltransferase involved in bacteriochlorophyll c Biosynthesis, and its complex with S-adenosylhomocysteine: Implications for reaction mechanism. J. Mol. Biol. 2006, 360, 839–849. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Staron, J.; Boron, B.; Karcz, D.; Szczygieł, M.; Fiedor, L. Recent progress in chemical modifications of chlorophylls and bacteriochlorophylls for the applications in photodynamic therapy. Curr. Med. Chem. 2015, 22, 3054–3074. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Liu, T.W.; Huynh, E.; MacDonald, T.D.; Zheng, G. Chapter 14—Porphyrins for imaging, photodynamic therapy, and photothermal therapy. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Academic Press: Oxford, UK, 2014; pp. 229–254. [Google Scholar]
- Grin, M.C.; Capital Em Cyrillicironov, C.A.; Shtil, A.C. Bacteriochlorophyll a, and its derivatives: Chemistry and perspectives for cancer therapy. Anticancer Agents Med. Chem. 2008, 8, 683–697. [Google Scholar] [CrossRef]
- Moore, C.M.; Pendse, D.; Emberton, M. Photodynamic therapy for prostate cancer—A review of current status and future promise. Nat. Clin. Pract. Urol. 2009, 6, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Escrig, J.L.; Casanova Ramón-Borja, J. Focal laser ablation and photodynamic vascular therapy with soluble TOOKAD® in the treatment of low risk prostate cancer. Arch. Esp. Urol. 2016, 69, 327–336. [Google Scholar]
- Kawczyk-Krupka, A.; Wawrzyniec, K.; Musiol, S.K.; Potempa, M.; Bugaj, A.M.; Sieroń, A. Treatment of localized prostate cancer using WST-09 and WST-11 mediated vascular targeted photodynamic therapy—A review. Photodiagnosis Photodyn. Ther. 2015, 12, 567–574. [Google Scholar] [CrossRef]
- Scherz, A.; Salomon, Y.; Fiedor, L. Chlorophyll and Bacteriochlorophyll Derivatives and Pharmaceutical Compositions Containing Them. AU674315B2, 26 July 1992. [Google Scholar]
- Brun, P.H.; Prudhomme, A.; Barnoux, J.L. Injectable Galenic Formulation for Use in a Diagnosis or a Photodynamic Therapy and Method for Preparing Same. EP1406616B1, 17 July 2001. [Google Scholar]
- Doron Eren, J.E.B.; Karine, N.; Goni, B.-A. Injectable Formulation for Photodynamic Therapy. WO2009000504A2, 25 June 2007. [Google Scholar]
- Avigdor Scherz, Y.S.; Brandis, A.; Scheer, H. Method for Tumor Diagnosis Comprising Administering of palladium-Substituted Bacteriochlorophyll Derivatives. US7045117B2, 16 May 2006. [Google Scholar]
- Brandis, A.S.; Salomon, Y.; Scherz, A. Bacteriochlorophyll sensitizers in photodynamic therapy. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 485–494. [Google Scholar]
- Neuschmelting, V.; Kim, K.; Malekzadeh-Najafabadi, J.; Jebiwott, S.; Prakash, J.; Scherz, A.; Coleman, J.A.; Kircher, M.F.; Ntziachristos, V. WST11 vascular targeted photodynamic therapy effect monitoring by multispectral optoacoustic tomography (MSOT) in mice. Theranostics 2018, 8, 723–734. [Google Scholar] [CrossRef]
- Noweski, A.; Roosen, A.; Lebdai, S.; Barret, E.; Emberton, M.; Benzaghou, F.; Apfelbeck, M.; Gaillac, B.; Gratzke, C.; Stief, C.; et al. Medium-term follow-up of vascular-targeted photodynamic therapy of localized prostate cancer using TOOKAD Soluble WST-11 (Phase II trials). Eur. Urol. Focus 2019, 5, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Aldahlawi, N.; Marcovich, A.L.; Brekelmans, J.; Goz, A.; Scherz, A.; Young, R.D.; Bell, J.S.; O’Brart, D.P.; Nuijts, R.; et al. The effect of bacteriochlorophyll derivative WST-D and near infrared light on the molecular and fibrillar architecture of the corneal stroma. Sci. Rep. 2020, 10, 9836. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Kumar, S.; Srivastava, A.; Maurya, P.K.; Singh, R.; Chandra, P. Chapter 14—Electrochemical immunosensors: Fundamentals and applications in clinical diagnostics. In Handbook of Immunoassay Technologies; Vashist, S.K., Luong, J.H.T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 359–414. [Google Scholar]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Hendrix, J.L. Fluorescent Chlorophyll Labeled Assay Reagents. US4707454A, 10 August 1981. [Google Scholar]
- Gross, S.; Brandis, A.; Chen, L.; Rosenbach-Belkin, V.; Roehrs, S.; Scherz, A.; Salomon, Y. Protein-A-mediated targeting of bacteriochlorophyll-IgG to Staphylococcus aureus: A model for enhanced site-specific photocytotoxicity. Photochem. Photobiol. 1997, 66, 872–878. [Google Scholar] [CrossRef]
- Fiedor, L.; Salomon, Y.; Scherz, A. Chlorophyll and Bacteriochlorophyll Derivatives, their Preparation and Pharmaceutical Compositions Comprising Them. US6740637B1, 3 March 1992. [Google Scholar]
- Eichwurzel, I.; Stiel, H.; Teuchner, K.; Leupold, D.; Scheer, H.; Salomon, Y.; Scherz, A. Photophysical consequences of coupling bacteriochlorophyll a with serine and its resulting solubility in water. Photochem. Photobiol. 2000, 72, 204–209. [Google Scholar] [CrossRef]
- Zilberstein, J.; Schreiber, S.; Bloemers, M.C.; Bendel, P.; Neeman, M.; Schechtman, E.; Kohen, F.; Scherz, A.; Salomon, Y. Antivascular treatment of solid melanoma tumors with bacteriochlorophyll-serine-based photodynamic therapy. Photochem. Photobiol. 2001, 73, 257–266. [Google Scholar] [CrossRef]
- Kelleher, D.K.; Thews, O.; Scherz, A.; Salomon, Y.; Vaupel, P. Combined hyperthermia and chlorophyll-based photodynamic therapy: Tumour growth and metabolic microenvironment. Br. J. Cancer 2003, 89, 2333–2339. [Google Scholar] [CrossRef]
- Zupcanova, A.; Arellano, J.B.; Bina, D.; Kopecky, J.; Psencik, J.; Vacha, F. The length of esterifying alcohol affects the aggregation properties of chlorosomal bacteriochlorophylls. Photochem. Photobiol. 2008, 84, 1187–1194. [Google Scholar] [CrossRef]
- Psencík, J.; Torkkeli, M.; Zupcanová, A.; Vácha, F.; Serimaa, R.E.; Tuma, R. The lamellar spacing in self-assembling bacteriochlorophyll aggregates is proportional to the length of the esterifying alcohol. Photosynth. Res. 2010, 104, 211–219. [Google Scholar] [CrossRef]
- Tamiaki, H.; Minqiu, J.; Tsukatani, Y.; Tsukaya, Y. New Enzyme and Method for Producing Bacteriochlorophyll Using the Same. JP2014003938A, 22 June 2012. [Google Scholar]
- Gratzel, M.; O’Regan, B. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Ghann, W.; Kang, H.; Sheikh, T.; Yadav, S.; Chavez-Gil, T.; Nesbitt, F.; Uddin, J. Fabrication, optimization and characterization of natural dye sensitized solar cell. Sci. Rep. 2017, 7, 41470. [Google Scholar] [CrossRef] [PubMed]
- Birel, Ö.; Nadeem, S.; Duman, H. Porphyrin-based dye-sensitized solar cells (DSSCs): A Review. J. Fluoresc. 2017, 27, 1075–1085. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundara Prabhu, R.; Prasanna, S.; Mallick, T.K.; Senthilarasu, S. Review on natural dye sensitized solar cells: Operation, materials and methods. Renew. Sust. Energ. Rev. 2015, 51, 1306–1325. [Google Scholar] [CrossRef]
- Wright, K.A.; Boxer, S.G. Solution properties of synthetic chlorophyllide--and bacteriochlorophyllide--apomyoglobin complexes. Biochemistry 1981, 20, 7546–7556. [Google Scholar] [CrossRef]
- Marković, D.; Pröll, S.; Bubenzer, C.; Scheer, H. Myoglobin with chlorophyllous chromophores: Influence on protein stability. Biochim. Biophys. Acta 2007, 1767, 897–904. [Google Scholar] [CrossRef]
- Cohen-Ofri, I.; van Gastel, M.; Grzyb, J.; Brandis, A.; Pinkas, I.; Lubitz, W.; Noy, D. Zinc-bacteriochlorophyllide dimers in de novo designed four-helix bundle proteins. A model system for natural light energy harvesting and dissipation. J. Am. Chem. Soc. 2011, 133, 9526–9535. [Google Scholar] [CrossRef]
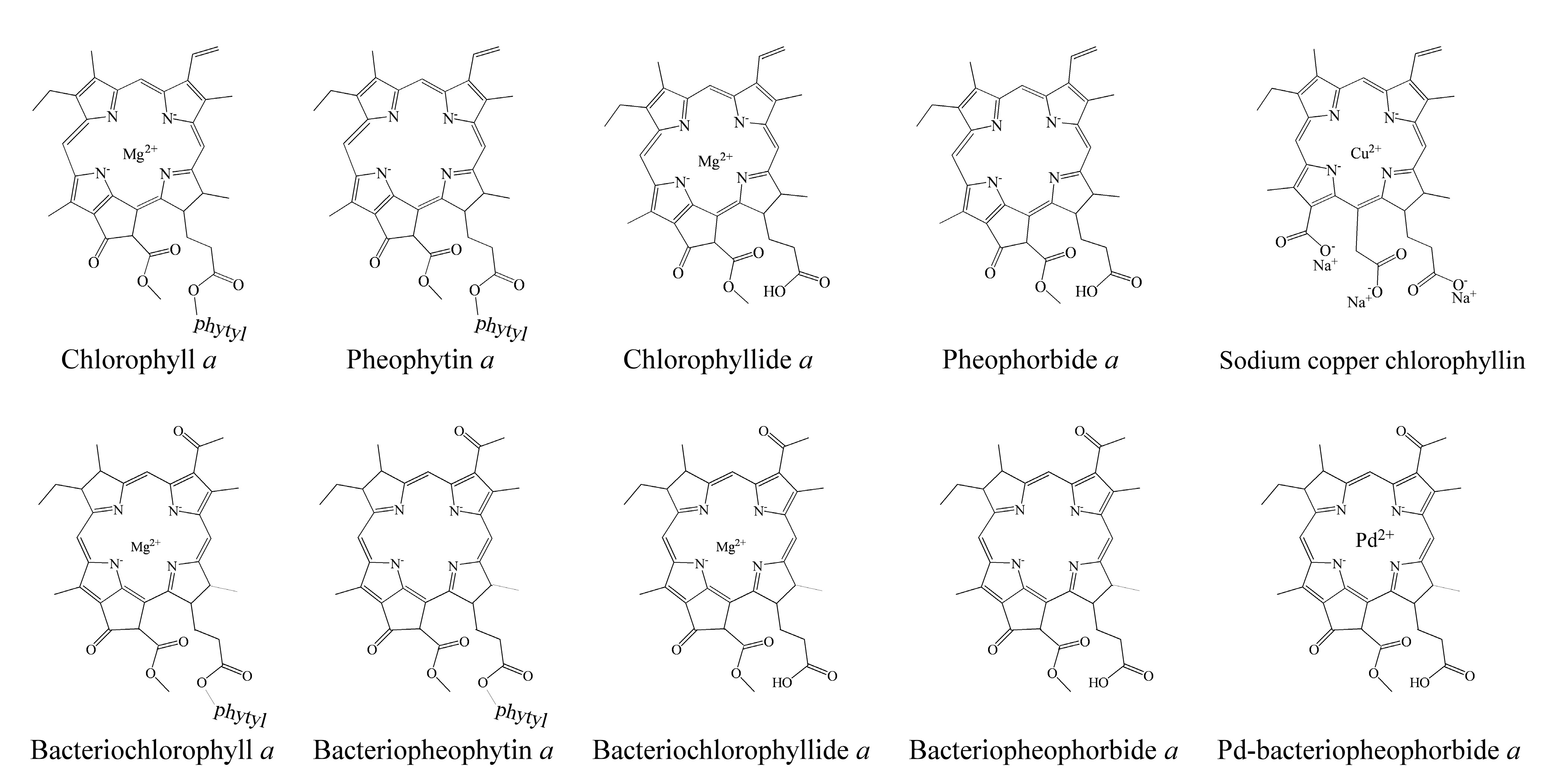
| Name | Molecular Formula | Molecular Weight (g/mol) | Ring Structure | Structure |
|---|---|---|---|---|
| Bacteriochlorophyllide a | C35H36MgN4O6 | 633 | Bacteriochlorin | 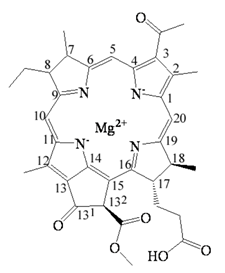 |
| Bacteriochlorophyllide b | C35H34MgN4O6 | 631 | Bacteriochlorin | 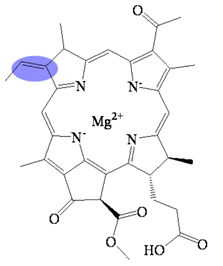 |
| Bacteriochlorophyllide c | C31H28MgN4O4(R1)(R2) | 559 (#) | Chlorin | 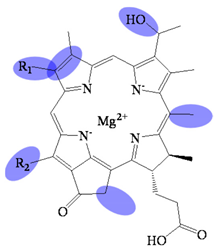 |
| Bacteriochlorophyllide d | C33H30MgN4O3(R1)(R2) | 545 (#) | Chlorin |  |
| Bacteriochlorophyllide e | C31H26MgN4O5(R1)(R2) | 572 (#) | Chlorin | 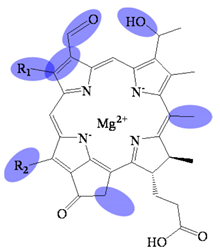 |
| Bacteriochlorophyllide f | C31H26MgN4O5(R1)(R2) | 559 (#) | Chlorin | 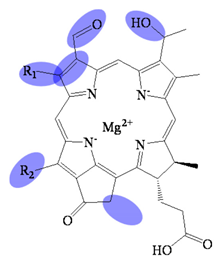 |
| Bacteriochlorophyllide g | C35H34MgN4O5 | 615 | Bacteriochlorin | 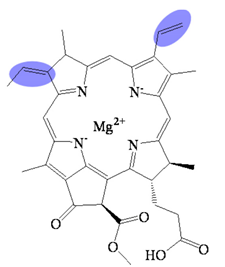 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Huang, K.-S.; Wang, Y.-T.; Shaw, J.-F. A Review of Bacteriochlorophyllides: Chemical Structures and Applications. Molecules 2021, 26, 1293. https://doi.org/10.3390/molecules26051293
Yang C-H, Huang K-S, Wang Y-T, Shaw J-F. A Review of Bacteriochlorophyllides: Chemical Structures and Applications. Molecules. 2021; 26(5):1293. https://doi.org/10.3390/molecules26051293
Chicago/Turabian StyleYang, Chih-Hui, Keng-Shiang Huang, Yi-Ting Wang, and Jei-Fu Shaw. 2021. "A Review of Bacteriochlorophyllides: Chemical Structures and Applications" Molecules 26, no. 5: 1293. https://doi.org/10.3390/molecules26051293
APA StyleYang, C.-H., Huang, K.-S., Wang, Y.-T., & Shaw, J.-F. (2021). A Review of Bacteriochlorophyllides: Chemical Structures and Applications. Molecules, 26(5), 1293. https://doi.org/10.3390/molecules26051293









