New Coumarin Dipicolinate Europium Complexes with a Rich Chemical Speciation and Tunable Luminescence
Abstract
1. Introduction
2. Results
2.1. Ligands and Tris Complexes Synthesis
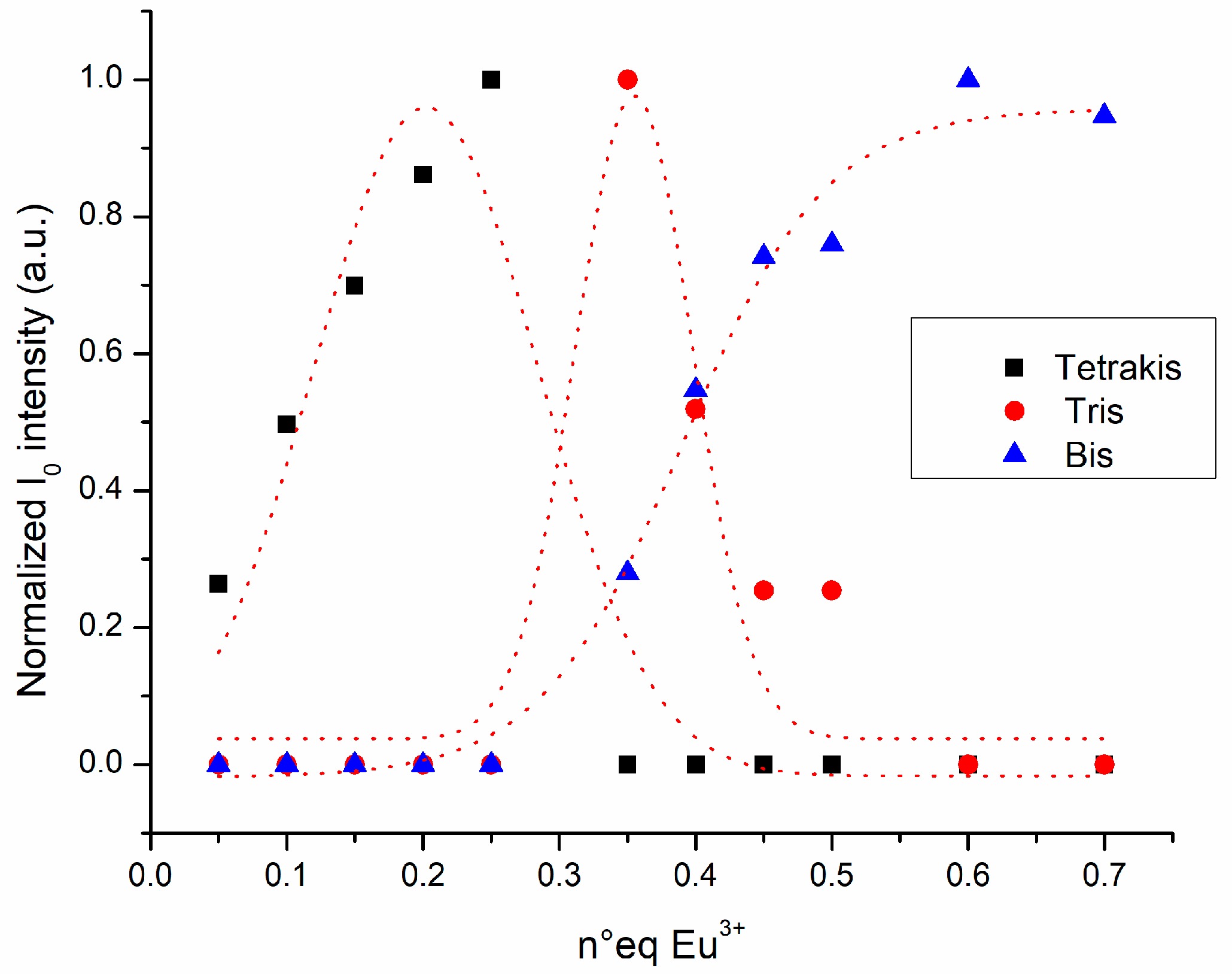
2.2. Chemical Speciation of the Eu/L1/2 System
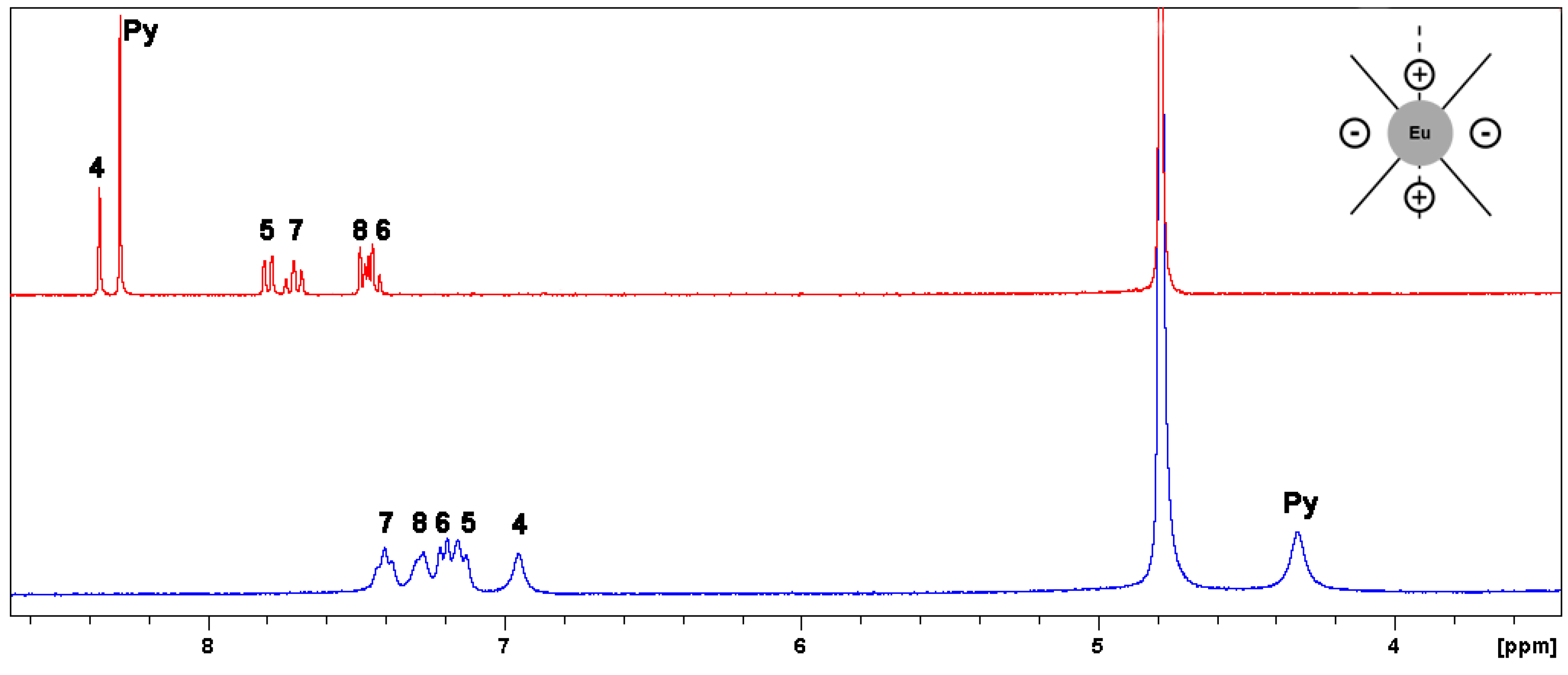
2.2.1. Paramagnetic NMR
2.2.2. Emission Spectroscopy and HMRS
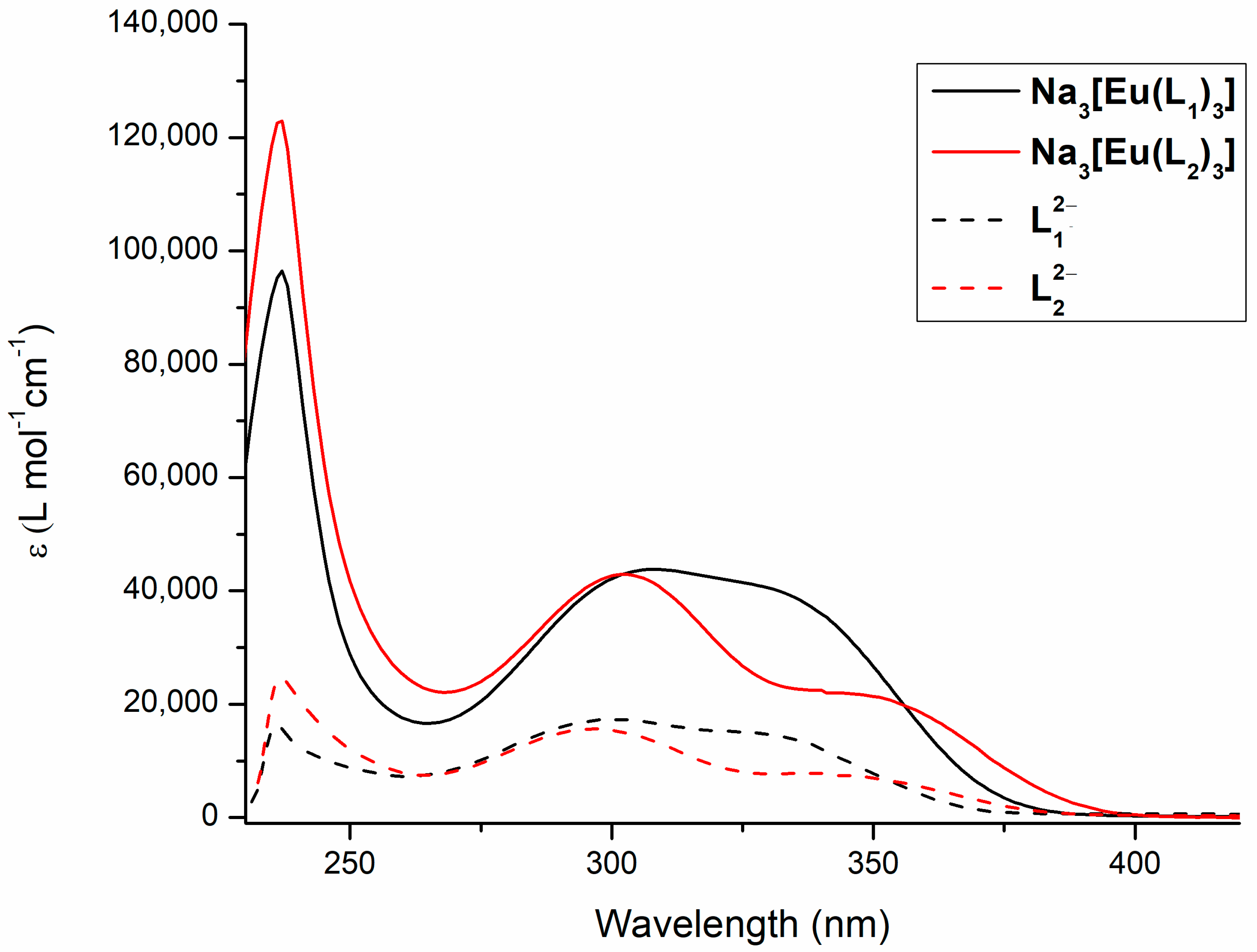
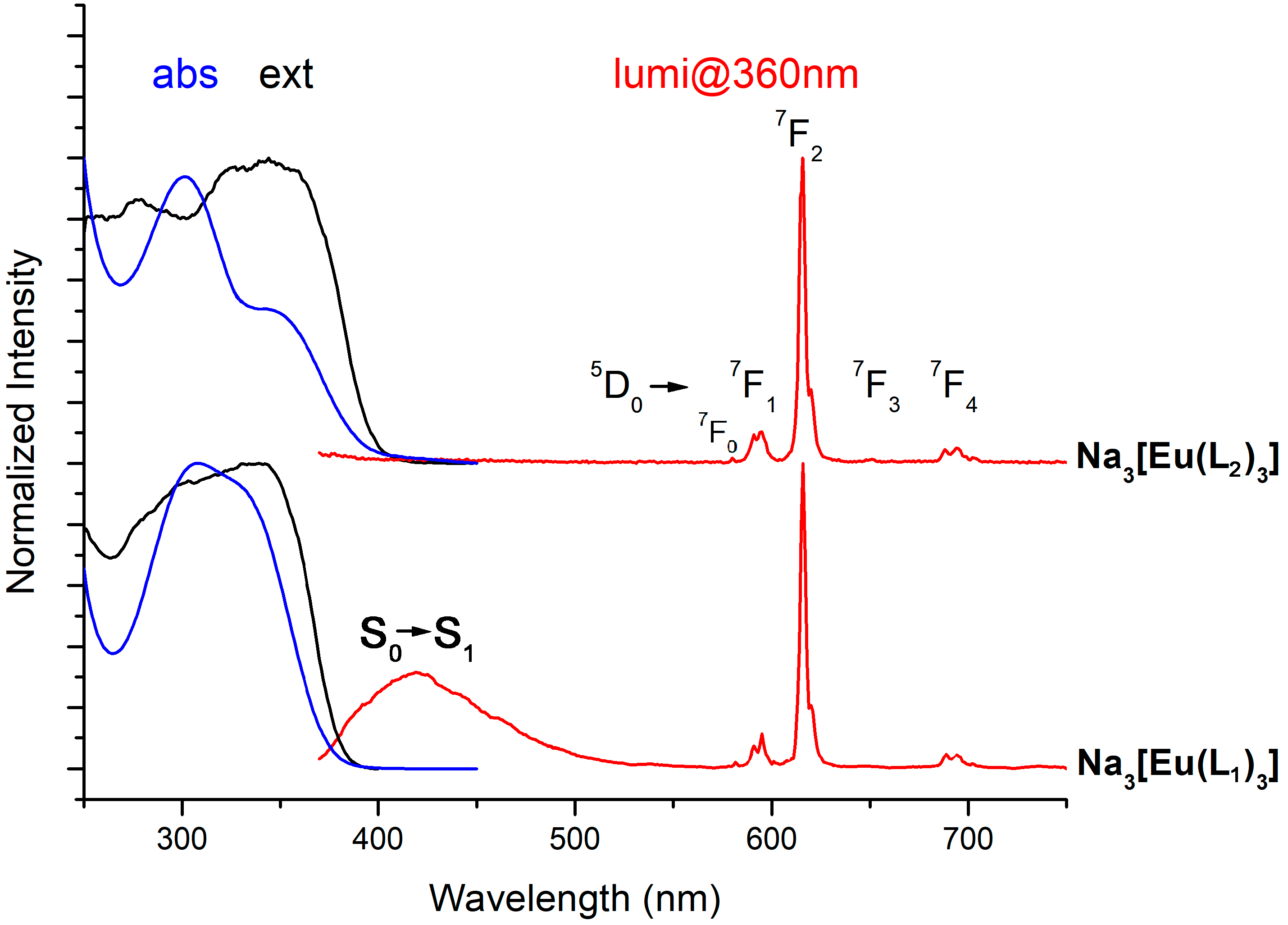
2.3. Photophysics of Na3[Eu(L1)3] and Na3[Eu(L2)3] Complexes
3. Materials and Methods
3.1. Synthetic Procedures and Characterization
3.2. NMR Titration of L12− with Eu3+ in D2O
3.3. NMR Titration of L12− with Eu3+ in (CD3)2SO
3.4. Fluorescence/Lifetime Titration of L12− with Eu3+ in Tris HCl
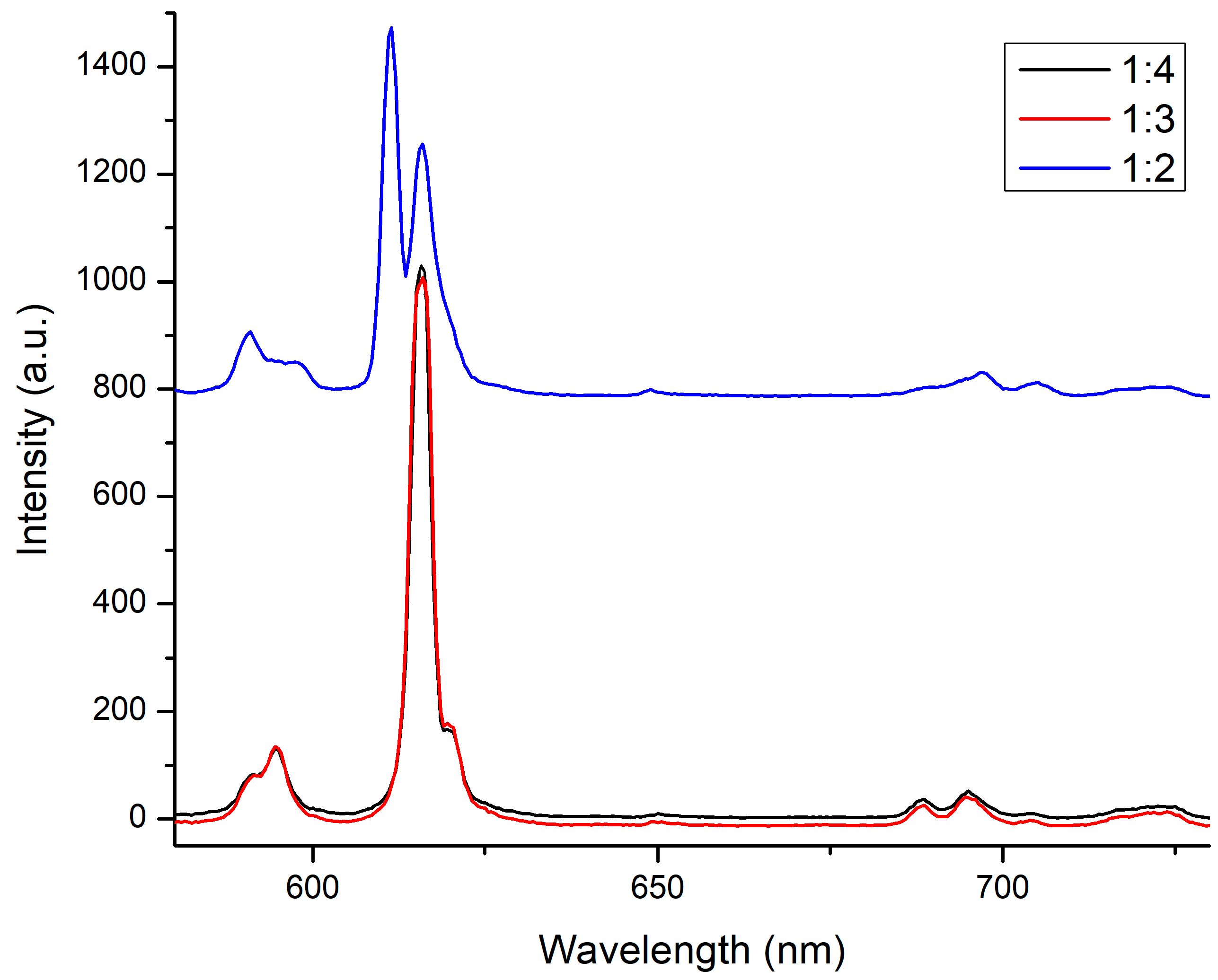
3.5. Fluorescence Spectra in DMSO of Eu3+/L1/22− Mixture of 1:2, 1:3 and 1:4 Molar Ratios
3.6. Absorption and Emission Spectra of L12−, L22−, Na3[Eu(L1)3] and Na3[Eu(L2)3]
3.7. Quantum Yield Determination of Na3[Eu(L1)3] and Na3[Eu(L2)3] Complexes
3.8. Sensitization Parameter (ηsens) for Na3[Eu(L1)3] and Na3[Eu(L2)3] Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De Bettencourt-Dias, A. Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials; de Bettencourt-Dias, A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2014; ISBN 9781118682760. [Google Scholar]
- Wei, C.; Ma, L.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Advances in luminescent lanthanide complexes and applications. Sci. China Technol. Sci. 2018, 61, 1265–1285. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.; Li, Z.; Lv, X.; Liang, L.; Yuan, Q. Recent Progress in Time-Resolved Biosensing and Bioimaging Based on Lanthanide-Doped Nanoparticles. Small 2019, 15, 1804969. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing. Coord. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide Probes for Bioresponsive Imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Signore, G.; Nifosì, R.; Albertazzi, L.; Bizzarri, R. A Novel Coumarin Fluorescent Sensor to Probe Polarity Around Biomolecules. J. Biomed. Nanotechnol. 2009, 5, 722–729. [Google Scholar] [CrossRef]
- Signore, G.; Nifosì, R.; Albertazzi, L.; Storti, B.; Bizzarri, R. Polarity-Sensitive Coumarins Tailored to Live Cell Imaging. J. Am. Chem. Soc. 2010, 132, 1276–1288. [Google Scholar] [CrossRef]
- Iacopini, D.; Moscardini, A.; Lessi, F.; Di Bussolo, V.; Di Pietro, S.; Signore, G. Coumarin-based fluorescent biosensor with large linear range for ratiometric measurement of intracellular pH. Bioorg. Chem. 2020, 105, 104372. [Google Scholar] [CrossRef]
- Roh, S.G.; Baek, N.S.; Hong, K.S.; Kim, H.K. Synthesis and Photophysical Properties of Luminescent Lanthanide Complexes Based on Coumarin-3-carboxylic Acid for Advanced Photonic Applications. Bull. Korean Chem. Soc. 2004, 25, 343–344. [Google Scholar] [CrossRef][Green Version]
- Borbas, K.E.; Bruce, J.I. Synthesis of asymmetrically substituted cyclen-based ligands for the controlled sensitisation of lanthanides. Org. Biomol. Chem. 2007, 5, 2274–2282. [Google Scholar] [CrossRef]
- Hanna, J.R.; Allan, C.; Lawrence, C.; Meyer, O.; Wilson, N.D.; Hulme, A.N. Optimizing the Readout of Lanthanide-DOTA Complexes for the Detection of Ligand-Bound Copper(I). Molecules 2017, 22, 802. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Méndez, Ó.; González, F.; Bernès, S.; Flores-Álamo, M.; Ordóñez-Hernández, J.; García-Ortega, H.; Guerrero, J.; Qian, W.; Aliaga-Alcalde, N.; Gasque, L. Coumarin Derivative Directly Coordinated to Lanthanides Acts as an Excellent Antenna for UV–Vis and Near-IR Emission. Inorg. Chem. 2018, 57, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Junker, A.K.R.; Hill, L.R.; Thompson, A.L.; Faulkner, S.; Sørensen, T.J. Shining light on the antenna chromophore in lanthanide based dyes. Dalton Trans. 2018, 47, 4794–4803. [Google Scholar] [CrossRef]
- Kiraev, S.R.; Mathieu, E.; Siemens, F.; Kovacs, D.; Demeyere, E.; Borbas, K.E. Lanthanide(III) Complexes of Cyclen Triacetates and Triamides Bearing Tertiary Amide-Linked Antennae. Molecules 2020, 25, 5282. [Google Scholar] [CrossRef] [PubMed]
- Féau, C.; Klein, E.; Kerth, P.; Lebeau, L. Synthesis of a coumarin-based europium complex for bioanalyte labeling. Bioorg. Med. Chem. Lett. 2007, 17, 1499–1503. [Google Scholar] [CrossRef]
- Andres, J.; Chauvin, A.-S. Europium Complexes of Tris(dipicolinato) Derivatives Coupled to Methylumbelliferone: A Double Sensitization. Eur. J. Inorg. Chem. 2010, 18, 2700–2713. [Google Scholar] [CrossRef]
- Szíjjártó, C.; Pershagen, E.; Ilchenko, N.O.; Borbas, K.E. A Versatile Long-Wavelength-Absorbing Scaffold for Eu-Based Responsive Probes. Chem. Eur. J. 2013, 19, 3099–3109. [Google Scholar] [CrossRef]
- Andres, J.; Chauvin, A.-S. Energy transfer in coumarin-sensitised lanthanide luminescence: Investigation of the nature of the sensitiser and its distance to the lanthanide ion. Phys. Chem. Chem. Phys. 2013, 15, 15981–15994. [Google Scholar] [CrossRef]
- Georgieva, I.; Mihaylov, T.; Trendafilova, N. Lanthanide and transition metal complexes of bioactive coumarins: Molecular modeling and spectroscopic studies. J. Inorg. Biochem. 2014, 135, 100–112. [Google Scholar] [CrossRef]
- Andres, J.; Borbas, K.E. Expanding the Versatility of Dipicolinate-Based Luminescent Lanthanide Complexes: A Fast Method for Antenna Testing. Inorg. Chem. 2015, 54, 8174–8176. [Google Scholar] [CrossRef]
- Kovacs, D.; Lu, X.; Mészáros, L.S.; Ott, M.; Andres, J.; Borbas, K.E. Photophysics of Coumarin and Carbostyril-Sensitized Luminescent Lanthanide Complexes: Implications for Complex Design in Multiplex Detection. J. Am. Chem. Soc. 2017, 139, 5756–5767. [Google Scholar] [CrossRef] [PubMed]
- Vithanarachchi, S.M.; Kovacs, D.; Borbas, K.E. Synthesis and photophysical characterization of luminescent lanthanide complexes of nucleotide-functionalized cyclen- and dipicolinate-based ligands. Inorg. Chim. Acta 2017, 460, 148–158. [Google Scholar] [CrossRef]
- Donato, H.; Martin, R.B. Dipolar shifts and structure in aqueous solutions of 3:1 lanthanide complexes of 2,6-dipicolinate. J. Am. Chem. Soc. 1972, 94, 4129–4131. [Google Scholar] [CrossRef]
- Desreux, J.F.; Reilley, C.N. Evaluation of contact and dipolar contributions to proton and carbon-13 paramagnetic NMR shifts in axially symmetric lanthanide chelates. J. Am. Chem. Soc. 1976, 98, 2105–2109. [Google Scholar] [CrossRef]
- Alsaadi, B.M.; Rossotti, F.J.C.; Williams, R.J.P. Structure of lanthanide(III) mono- and bis-dipicolinates in solution. J. Chem. Soc. Chem. Commun. 1977, 527–529. [Google Scholar] [CrossRef]
- An, Y.; Berry, M.T.; van Veggel, F.C.J.M. Aqueous Solutions of Europium(III) Dipicolinate Complexes: Estimates of Water Coordination Based on Molecular Dynamics Simulations and Excited State Decay Rate Constants. J. Phys. Chem. A 2000, 104, 11243–11247. [Google Scholar] [CrossRef]
- Ouali, N.; Bocquet, B.; Rigault, S.; Morgantini, P.-Y.; Weber, J.; Piguet, C. Analysis of Paramagnetic NMR Spectra of Triple-Helical Lanthanide Complexes with 2,6-Dipicolinic Acid Revisited: A New Assignment of Structural Changes and Crystal-Field Effects 25 Years Later. Inorg. Chem. 2002, 41, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, P.R.; Kofod, N.; Juelsholt, M.; Jensen, K.M.Ø.; Sørensen, T.J. The effect of weighted averages when determining the speciation and structure–property relationships of europiumIII dipicolinate complexes. Phys. Chem. Chem. Phys. 2020, 22, 12794–12805. [Google Scholar] [CrossRef]
- Di Bari, L.; Salvadori, P. Solution structure of chiral lanthanide complexes. Coord. Chem. Rev. 2005, 249, 2854–2879. [Google Scholar] [CrossRef]
- Di Pietro, S.; Piano, S.L.; Di Bari, L. Pseudocontact shifts in lanthanide complexes with variable crystal field parameters. Coord. Chem. Rev. 2011, 255, 2810–2820. [Google Scholar] [CrossRef]
- Di Bari, L.; Di Pietro, S.; Pescitelli, G.; Tur, F.; Mansilla, J.; Saá, J.M. [Ln(binolam)3] (OTf)3, a New Class of Propeller-Shaped Lanthanide(III) Salt Complexes as Enantioselective Catalysts: Structure, Dynamics and Mechanistic Insight. Chem. Eur. J. 2010, 16, 14190–14201. [Google Scholar] [CrossRef]
- Di Pietro, S.; Di Bari, L. The structure of MLn(hfbc)4 and a key to high circularly polarized luminescence. Inorg. Chem. 2012, 51, 12007–12014. [Google Scholar] [CrossRef] [PubMed]
- Freund, C.; Porzio, W.; Giovanella, U.; Vignali, F.; Pasini, M.; Destri, S.; Mech, A.; Di Pietro, S.; Di Bari, L.; Mineo, P. Thiophene Based Europium β-Diketonate Complexes: Effect of the Ligand Structure on the Emission Quantum Yield. Inorg. Chem. 2011, 50, 5417–5429. [Google Scholar] [CrossRef]
- Di Pietro, S.; Imbert, D.; Mazzanti, M. An efficient triazole-pyridine-bistetrazolate platform for highly luminescent lanthanide complexes. Chem. Commun. 2014, 50, 10323–10326. [Google Scholar] [CrossRef]
- Di Pietro, S.; Gautier, N.; Imbert, D.; Pécaut, J.; Mazzanti, M. Versatile pyridine-2,6-bis-tetrazolate scaffolds for the formation of highly luminescent lanthanide complexes. Dalton Trans. 2016, 45, 3429–3442. [Google Scholar] [CrossRef]
- Gendron, F.; Di Pietro, S.; Abad Galán, L.; Riobé, F.; Placide, V.; Guy, L.; Zinna, F.; Di Bari, L.; Bensalah-Ledoux, A.; Guyot, Y.; et al. Luminescence, chiroptical, magnetic and ab initio crystal-field characterizations of an enantiopure helicoidal Yb (III) complex. Inorg. Chem. Front. 2021, 8, 914–926. [Google Scholar] [CrossRef]
- Mcconnell, H.M.; Robertson, R.E. Isotropic nuclear resonance shifts. J. Chem. Phys. 1958, 29, 1361–1365. [Google Scholar] [CrossRef]
- Bleaney, B. Nuclear magnetic resonance shifts in solution due to lanthanide ions. J. Magn. Reson. 1972, 8, 91–100. [Google Scholar] [CrossRef]
- Harrowfield, J.; Kim, Y.; Skelton, B.; White, A. Mixed Transition Metal/Lanthanide Complexes: Structural Characterization of Solids Containing Cage Amine Chromium(III) Cations and Tris(dipicolinato)lanthanide Anions. Aust. J. Chem. 1995, 48, 807–823. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 9780470010082. [Google Scholar]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Li, L.-D.; Yang, S.-Z. Room temperature phosphorescence properties of 27 coumarin derivatives on filter paper. Anal. Chim. Acta 1994, 296, 99–105. [Google Scholar] [CrossRef]
- Horrocks, W.; Sudnick, D. Time-resolved europium(III) excitation spectroscopy: A luminescence probe of metal ion binding sites. Science 1979, 206, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietro, S.; Iacopini, D.; Moscardini, A.; Bizzarri, R.; Pineschi, M.; Di Bussolo, V.; Signore, G. New Coumarin Dipicolinate Europium Complexes with a Rich Chemical Speciation and Tunable Luminescence. Molecules 2021, 26, 1265. https://doi.org/10.3390/molecules26051265
Di Pietro S, Iacopini D, Moscardini A, Bizzarri R, Pineschi M, Di Bussolo V, Signore G. New Coumarin Dipicolinate Europium Complexes with a Rich Chemical Speciation and Tunable Luminescence. Molecules. 2021; 26(5):1265. https://doi.org/10.3390/molecules26051265
Chicago/Turabian StyleDi Pietro, Sebastiano, Dalila Iacopini, Aldo Moscardini, Ranieri Bizzarri, Mauro Pineschi, Valeria Di Bussolo, and Giovanni Signore. 2021. "New Coumarin Dipicolinate Europium Complexes with a Rich Chemical Speciation and Tunable Luminescence" Molecules 26, no. 5: 1265. https://doi.org/10.3390/molecules26051265
APA StyleDi Pietro, S., Iacopini, D., Moscardini, A., Bizzarri, R., Pineschi, M., Di Bussolo, V., & Signore, G. (2021). New Coumarin Dipicolinate Europium Complexes with a Rich Chemical Speciation and Tunable Luminescence. Molecules, 26(5), 1265. https://doi.org/10.3390/molecules26051265








