A Simple Turn-off Schiff Base Fluorescent Sensor for Copper (II) Ion and Its Application in Water Analysis
Abstract
1. Introduction
2. Results and Discussion
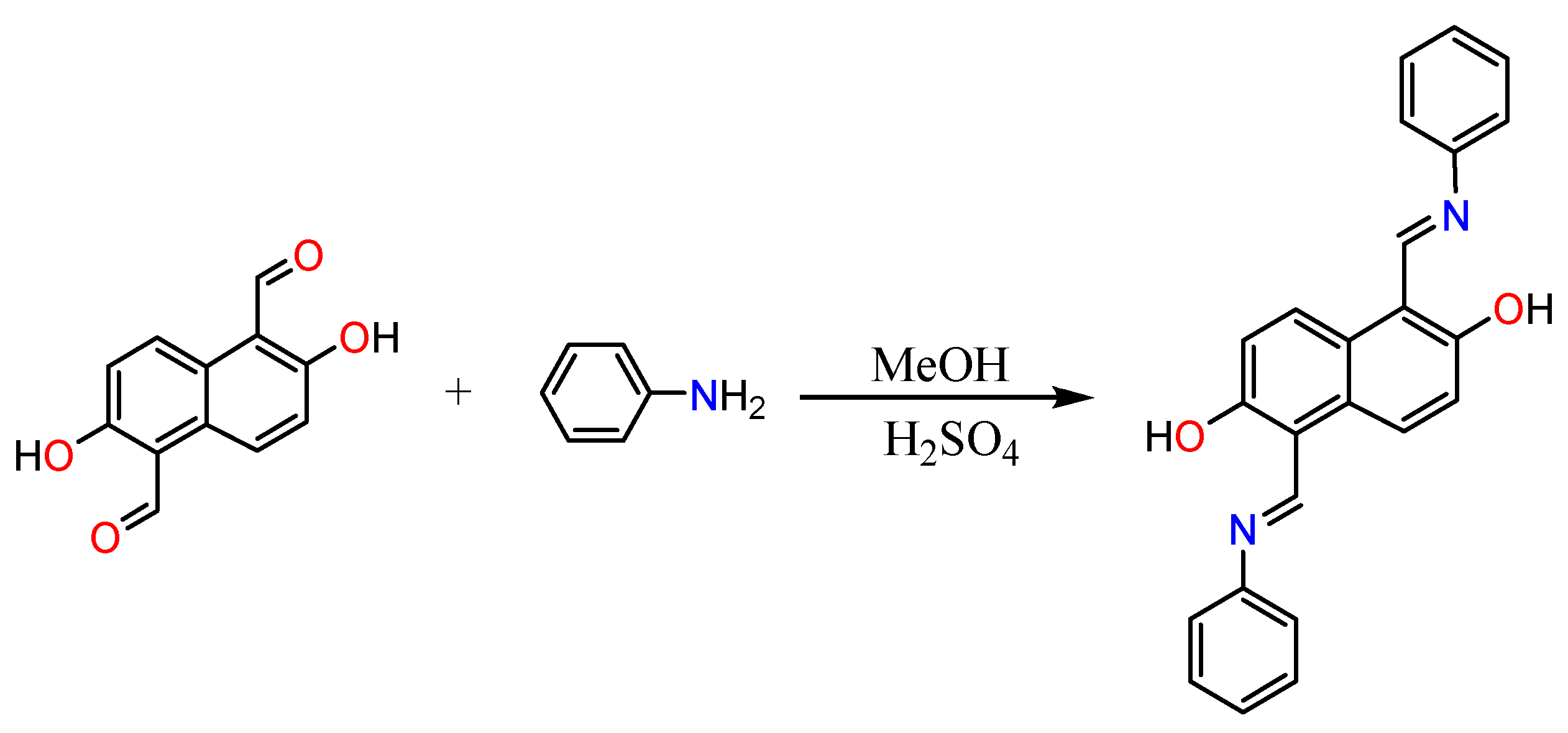
2.1. Synthesis
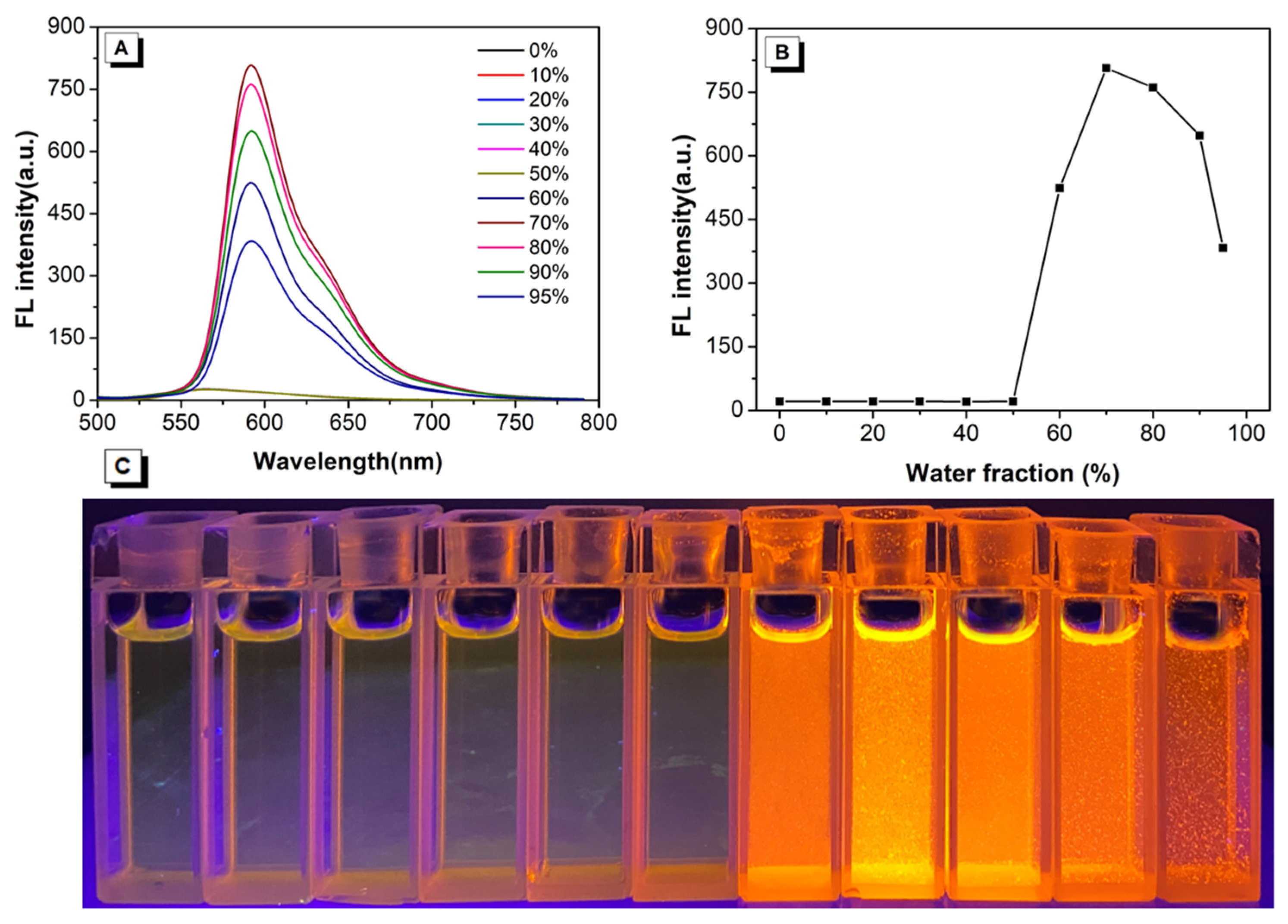
2.2. AIEE Properties
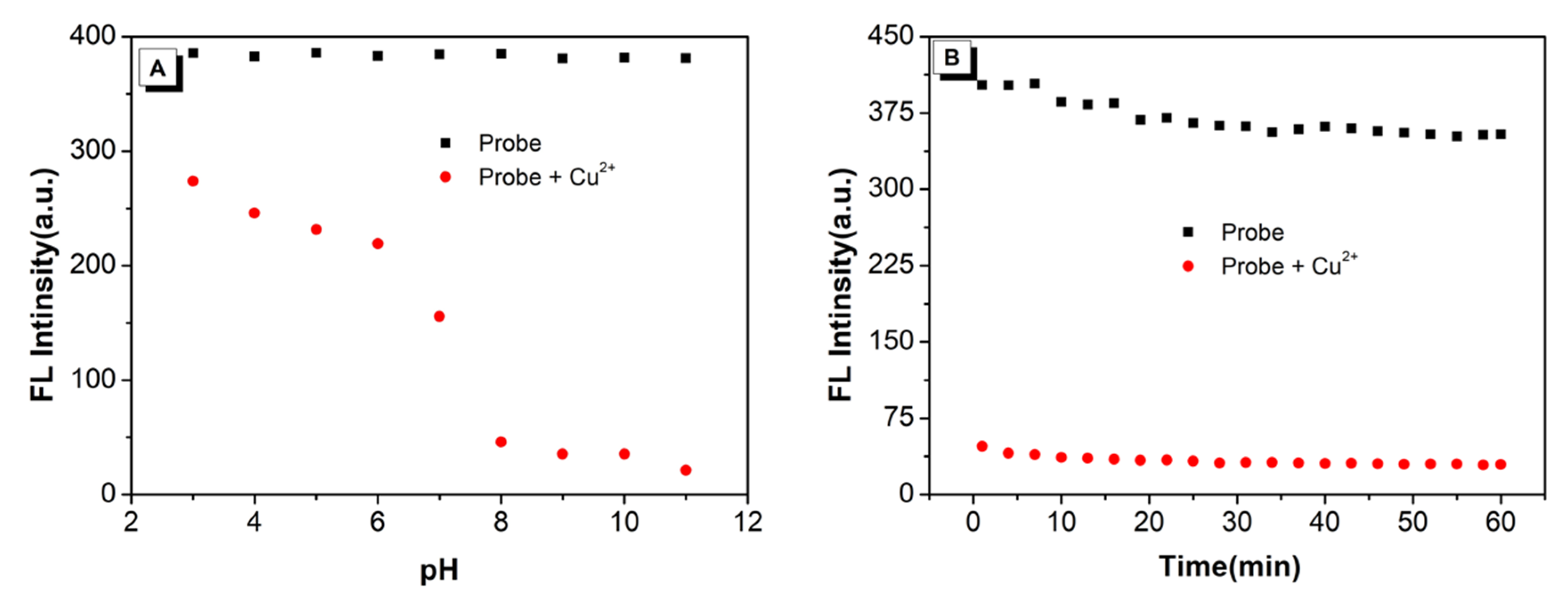
2.3. Stability of Detection
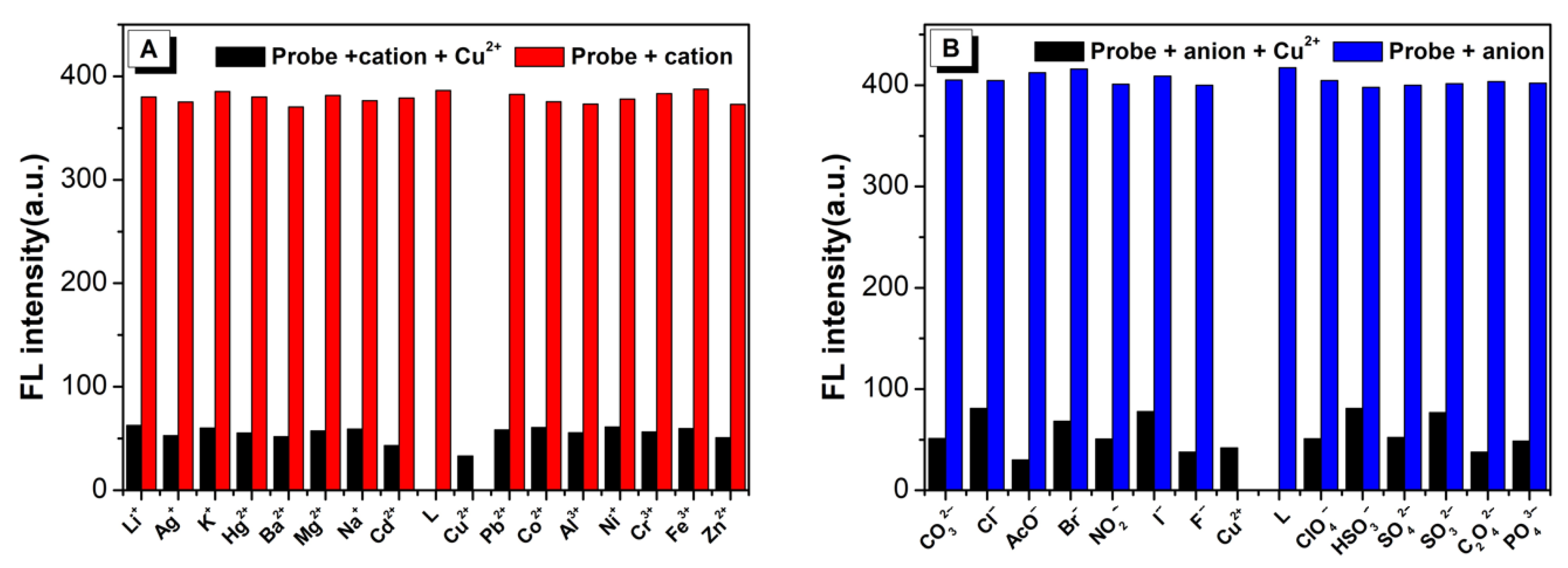
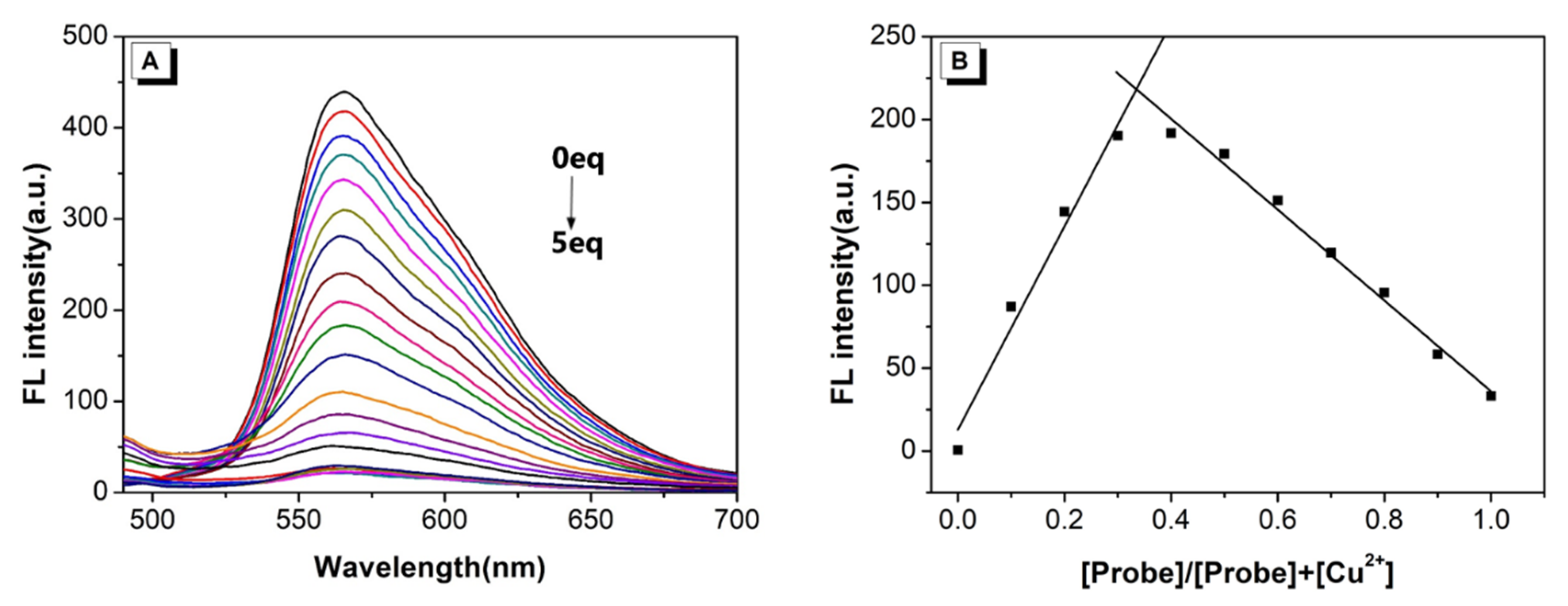
2.4. Cation Sensing Study
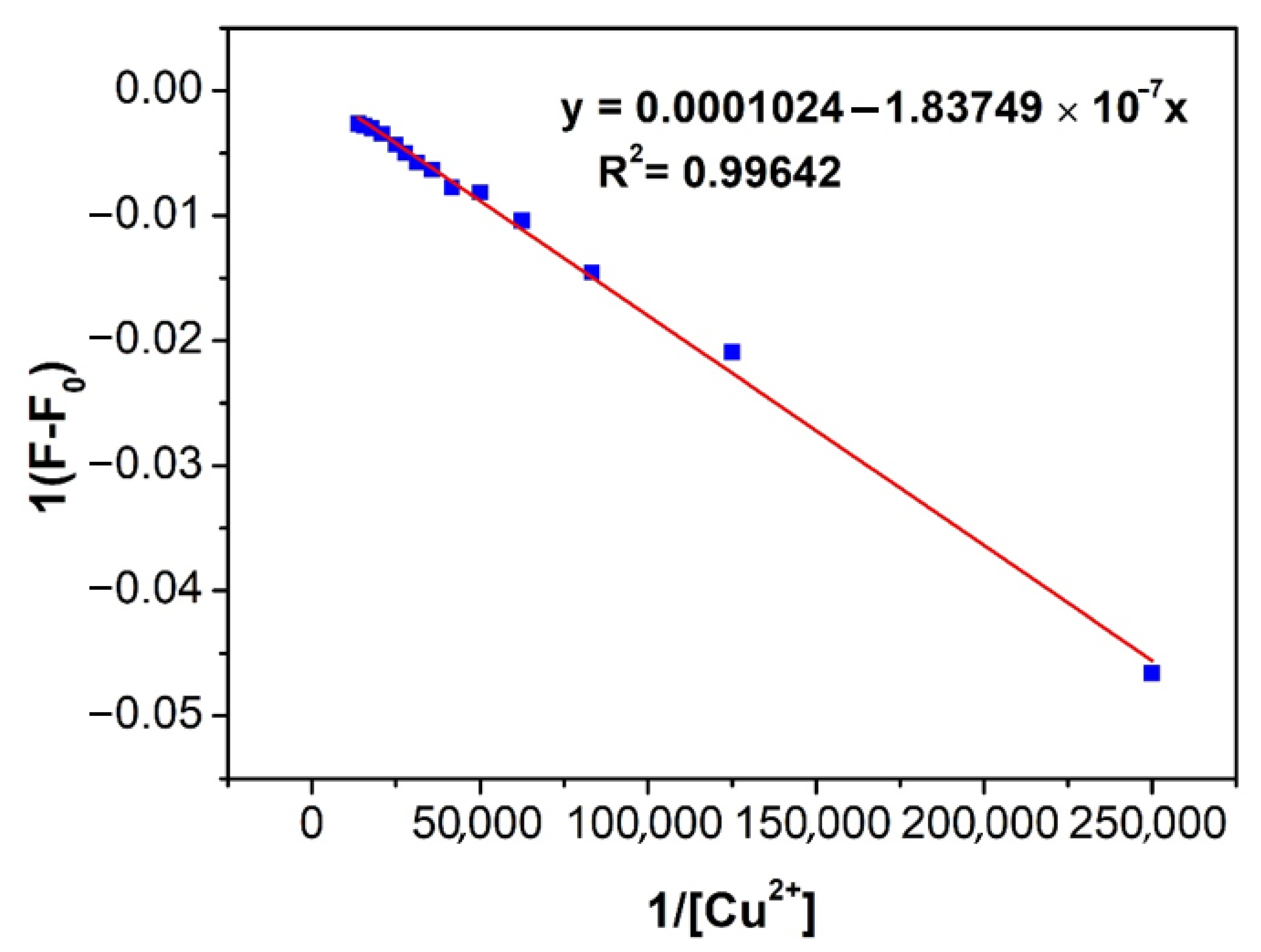
2.5. Job’s Plot and Binding Constant
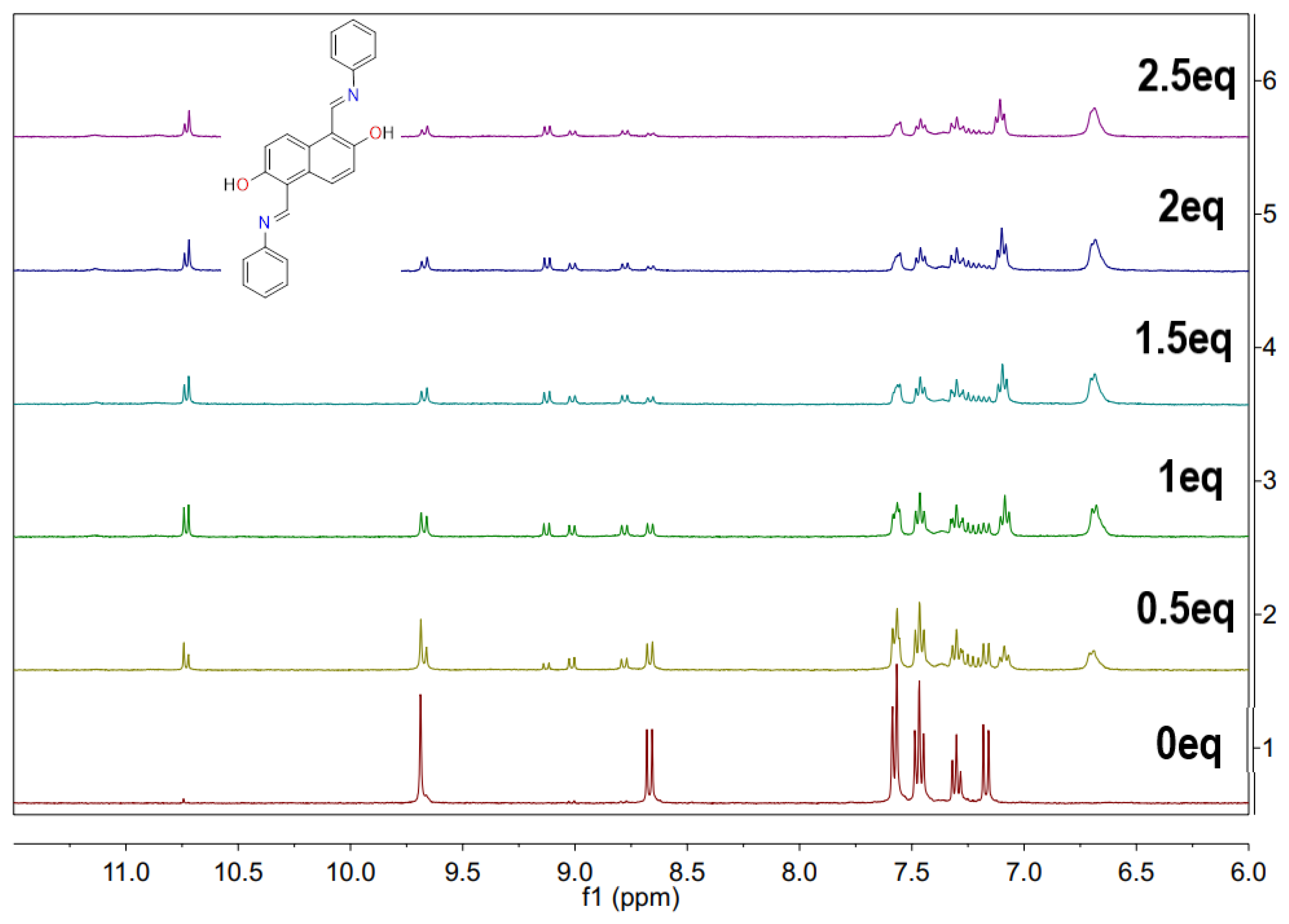
2.6. A Possible Mechanism for Detection Cu2+
2.7. Applications
3. Experimental Section
3.1. Materials and Characterization
3.2. Synthesis of the Compound L
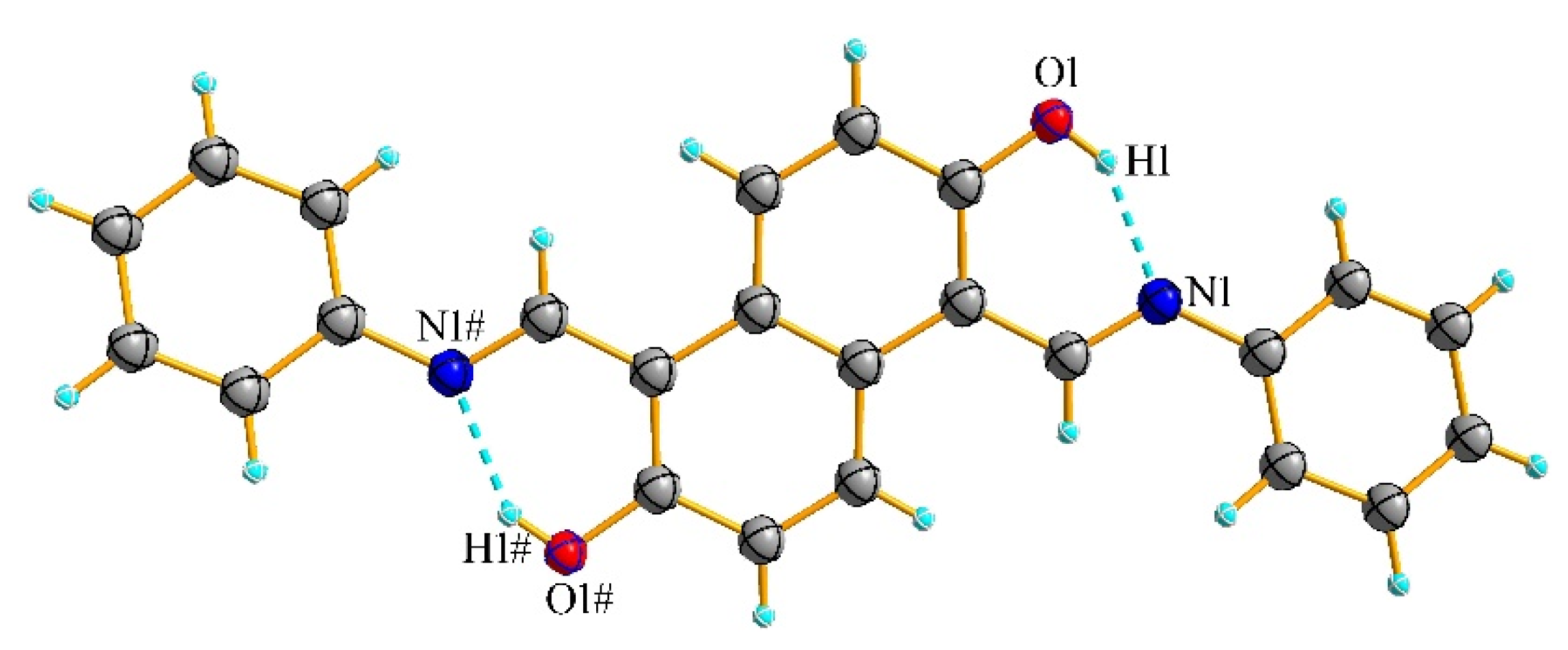
3.3. X-ray Crystallography
3.4. General Methods for Optical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, N.; Wang, H.; Li, Y.; Yang, S.; Tian, H.; Sun, B. The research progress of organic fluorescent probe applied in food and drinking water detection. Coord. Chem. Rev. 2021, 427, 213557. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rooj, B.; Dutta, A.; Mandal, U. Review on recent advances in metal ions sensing using different fluorescent probes. J. Fluoresc. 2018, 28, 999–1021. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Yue, D.; Wang, M.; Deng, F.; Yin, W.; Zhao, H.; Zhao, X.; Xu, Z. Biomarker-targeted fluorescent probes for breast cancer imaging. Chin. Chem. Lett. 2018, 29, 648–656. [Google Scholar] [CrossRef]
- O’gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Olivares, M.; Pizarro, F. Copper in human health. Int. J. Environ. Health 2007, 1, 608–620. [Google Scholar] [CrossRef]
- Hadjipanagiotou, C.; Christou, A.; Zissimos, A.M.; Chatzitheodoridis, E.; Varnavas, S.P. Contamination of stream waters, sediments, and agricultural soil in the surroundings of an abandoned copper mine by potentially toxic elements and associated environmental and potential human health–derived risks: A case study from Agrokipia, Cyprus. Environ. Sci. Pollut. Res. 2020, 27, 41279–41298. [Google Scholar] [CrossRef] [PubMed]
- Loland, J.; Singh, B. Copper contamination of soil and vegetation in coffee orchards after long-term use of Cu fungicides. Nutr. Cycl. Agroecosyst. 2004, 69, 203–211. [Google Scholar] [CrossRef]
- Moghaddam, M.R.; Carrara, S.; Hogan, C.F. Multi-colour bipolar electrochemiluminescence for heavy metal ion detection. Chem. Commun. 2019, 55, 1024–1027. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Walekar, L.; Dutta, T.; Kumar, P.; Ok, Y.S.; Pawar, S.; Deep, A.; Kim, K.-H. Functionalized fluorescent nanomaterials for sensing pollutants in the environment: A critical review. TrAC Trends Anal. Chem. 2017, 97, 458–467. [Google Scholar] [CrossRef]
- Qing, T.; Zhang, K.; Qing, Z.; Wang, X.; Long, C.; Zhang, P.; Hu, H.; Feng, B. Recent progress in copper nanocluster-based fluorescent probing: A review. Microchim. Acta 2019, 186, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo Jr, J.A.; Aron, A.T.; Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar] [CrossRef]
- Islam, M.M.; Hu, Z.; Wang, Q.; Redshaw, C.; Feng, X. Pyrene-based aggregation-induced emission luminogens and their applications. Mater. Chem. Front. 2019, 3, 762–781. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-based AIE-active probes for sensing applications. ACS Appl. Mater. Interfaces 2017, 10, 12189–12216. [Google Scholar] [CrossRef]
- Sahana, A.; Banerjee, A.; Lohar, S.; Panja, S.; Mukhopadhyay, S.K.; Matalobos, J.S.; Das, D. Fluorescence sensing of arsenate at nanomolar level in a greener way: Naphthalene based probe for living cell imaging. Chem. Commun. 2013, 49, 7231–7233. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Song, Y.; Li, Q.; Li, Z. A “turn-on” fluorescence probe towards copper ions based on core-substitued naphthalene diimide. Sens. Actuators B Chem. 2016, 226, 239–244. [Google Scholar] [CrossRef]
- Chaichana, K.; Phutlaprungrueang, N.; Chaicharoenwimolkul, L.; Promkatkaew, M.; Kongsriprapan, S. A selective fluorescence probe based on naphthalene for the detection of barium (ii). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yuan, X.; Cao, S.; Han, Y.; Liu, W.; Chen, Q.; Han, Z.; Wang, K. Determination of Cyanide in Water and Food Samples Using an Efficient Naphthalene-Based Ratiometric Fluorescent Probe. ACS Omega 2019, 4, 10784–10790. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Bhattacharjee, A.; Roy, A.; Chatterjee, S.; Roy, P. Chromogenic and fluorescence sensing of pH with a Schiff-base molecule. RSC Adv. 2016, 6, 39118–39124. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, A.K.; Kumawat, L.K. Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion. Sens. Actuators B Chem. 2014, 195, 98–108. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.; Li, J.; Zhang, Y.; Li, J.; Song, J.; Qu, J.; Wong, W.-Y. A simple Schiff base as dual-responsive fluorescent sensor for bioimaging recognition of Zn 2+ and Al 3+ in living cells. J. Mater. Chem. B 2018, 6, 5435–5442. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.M.; Saleh, M.B.; Ahmed, S.A. New solid phase extractors for selective separation and preconcentration of mercury (II) based on silica gel immobilized aliphatic amines 2-thiophenecarboxaldehyde Schiff’s bases. Anal. Chim. Acta 2004, 523, 133–140. [Google Scholar] [CrossRef]
- Ghaedi, M.; Montazerozohori, M.; Rahimi, N.; Biysreh, M.N. Chemically modified carbon nanotubes as efficient and selective sorbent for enrichment of trace amount of some metal ions. J. Ind. Eng. Chem. 2013, 19, 1477–1482. [Google Scholar] [CrossRef]
- Kumar, R.; Semwal, S.; Choudhury, J.; Srivastava, A. Heli (aza) cene: A Helical Molecular Tweezer with Tunable Intraand Inter-molecular Charge Transfer. Chem. Eur. J. 2017, 23, 15012–15016. [Google Scholar] [CrossRef] [PubMed]
- Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P. Ion recognition: Application of symmetric and asymmetric Schiff bases and their complexes for the fabrication of cationic and anionic membrane sensors to determine ions in real samples. Comb. Chem. High Throughput Screen. 2007, 10, 527–546. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Ali, I.A. A novel, highly sensitive, selective, reversible and turn-on chemi-sensor based on Schiff base for rapid detection of Cu (II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 225–231. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, H.; Chen, Y.; Wang, Q.; Elsegood, M.R.; Teat, S.J.; Feng, X.; Islam, M.M.; Wu, F.; Tang, B.Z. Tetraphenylethylene-based color-tunable AIE-ESIPT chromophores. Dyes Pigment. 2020, 175, 108175. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Chen, Y.; Sun, T.; Tang, Y.; Huang, Y.; Yang, Q.; Ma, D.; Wang, Y.; Wang, M. A Schiff base fluorescence probe for highly selective turn-on recognition of Zn2+. Tetrahedron Lett. 2017, 58, 365–370. [Google Scholar] [CrossRef]
- Ganguly, A.; Ghosh, S.; Kar, S.; Guchhait, N. Selective fluorescence sensing of Cu (II) and Zn (II) using a simple Schiff base ligand: Naked eye detection and elucidation of photoinduced electron transfer (PET) mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 72–80. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Wang, L.; Sun, T.; Wang, M.; Wang, Y.; Ma, D.; Yang, Q.; Tang, Y. A simple turn-on Schiff base fluorescence sensor for aluminum ion. Tetrahedron Lett. 2016, 57, 3535–3539. [Google Scholar] [CrossRef]
- Feng, H.-T.; Song, S.; Chen, Y.-C.; Shen, C.-H.; Zheng, Y.-S. Self-assembled tetraphenylethylene macrocycle nanofibrous materials for the visual detection of copper (II) in water. J. Mater. Chem. C 2014, 2, 2353–2359. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


| Sample | Measured | Added | Detected | Recovery | RSD |
|---|---|---|---|---|---|
| (μmol·L−1) | (μmol·L−1) | (μmol·L−1) | (n = 3, %) | (n = 3, %) | |
| Running water | 8.99 | 5.00 | 14.03 | 99.71 | 0.93 |
| 10.00 | 19.28 | 98.49 | 2.01 | ||
| 20.00 | 28.32 | 102.37 | 1.15 | ||
| Artificial lake | 15.92 | 5.00 | 21.11 | 99.10 | 1.48 |
| 10.00 | 25.19 | 102.90 | 1.72 | ||
| 20.00 | 35.99 | 99.81 | 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Shen, L.-Y.; Zhang, Q.-L.; Yang, X.-J.; Huang, Y.-L.; Redshaw, C.; Xu, H. A Simple Turn-off Schiff Base Fluorescent Sensor for Copper (II) Ion and Its Application in Water Analysis. Molecules 2021, 26, 1233. https://doi.org/10.3390/molecules26051233
Zhang X, Shen L-Y, Zhang Q-L, Yang X-J, Huang Y-L, Redshaw C, Xu H. A Simple Turn-off Schiff Base Fluorescent Sensor for Copper (II) Ion and Its Application in Water Analysis. Molecules. 2021; 26(5):1233. https://doi.org/10.3390/molecules26051233
Chicago/Turabian StyleZhang, Xing, Ling-Yi Shen, Qi-Long Zhang, Xian-Jiong Yang, Ya-Li Huang, Carl Redshaw, and Hong Xu. 2021. "A Simple Turn-off Schiff Base Fluorescent Sensor for Copper (II) Ion and Its Application in Water Analysis" Molecules 26, no. 5: 1233. https://doi.org/10.3390/molecules26051233
APA StyleZhang, X., Shen, L.-Y., Zhang, Q.-L., Yang, X.-J., Huang, Y.-L., Redshaw, C., & Xu, H. (2021). A Simple Turn-off Schiff Base Fluorescent Sensor for Copper (II) Ion and Its Application in Water Analysis. Molecules, 26(5), 1233. https://doi.org/10.3390/molecules26051233





