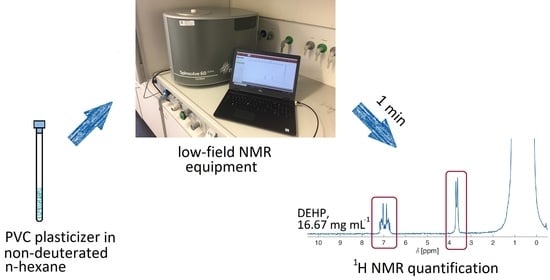
Compact NMR Spectroscopy for Low-Cost Identification and Quantification of PVC Plasticizers
Abstract
1. Introduction
2. Results and Discussions
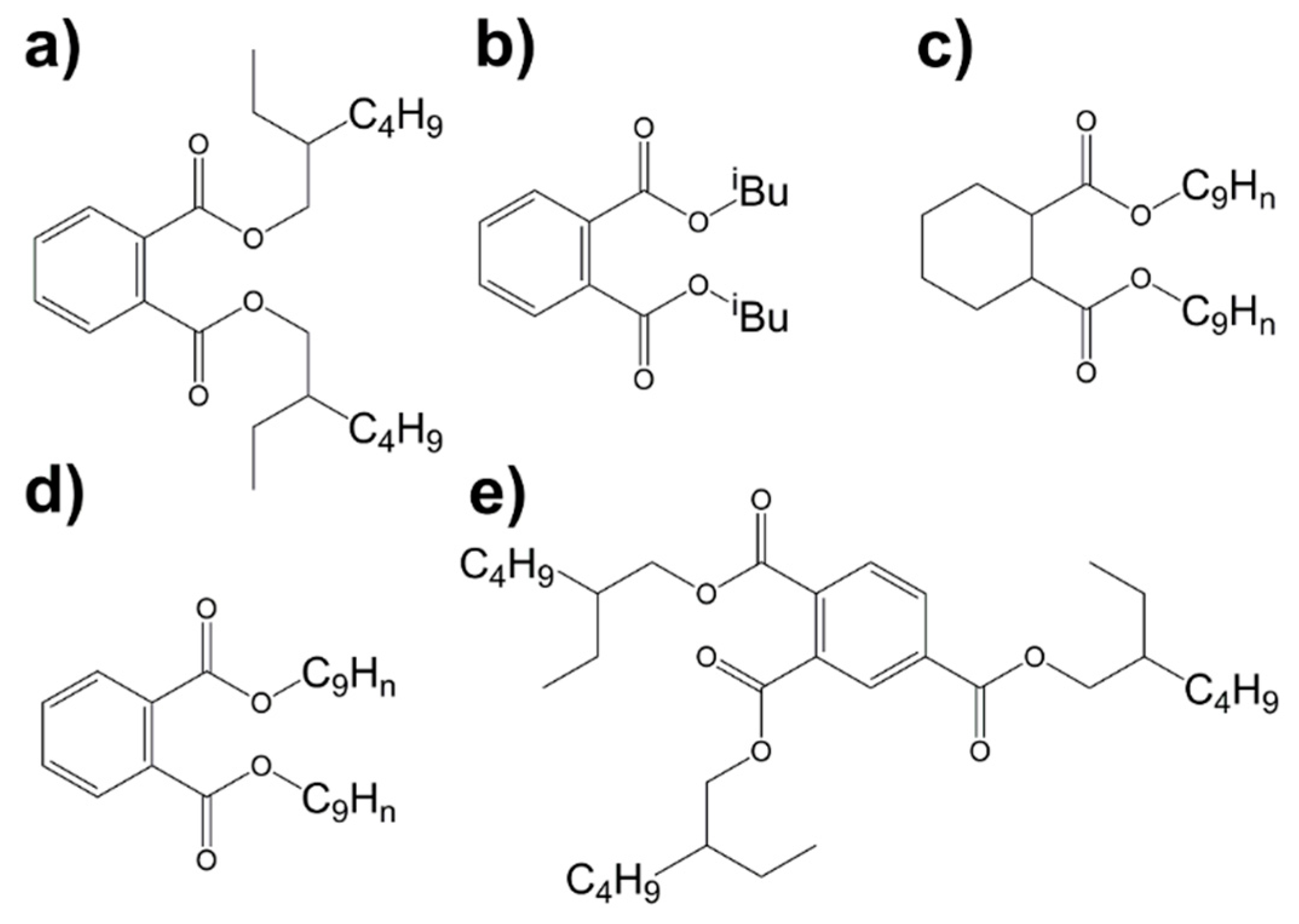
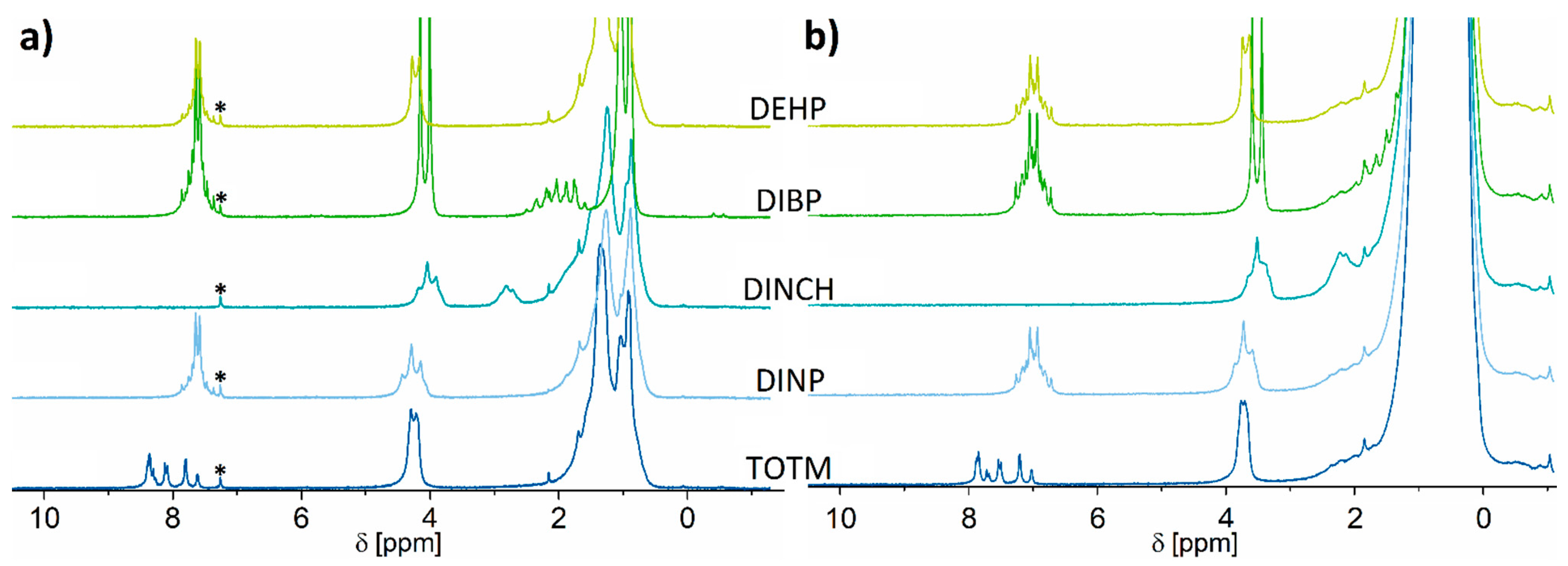
2.1. 1H NMR Spectroscopy
2.2. 13C NMR Spectroscopy
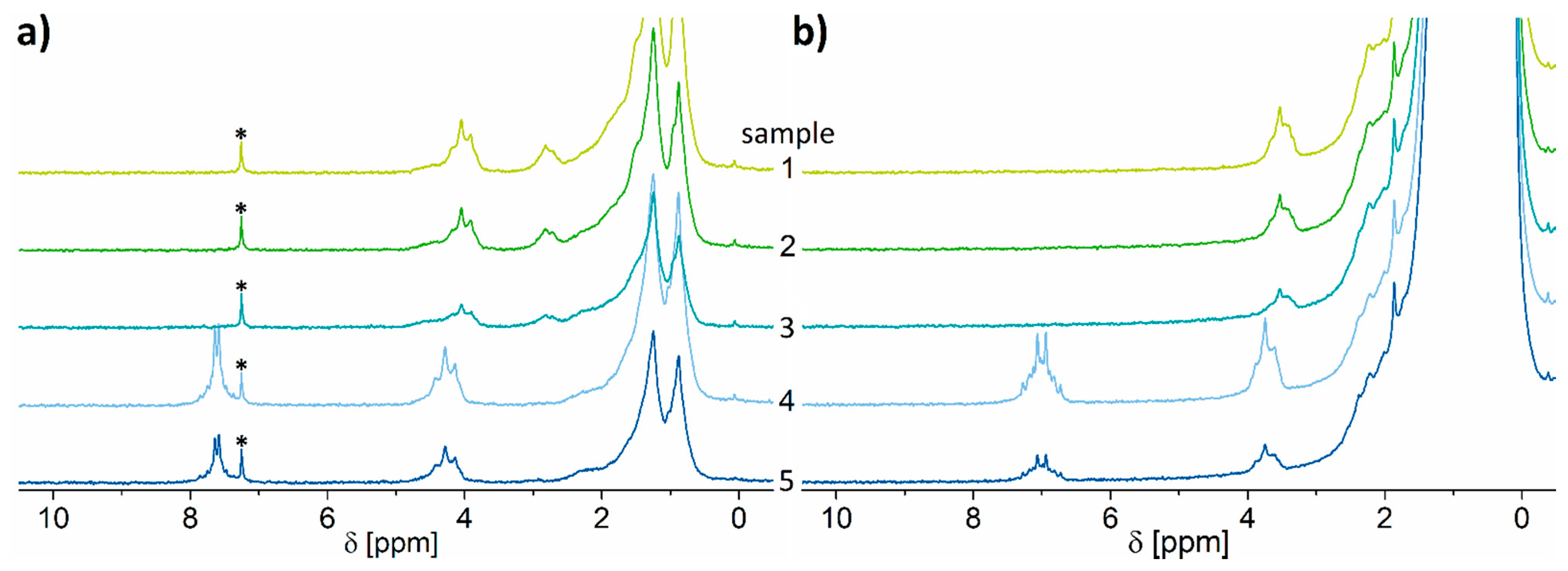
2.3. Test of the Proposed Method
2.4. How to Further Improve the Low-Field NMR Identification and Quantification
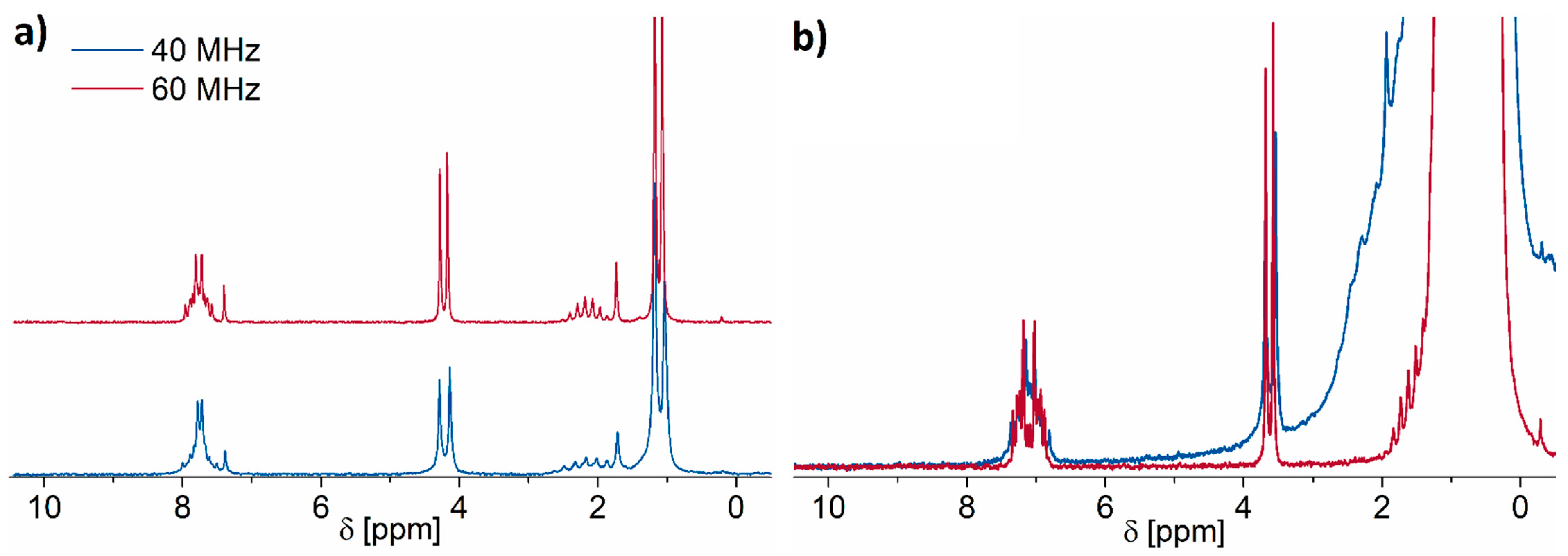
2.5. Low-Field NMR versus Conventional High-Field NMR
3. Materials and Methods
3.1. Samples
3.2. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Plastic antioxidants market projected to reach US$2.11 billion by 2022. Addit. Polym. 2018. [CrossRef]
- Organic peroxide market forecast to be worth US$1.20 billion by 2025. Addit. Polym. 2018. [CrossRef]
- Rahman, M.; Brazel, C. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Global demand for plasticizers continues to rise. Addit. Polym. 2017, 10–11. [CrossRef]
- Wilkes, C.E.; Summers, J.W.; Daniels, C.A.; Berard, M.T. PVC Handbook, Hanser, Munich, Cincinnati. In PVC Formulary; Wypych, G., Ed.; ChemTec Publishing: Scarborough, ON, Canada, 2005. [Google Scholar]
- Wypych, G. PVC Formulary, 3rd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2020. [Google Scholar]
- Bernard, L.; Cueff, R.; Bourdeaux, D.; Breysse, C.; Sautou, V. Analysis of plasticizers in poly (vinyl chloride) medical devices for infusion and artificial nutrition: Comparison and optimization of the extraction procedures, a pre-migration test step. Anal. Bioanal. Chem. 2015, 407, 1651–1659. [Google Scholar] [CrossRef]
- Messadi, D.; Vergnaud, J.M. Plasticizer transfer from plasticized PVC into ethanol–water mixtures. J. Appl. Polym. Sci. 1982, 27, 3945–3955. [Google Scholar] [CrossRef]
- Messadi, D.; Taverdet, J.L.; Vergnaud, J.M. Plasticizer migration from plasticized poly (vinyl chloride) into liquids. Effect of several parameters on the transfer. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 142–146. [Google Scholar] [CrossRef]
- Demir, A.P.T.; Ulutan, S. Migration of phthalate and non-phthalate plasticizers out of plasticized PVC films into air. J. Appl. Polym. Sci. 2012. [Google Scholar] [CrossRef]
- Kovačić, T.; Mrklić, Ž. The kinetic parameters for the evaporation of plasticizers from plasticized poly (vinyl chloride). Thermochim. Acta 2002, 381, 49–60. [Google Scholar] [CrossRef]
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Vimala, K.; Raju, K.M. Surface treatment of plasticized poly (vinyl chloride) to prevent plasticizer migration. J. Appl. Polym. Sci. 2010, 115, 1589–1597. [Google Scholar] [CrossRef]
- Kastner, J.; Cooper, D.G.; Marić, M.; Dodd, P.; Yargeau, V. Aqueous leaching of di-2-ethylhexyl phthalate and “green” plasticizers from poly (vinyl chloride). Sci. Total. Environ. 2012, 432, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Linde, E.; Gedde, U. Plasticizer migration from PVC cable insulation—The challenges of extrapolation methods. Polym. Degrad. Stab. 2014, 101, 24–31. [Google Scholar] [CrossRef]
- Ekelund, M.; Azhdar, B.; Hedenqvist, M.; Gedde, U. Long-term performance of poly (vinyl chloride) cables, Part 2: Migration of plasticizer. Polym. Degrad. Stab. 2008, 93, 1704–1710. [Google Scholar] [CrossRef]
- Taverdet, J.L.; Vergnaud, J.M. Modelization of matter transfers between plasticized PVC and liquids in case of a maximum for liquid-time curves. J. Appl. Polym. Sci. 1986, 31, 111–122. [Google Scholar] [CrossRef]
- Audouin, L.; Dalle, B.; Metzger, G.; Verdu, J. Thermal Aging of Plasticized PVC. 11. Effect of Plasticizer Loss on Electrical and Mechanical Properties. J. Appl. Polym. Sci. 1992, 45, 2097–2103. [Google Scholar] [CrossRef]
- Ekelund, M.; Edin, H.; Gedde, U. Long-term performance of poly(vinyl chloride) cables. Part 1: Mechanical and electrical performances. Polym. Degrad. Stab. 2007, 92, 617–629. [Google Scholar] [CrossRef]
- Jakubowicz, I.; Yarahmadi, N.; Gevert, T. Effects of accelerated and natural ageing on plasticized PVC. Polym. Degrad. Stab. 1999, 66, 415–421. [Google Scholar] [CrossRef]
- Yu, Q.; Selvadurai, A. Mechanical behaviour of a plasticized PVC subjected to ethanol exposure. Polym. Degrad. Stab. 2005, 89, 109–124. [Google Scholar] [CrossRef]
- Latini, G.; Ferri, M.; Chiellini, F. Materials Degradation in PVC Medical Devices, DEHP Leaching and Neonatal Outcomes. Curr. Med. Chem. 2010, 17, 2979–2989. [Google Scholar] [CrossRef]
- Mersiowsky, I. Long-term fate of PVC products and their additives in landfills. Prog. Polym. Sci. 2002, 27, 2227–2277. [Google Scholar] [CrossRef]
- Abb, M.; Heinrich, T.; Sorkau, E.; Lorenz, W. Phthalates in house dust. Environ. Int. 2009, 35, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Yost, E.E.; Euling, S.Y.; Weaver, J.A.; Beverly, B.E.; Keshava, N.; Mudipalli, A.; Arzuaga, X.; Blessinger, T.; Dishaw, L.; Hotchkiss, A.; et al. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ. Int. 2019, 125, 579–594. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Decision (EU) 2017/1210 of 4 July 2017 on the Identification Of Bis(2-Ethylhexyl) Phthalate (DEHP), Dibutyl Phthalate (DBP), Benzyl Butyl Phthalate (BBP) and Diisobutyl Phthalate (DIBP) as Substances of very High Concern According to Article 57(F) of Regulation (EC) No 1907/2006 of the European Parliament and of the Council (Notified Under Document C(2017) 4462) (Text with EEA Relevance), OPOCE, Brussels EU/2017/4462; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Sazan, P.; Karin, A.; Orna, C.; Bert-Ove, L.; Ana, P.P.; Stefania, V. European Union Summary Risk Assessment Report-Bis (2-ethylhexyl) Phthalate (DEHP); European Chemicals Bureau: Ispra, Italy, 2008.
- Allanou, R.; Munn, S.J.; Hansen, B.G. European Union Risk Assessment Report Dibutyl Phthalate; European Chemicals Bureau: Ispra, Italy, 2009.
- Navarro, R.; Perrino, M.P.; Tardajos, M.G.; Reinecke, H. Phthalate Plasticizers Covalently Bound to PVC: Plasticization with Suppressed Migration. Macromolecules 2010, 43, 2377–2381. [Google Scholar] [CrossRef]
- Greco, A.; Brunetti, D.; Renna, G.; Mele, G.; Maffezzoli, A. Plasticizer for poly(vinyl chloride) from cardanol as a renewable resource material. Polym. Degrad. Stab. 2010, 95, 2169–2174. [Google Scholar] [CrossRef]
- Chiellini, F.; Ferri, M.; Morelli, A.; Dipaola, L.; Latini, G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013, 38, 1067–1108. [Google Scholar] [CrossRef]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total. Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef]
- Weiss, J.M.; Gustafsson, Å.; Gerde, P.; Bergman, Å.; Lindh, C.H.; Krais, A.M. Daily intake of phthalates, MEHP, and DINCH by ingestion and inhalation. Chemosphere 2018, 208, 40–49. [Google Scholar] [CrossRef]
- Wypych, G. (Ed.) 15 Specialized Analytical Methods in Plasticizer Testing. In Handbook of Plasticizers, 3rd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2017; pp. 661–669. [Google Scholar]
- Marcilla, A.; Garcia, S.; Garcia-Quesada, J. Migrability of PVC plasticizers. Polym. Test. 2008, 27, 221–233. [Google Scholar] [CrossRef]
- Gimeno, P.; Thomas, S.; Bousquet, C.; Maggio, A.-F.; Civade, C.; Brenier, C.; Bonnet, P.-A. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC–MS. J. Chromatogr. B 2014, 949–950, 99–108. [Google Scholar] [CrossRef]
- Bernard, L.; Bourdeaux, D.; Pereira, B.; Azaroual, N.; Barthelemy, C.; Breysse, C.; Chennell, P.; Cueff, R.; Dine, T.; Eljezi, T.; et al. Analysis of plasticizers in PVC medical devices: Performance comparison of eight analytical methods. Talanta 2017, 162, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Barendswaard, W.; Litvinov, V.M.; Souren, F.; Scherrenberg, R.L.; Gondard, C.; Colemonts, C. Crystallinity and Microstructure of Plasticized Poly (vinyl chloride). A 13C and 1H Solid State NMR Study. Macromolecules 1999, 32, 167–180. [Google Scholar] [CrossRef]
- Giachet, M.T.; Schilling, M.R.; McCormick, K.; Mazurek, J.; Richardson, E.; Khanjian, H.; Learner, T. Assessment of the composition and condition of animation cels made from cellulose acetate. Polym. Degrad. Stab. 2014, 107, 223–230. [Google Scholar] [CrossRef][Green Version]
- Adams, A.; Kwamen, R.; Woldt, B.; Grass, M. Nondestructive Quantification of Local Plasticizer Concentration in PVC by (1)H NMR Relaxometry. Macromol. Rapid Commun. 2015, 36, 2171–2175. [Google Scholar] [CrossRef]
- Sommer, S.; Koch, M.; Adams, A. Terahertz Time-Domain Spectroscopy of Plasticized Poly (vinyl chloride). Anal. Chem. 2018, 90, 2409–2413. [Google Scholar] [CrossRef] [PubMed]
- Genay, S.; Feutry, F.; Masse, M.; Barthelemy, C.; Sautou, V.; Odou, P.; Decaudin, B.; Azaroual, N. Armed Study Group. Identification and quantification by (1)H nuclear magnetic resonance spectroscopy of seven plasticizers in PVC medical devices. Anal. Bioanal. Chem. 2017, 409, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Gladden, L.F.; Chandrasekera, T.C.; Fordham, E. Low-Field Permanent Magnets for Industrial Process and Quality Control. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 76, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Blümich, B.; Singh, K. Desktop NMR and Its Applications from Materials Science to Organic Chemistry. Angew. Chem. Int. Ed. 2018, 57, 6996–7010. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.; Limer, E.; Watson, A.; Defernez, M.; Williamson, D.; Kemsley, E.K. 60MHz 1H NMR spectroscopy for the analysis of edible oils. TrAC Trends Anal. Chem. 2014, 57, 147–158. [Google Scholar] [CrossRef]
- Dalitz, F.; Cudaj, M.; Maiwald, M.; Guthausen, G. Process and reaction monitoring by low-field NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 52–70. [Google Scholar] [CrossRef]
- Adams, A. Analysis of solid technical polymers by compact NMR. TrAC Trends Anal. Chem. 2016, 83, 107–119. [Google Scholar] [CrossRef]
- Kwamen, R.; Blumich, B.; Adams, A. Estimation of Self-Diffusion Coefficients of Small Penetrants in Semicrystalline Polymers Using Single-Sided NMR. Macromol. Rapid Commun. 2012, 33, 943–947. [Google Scholar] [CrossRef]
- Duffy, J.; Urbas, A.; Niemitz, M.; Lippa, K.; Marginean, I. Differentiation of fentanyl analogues by low-field NMR spectroscopy. Anal. Chim. Acta 2019, 1049, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Piechatzek, A.; Schmitt, G.; Siegmund, G. Single-sided Nuclear Magnetic Resonance for condition monitoring of cross-linked polyethylene exposed to aggressive media. Anal. Chim. Acta 2015, 887, 163–171. [Google Scholar] [CrossRef]
- Adams, A. Non-destructive analysis of polymers and polymer-based materials by compact NMR. Magn. Reson. Imaging 2019, 56, 119–125. [Google Scholar] [CrossRef]
- Höpfner, J.; Ratzsch, K.-F.; Botha, C.; Wilhelm, M. Medium Resolution 1 H-NMR at 62 MHz as a New Chemically Sensitive Online Detector for Size-Exclusion Chromatography (SEC-NMR). Macromol. Rapid Commun. 2018, 39, e1700766. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Blümich, B. Compact low-field NMR spectroscopy and chemometrics: A tool box for quality control of raw rubber. Polymer 2018, 141, 154–165. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.; Gibson, M.; Osman, Y.; Edgar, M.; Molinari, M.; Mather, M.L.; Casanova, F.; Wilson, P.B. Progress in low-field benchtop NMR spectroscopy in chemical and biochemical analysis. Anal. Chim. Acta 2019, 1067, 11–30. [Google Scholar] [CrossRef]
- Chakrapani, S.B.; Minkler, M.J.; Beckingham, B.S. Low-field 1H-NMR spectroscopy for compositional analysis of multicomponent polymer systems. Anal. Chim. Acta 2019, 144, 1679–1686. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Ling, Y.-C. Sulfonated graphene as highly efficient and reusable acid carbocatalyst for the synthesis of ester plasticizers. RSC Adv. 2014, 4, 57297–57307. [Google Scholar] [CrossRef]
- Bernard, L.; Décaudin, B.; Lecoeur, M.; Richard, D.; Bourdeaux, D.; Cueff, R.; Sautou, V. Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: A review. Talanta 2014, 129, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- European Commission. REGULATION (EC) No 1907/2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC, OPOCE; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
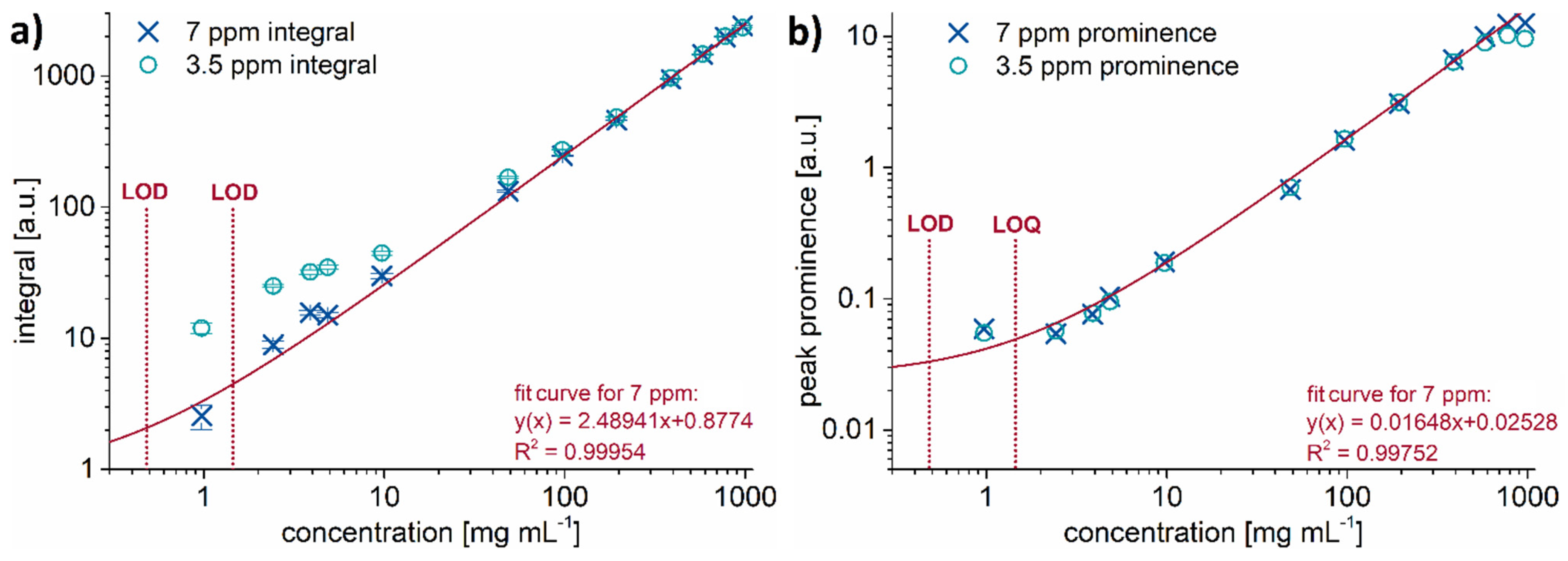
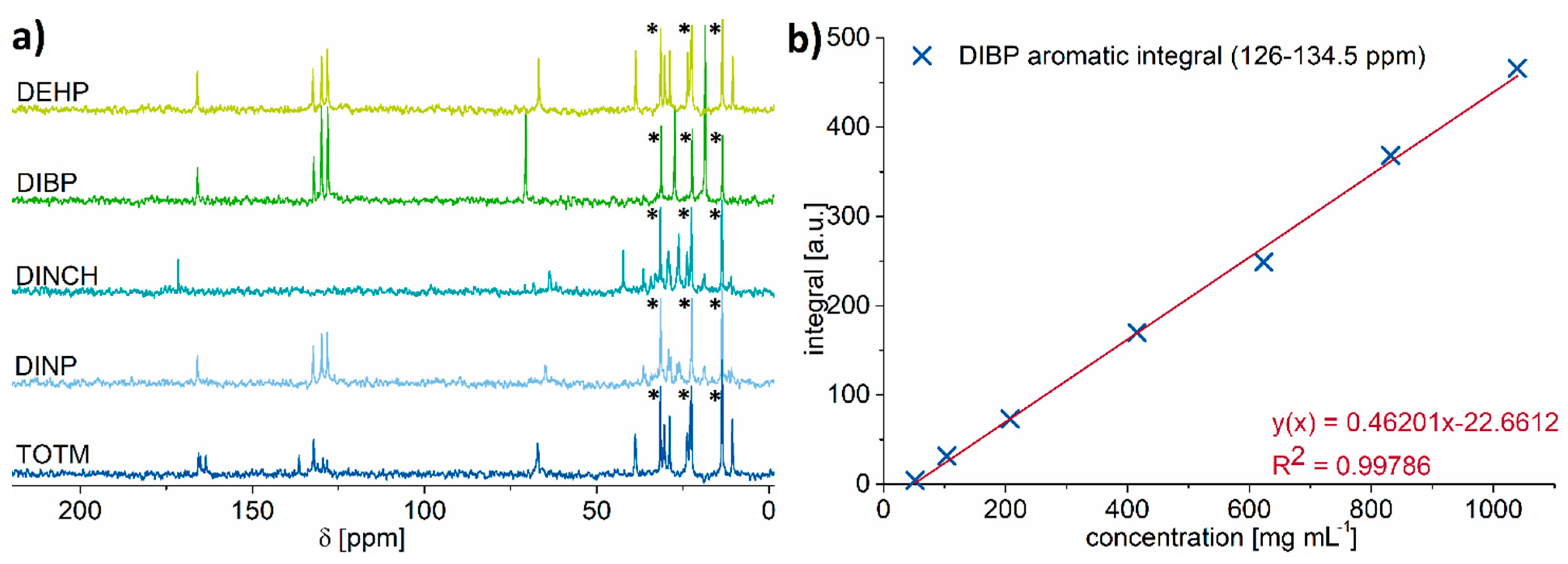

| DINP | DIBP | DEHP | TOTM | DINCH | |
|---|---|---|---|---|---|
| LOD [mg mL−1] | 0.48 | 0.42 | 0.57 | 1.52 | 0.63 |
| LOQ [mg mL−1] | 1.45 | 1.25 | 1.70 | 4.58 | 1.90 |
| LOD [wt% in PVC] | 0.96 | 0.83 | 1.13 | 3.05 | 1.27 |
| LOQ [wt% in PVC] | 2.89 | 2.49 | 3.39 | 9.15 | 3.80 |
| Sample | Identified Plasticizer Type | Determined Plasticizer Content [wt.%] from CDCl3 Extraction | Determined Plasticizer Content [wt.%] from n-hexane Extraction | ||
|---|---|---|---|---|---|
| 1 | DINCH | 38.49 | ±1.93 | 42.69 | ±1.64 |
| 2 | DINCH | 31.68 | ±1.83 | 34.14 | ±1.19 |
| 3 | DINCH | 15.92 | ±3.63 | 17.97 | ±2.60 |
| 4 | DINP | 40.94 | ±0.10 | 43.26 | ±3.98 |
| 5 | DINP | 23.85 | ±0.95 | 22.64 | ±1.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchowny, A.; Adams, A. Compact NMR Spectroscopy for Low-Cost Identification and Quantification of PVC Plasticizers. Molecules 2021, 26, 1221. https://doi.org/10.3390/molecules26051221
Duchowny A, Adams A. Compact NMR Spectroscopy for Low-Cost Identification and Quantification of PVC Plasticizers. Molecules. 2021; 26(5):1221. https://doi.org/10.3390/molecules26051221
Chicago/Turabian StyleDuchowny, Anton, and Alina Adams. 2021. "Compact NMR Spectroscopy for Low-Cost Identification and Quantification of PVC Plasticizers" Molecules 26, no. 5: 1221. https://doi.org/10.3390/molecules26051221
APA StyleDuchowny, A., & Adams, A. (2021). Compact NMR Spectroscopy for Low-Cost Identification and Quantification of PVC Plasticizers. Molecules, 26(5), 1221. https://doi.org/10.3390/molecules26051221