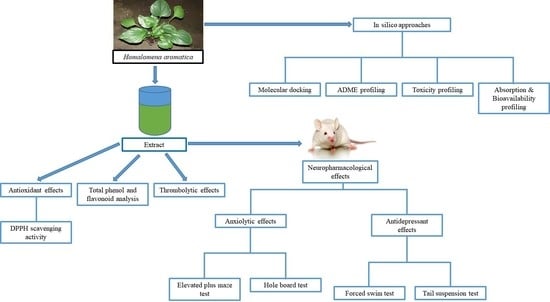
Investigation of Potential Antioxidant, Thrombolytic and Neuropharmacological Activities of Homalomena aromatica Leaves Using Experimental and In Silico Approaches
Abstract
:1. Introduction
2. Results and Discussion
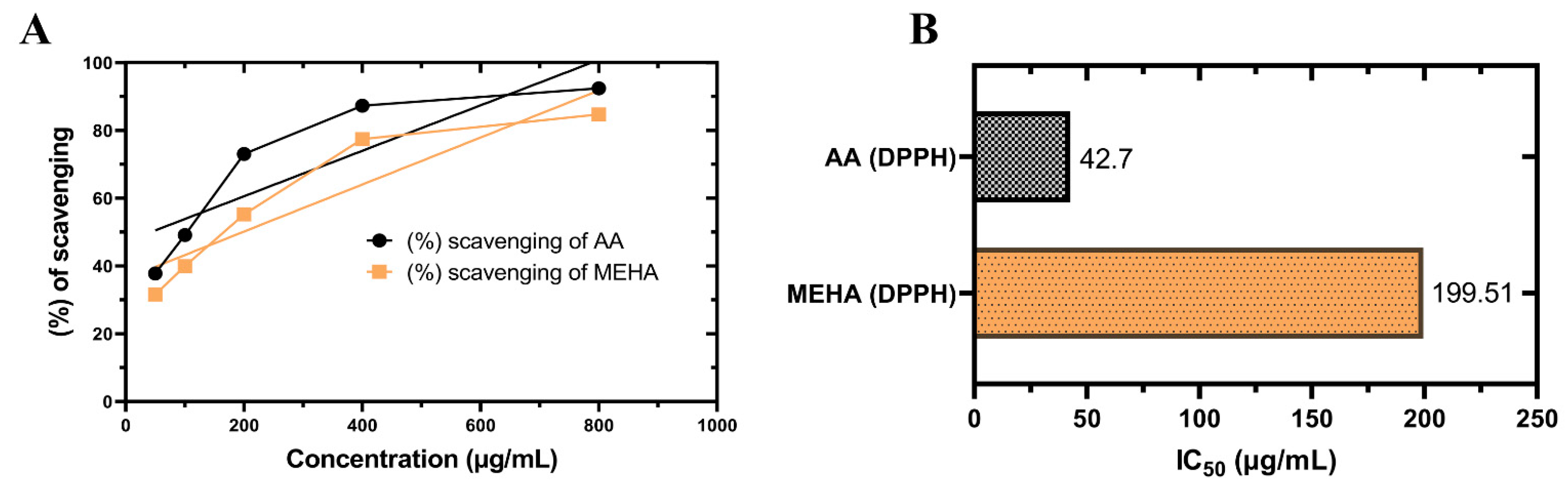
2.1. In Vitro Antioxidant Activity
DPPH Free Radical Scavenging Assay
2.2. Total Phenolics and Flavonoids Content
2.3. Thrombolytic Activity
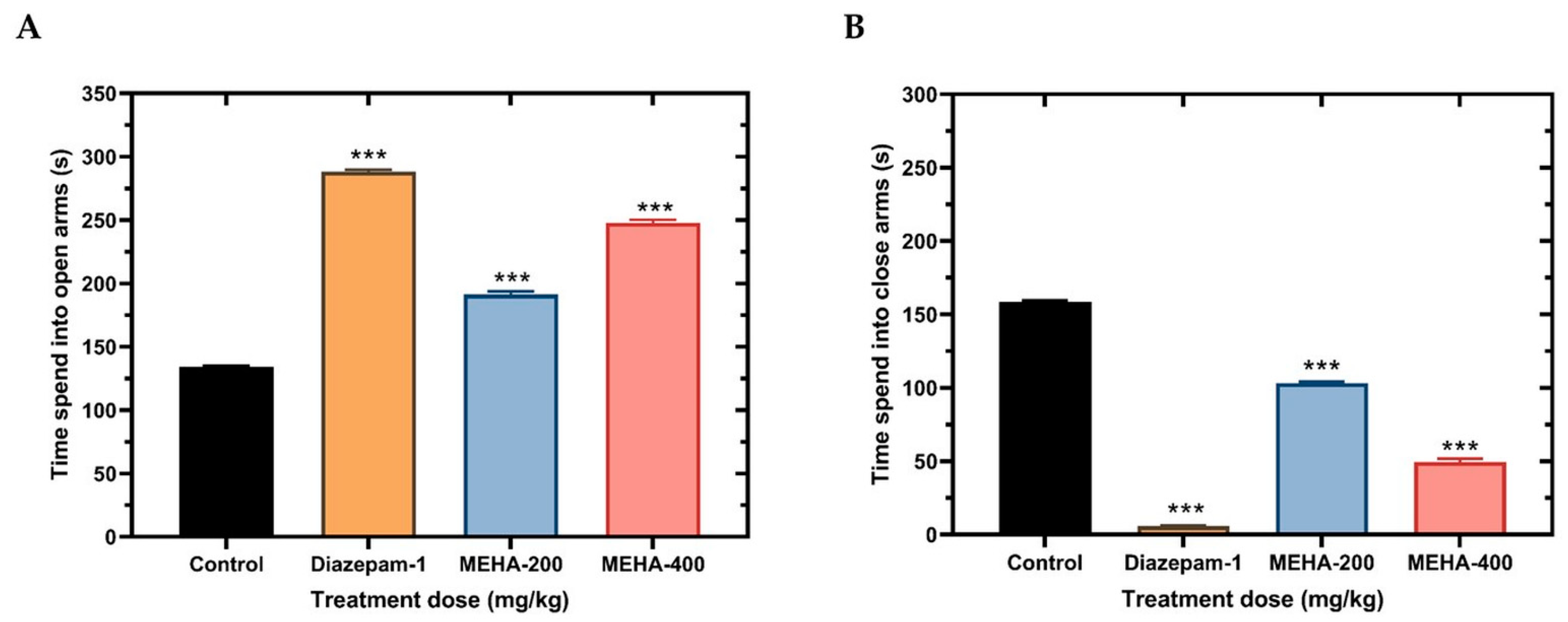
2.4. Anxiolytic Activity
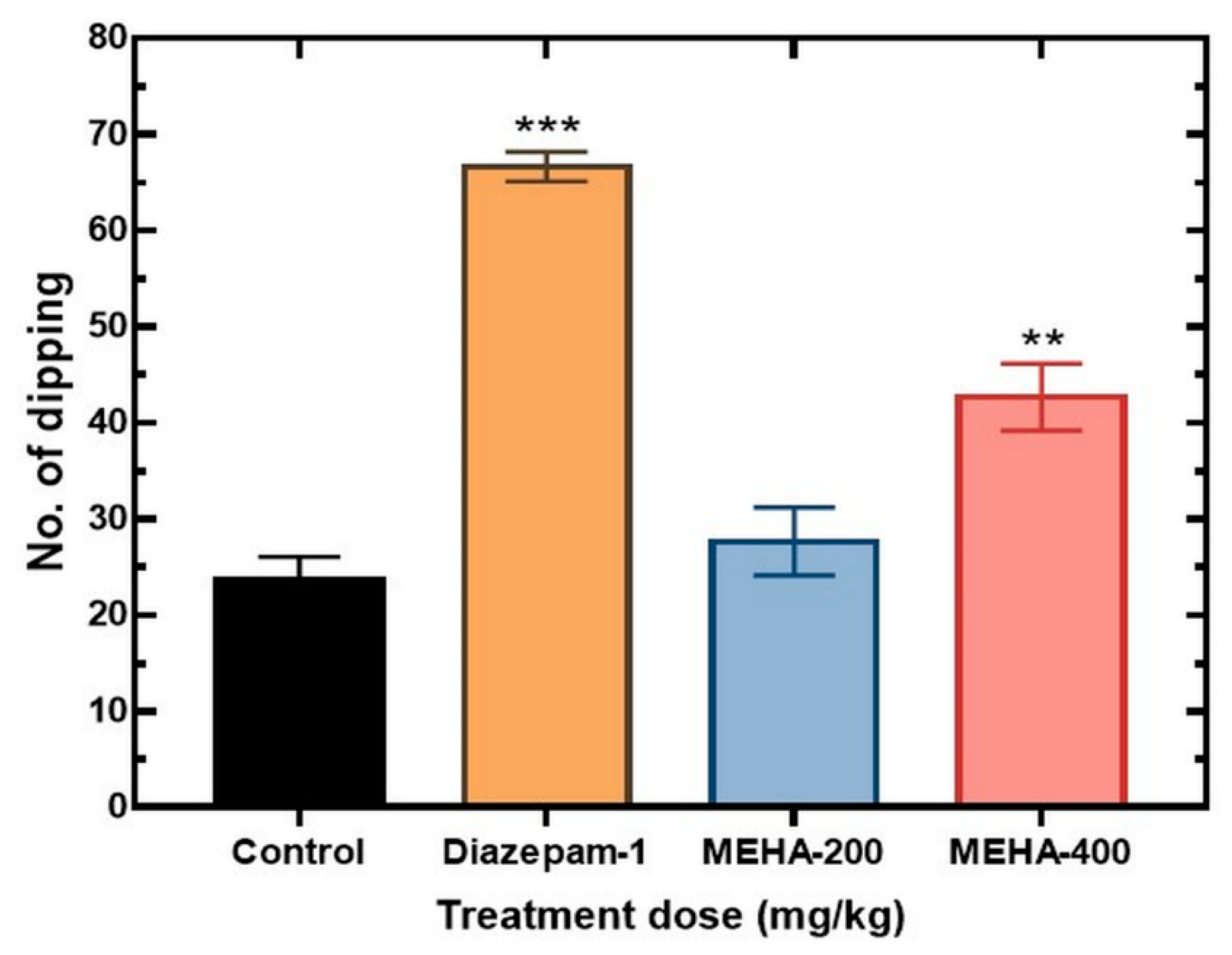
2.5. Antidepressant Activity
2.6. In Silico Studies
3. Materials and Methods
3.1. Reagents, Chemicals and Instruments
3.2. Collection, Identification and Preparation of Extract
3.3. Antioxidant Activity
DPPH (1,1-diphenyl-2-picrylydrazyl) Radical Scavenging Activity
3.4. Total Phenolics Content
3.5. Total Flavonoids Content
3.6. Thrombolytic Activity
3.7. In Vivo Neuropharmacological Activity
3.7.1. Experimental Animals
3.7.2. Acute Toxicity Study
3.7.3. Anxiolytic Activity
Elevated Plus Maze Test
Hole Board Test
3.7.4. Antidepressant Activity
Tail Suspension Test
Forced Swimming Test
3.8. Protein and Chemical Compounds Studied in This Investigation
3.9. In Silico Studies
3.9.1. Molecular Docking: Preparation of Ligands
3.9.2. Molecular Docking: Preparation of Proteins/Enzymes
3.9.3. Molecular Docking: Glide Standard Precision Docking
3.9.4. Prediction of the Pharmacokinetic Parameters (ADME)
3.9.5. Prediction of Toxicological Properties
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| PDB | Protein data bank |
| GABA | Gamma amino butyric acid |
| p.o | per oral |
| i.p | intraperitoneal |
| ADME/T | Absorption, distribution, metabolism, excretion and toxicity |
| BMI | Basal metabolic rate |
| ANOVA | Analysis of variance |
| SPSS | Statistical package for social science |
| SEM | Standard error of mean |
References
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Kara, S.; Yazici, K.M.; Güleç, C.; Ünsal, I. Mixed anxiety-depressive disorder and major depressive disorder: Comparison of the severity of illness and biological variables. Psychiatry Res. 2000, 94, 59–66. [Google Scholar] [CrossRef]
- Hassan, W.; Eduardo Barroso Silva, C.; Mohammadzai, I.U.; Batista Teixeira da Rocha, J.; Landeira-Fernandez, J. Association of oxidative stress to the genesis of anxiety: Implications for possible therapeutic interventions. Curr. Neuropharmacol. 2014, 12, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity: Supplementary issue: Brain plasticity and repair. J. Exp. Neurosci. 2016, 10, S39887. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, M.A.; Barua, N.; Hossain, M.S.; Hoque, M.; Bristy, T.A.; Mahmud, S.; Adnan, M.; Chy, M.; Uddin, N.; Paul, A. Unravelling the biological activities of the Byttneria pilosa leaves using experimental and computational approaches. Molecules 2020, 25, 4737. [Google Scholar] [CrossRef] [PubMed]
- Kehie, P.; Pfoze, N.L. Phytochemical and ethnopharmacological overview of endangered Homalomena aromatica Schott: An aromatic medicinal herb of Northeast India. Indian J. Nat. Prod. Resour. 2017, 8, 18–31. [Google Scholar]
- Van, T.H.; Nguyen, Q.P.; Tran, G.B.; Huynh, N.T.A. Chemical composition and antibacterial activities of Homalomena vietnamensis bogner & vd nguyen (Araceae). J. Microbiol. Biotechnol. Food Sci. 2020, 10, 201–204. [Google Scholar]
- Chandana, C.B.; Anindita, T.; Anindhya, S.D.; Acheenta, G.B.; Debesh, C.P. Ulcer protective activity of ethanolic extract of Homalomena aromatica Schott. (Araceae) Root. Adv. Tech. Biol. Med. 2014, 2, 117. [Google Scholar]
- Dutta, B.; Lahkar, M.; Augustine, B.B.; Lihite, R.J. Hepatoprotective activity of Tamarind indica and Homalomena aromatica in rats. Carbon N. Y. 2013, 100, 2. [Google Scholar]
- Policegoudra, R.S.; Goswami, S.; Aradhya, S.M.; Chatterjee, S.; Datta, S.; Sivaswamy, R.; Chattopadhyay, P.; Singh, L. Bioactive constituents of Homalomena aromatica essential oil and its antifungal activity against dermatophytes and yeasts. J. Mycol. Med. 2012, 22, 83–87. [Google Scholar] [CrossRef]
- Roy, S.J.; Baruah, P.S.; Lahkar, L.; Gurung, L.; Saikia, D.; Tanti, B. Phytochemical analysis and antioxidant activities of Homalomena aromatic Schott. J. Pharmacogn. Phytochem. 2019, 8, 1379–1385. [Google Scholar]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhong, Y.; Ke, G.; Liu, X.; Li, H.; Li, X.; Zheng, Q.; Yang, M. The mechanism of compound anshen essential oil in the treatment of insomnia was examined by network pharmacology. Evid. Based Complement. Altern. Med. 2019, 2019, 9241403. [Google Scholar] [CrossRef] [Green Version]
- Wolffenbüttel, A.N.; Zamboni, A.; Becker, G.; Dos Santos, M.K.; Borille, B.T.; de Cássia Mariotti, K.; Fagundes, A.C.; de Oliveira Salomón, J.L.; Coelho, V.R.; Ruiz, L.V. Citrus essential oils inhalation by mice: Behavioral testing, GCMS plasma analysis, corticosterone, and melatonin levels evaluation. Phyther. Res. 2018, 32, 160–169. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Bruhn, J.G.; Bohlin, L. Molecular pharmacognosy: An explanatory model. Drug Discov. Today 1997, 2, 243–246. [Google Scholar] [CrossRef]
- Berton, O.; Nestler, E.J. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef]
- Chen, C.; Yang, F.-Q.; Zhang, Q.; Wang, F.-Q.; Hu, Y.-J.; Xia, Z.-N. Natural products for antithrombosis. Evid. Based Complement. Altern. Med. 2015, 2015, 876426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngan, A.; Conduit, R. A double-blind, placebo-controlled investigation of the effects of Passiflora incarnata (passionflower) herbal tea on subjective sleep quality. Phytother. Res. 2011, 25, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, M.; Kashani, L.; Aleyaseen, A.; Ghoreishi, A.; Rahmanpour, H.; Zarrinara, A.R.; Akhondzadeh, S. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: A double-blind, randomised and placebo-controlled trial. BJOG 2008, 115, 515–519. [Google Scholar] [CrossRef]
- Ramjan, A.; Hossain, M.; Runa, J.F.; Md, H.; Mahmodul, I. Evaluation of thrombolytic potential of three medicinal plants available in Bangladesh, as a potent source of thrombolytic compounds. Avicenna J. Phytomed. 2014, 4, 430–436. [Google Scholar]
- Violi, F.; Pignatelli, P. Platelet oxidative stress and thrombosis. Thromb. Res. 2012, 129, 378–381. [Google Scholar] [CrossRef]
- Loscalzo, J. Oxidant stress: A key determinant of atherothrombosis. Biochem. Soc. Trans. 2003, 31, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Rammal, H.; Bouayed, J.; Younos, C.; Soulimani, R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav. Immun. 2008, 22, 1156–1159. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, Y.; Wang, Q.; Sun, L.; Li, G.; Zhang, C.; Huang, J.; Chen, L.; Zhai, H. Support for natural small-molecule phenols as anxiolytics. Molecules 2017, 22, 2138. [Google Scholar] [CrossRef] [Green Version]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant flavonoids and their relationship with oxidative stress. Oxid. Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opie, L.H.; Lecour, S. The red wine hypothesis: From concepts to protective signalling molecules. Eur. Heart J. 2007, 28, 1683–1693. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Ascherio, A.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Hennekens, C.H.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999, 282, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Manson, J.E.; Lee, I.M.; Cole, S.R.; Hennekens, C.H.; Willett, W.C.; Buring, J.E. Fruit and vegetable intake and risk of cardiovascular disease: The Women’s Health Study. Am. J. Clin. Nutr. 2000, 72, 922–928. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Nic Dhonnchadha, B.A.; Bourin, M.; Hascoët, M. Anxiolytic-Like effects of 5-HT2 ligands on three mouse models of anxiety. Behav. Brain Res. 2003, 140, 203–214. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327. [Google Scholar]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Tallman, J.F.; Cassella, J.; Kehne, J.; Corpora, N. Mechanism of Action of Anxiolytics; American College of Neuropsychopharmacology: Brentwood, TN, USA, 2002. [Google Scholar]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef] [Green Version]
- Arborelius, L.; Owens, M.J.; Plotsky, P.M.; Nemeroff, C. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999, 160, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bordia, A.K.; Sanadhya, S.K.; Rathore, A.S.; Bhu, N. Essential oil of garlic on blood lipids and fibrinolytic activity in patients of coronary artery disease. Part I. J. Assoc. Physicians India 1978, 26, 327–331. [Google Scholar] [PubMed]
- Setzer, W.N. Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun. 2009, 4. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, D.P.; Silva, R.H.N.; da Silva, E.F.; Gavioli, E.C. Essential oils and their constituents: An alternative source for novel antidepressants. Molecules 2017, 22, 1290. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Chy, M.; Uddin, N.; Kamal, A.T.M.; Chowdhury, K.A.A.; Rahman, M.; Reza, A.S.M.; Moniruzzaman, M.; Rony, S.R.; Nasrin, M. Intervention in neuropsychiatric disorders by suppressing inflammatory and oxidative stress signal and exploration of in silico studies for potential lead compounds from Holigarna caustica (Dennst.) Oken leaves. Biomolecules 2020, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Adnan, M.; Chy, M.; Uddin, N.; Kamal, A.T.M.; Chowdhury, M.; Islam, M.; Hossain, M.; Tareq, A.M.; Bhuiyan, M.; Hossain, I. Unveiling pharmacological responses and potential targets insights of identified bioactive constituents of Cuscuta reflexa Roxb. leaves through in vivo and in silico approaches. Pharmaceuticals 2020, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Clark, G.; Koester, A.G.; Pearson, D.W. Exploratory behavior in chronic disulfoton poisoning in mice. Psychopharmacologia 1971, 20, 169–171. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Bhachoo, J.; Beuming, T. Investigating protein–peptide interactions using the Schrödinger computational suite. In Modeling Peptide-Protein Interactions; Springer: Cham, Switzerland, 2017; pp. 235–254. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]

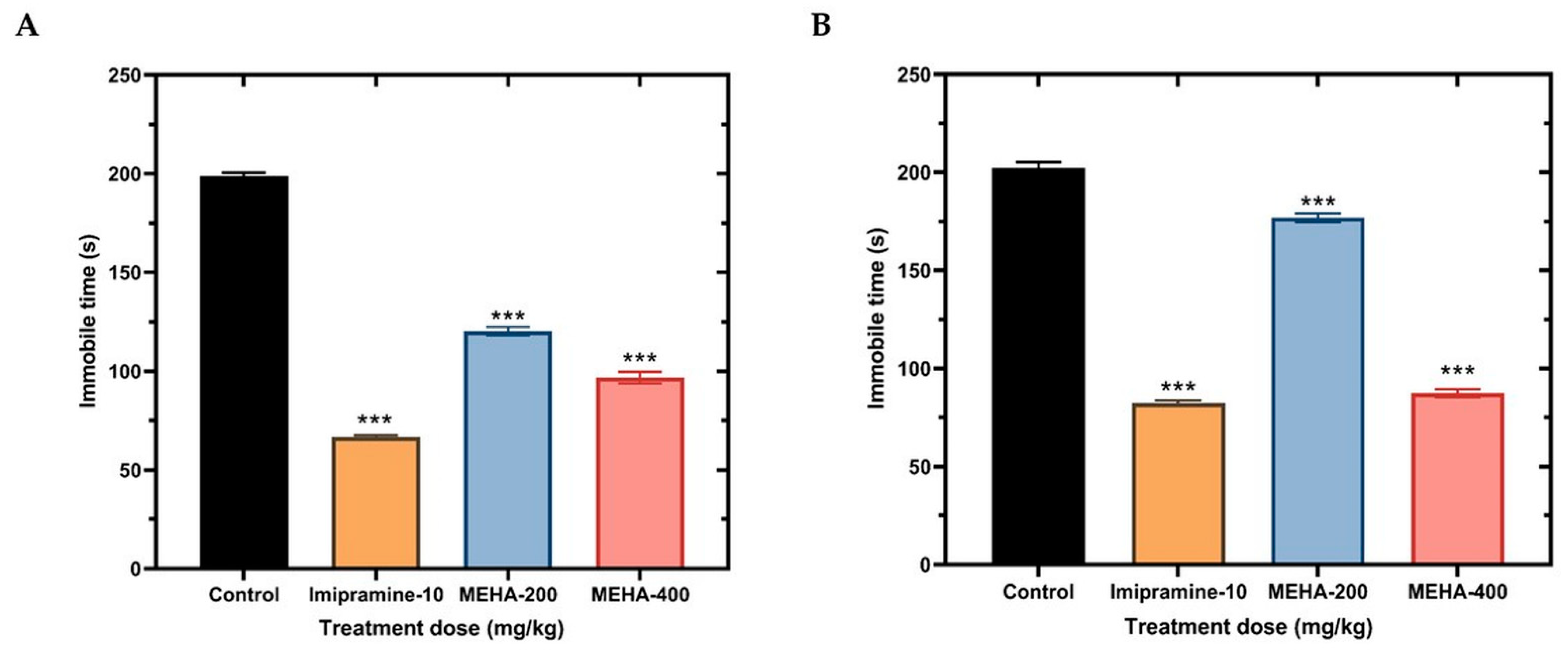
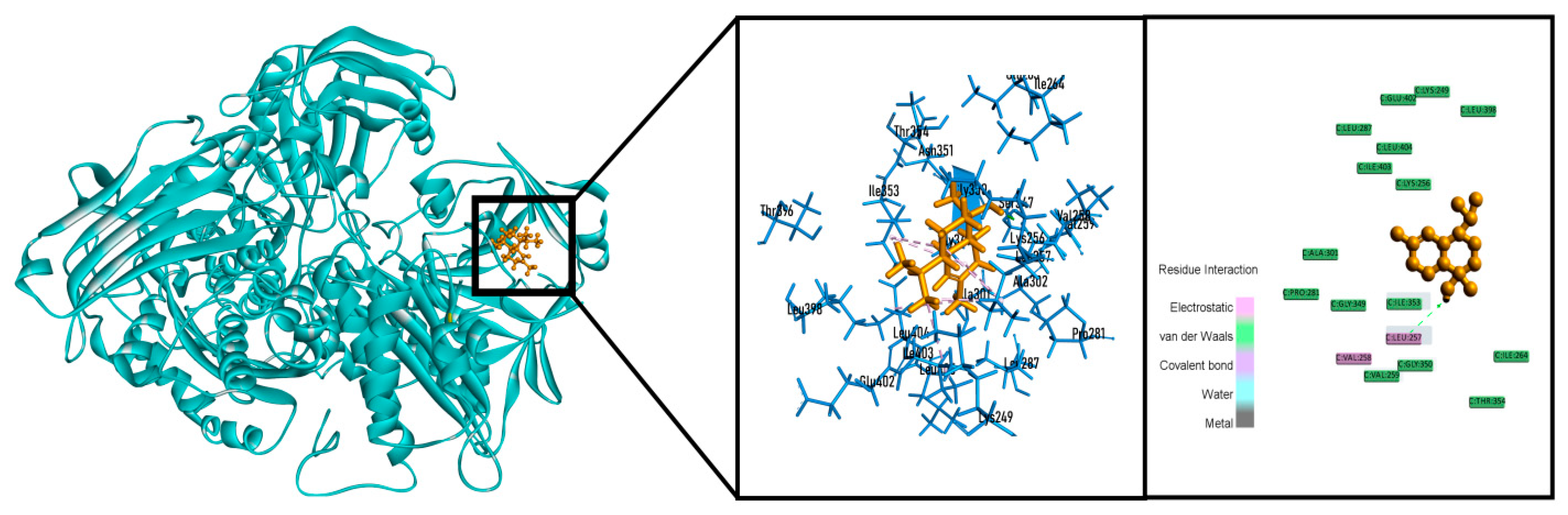

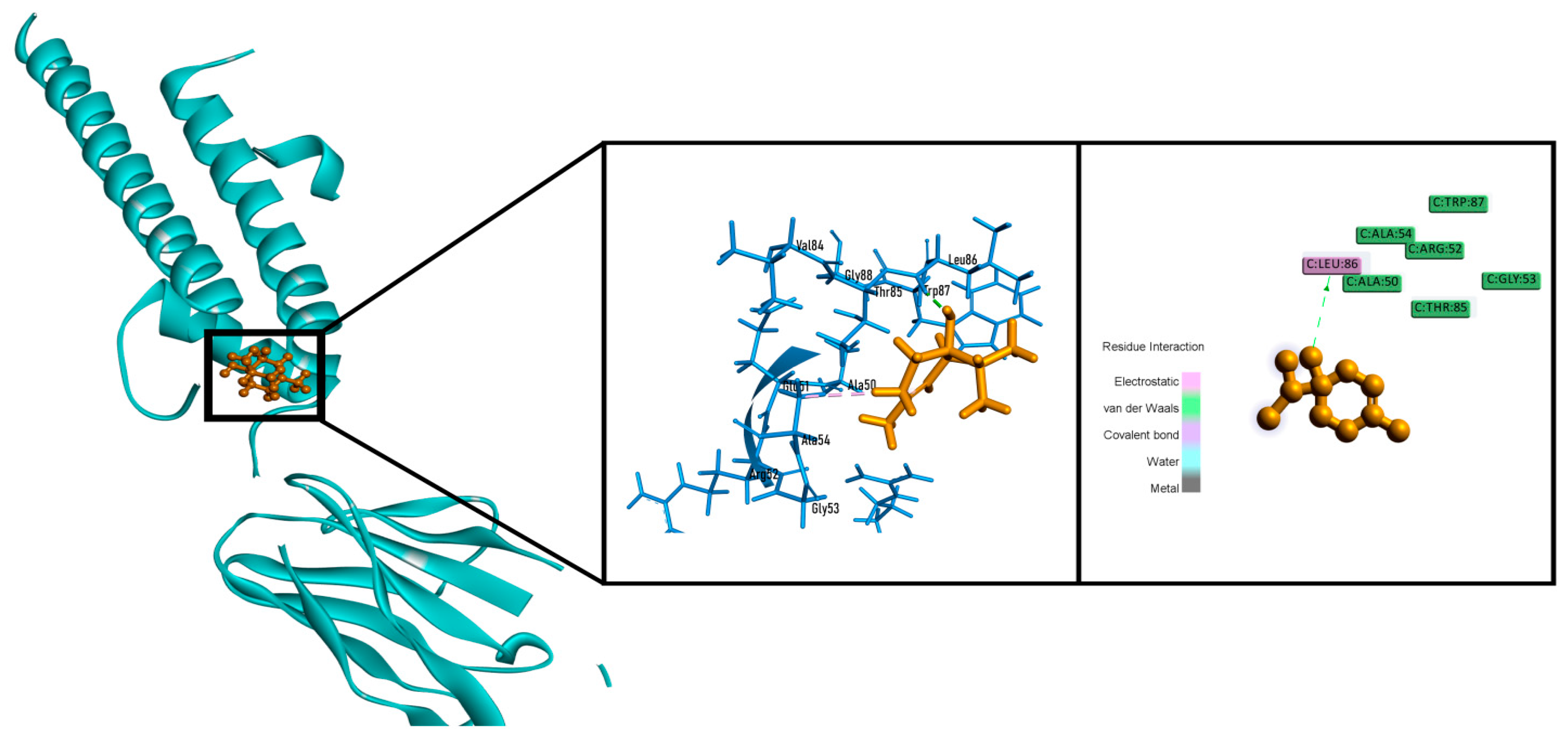
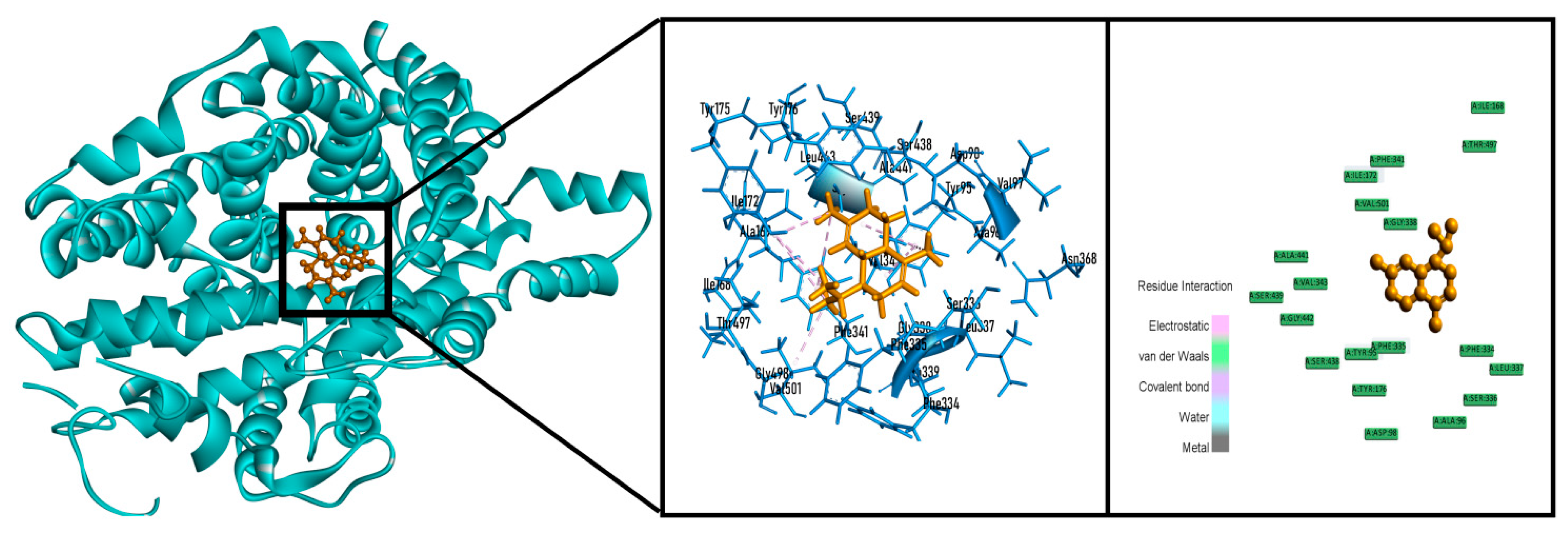
| Sample | Total Phenolics Content (mg GAE/g Extract) | Total Flavonoids Content (mg QE/g Extract) |
|---|---|---|
| MEHA | 147.716 ± 5.07 | 66.65 ± 6.208 |
| Compounds | Binding Score (kcal/mol) | Glide Emodel (kcal/mol) | Glide Energy (kcal/mol) |
|---|---|---|---|
| 1,2-Dimethylbenzene | −4.214 | −15.464 | −13.480 |
| 4-Terpineol | −6.457 | −23.043 | −16.968 |
| α-Cadinene | - | - | - |
| α-Pinene | −4.487 | −12.354 | −11.451 |
| Bullatantriol | −6.260 | −34.646 | −27.570 |
| Camphene | −4.470 | −7.083 | −6.289 |
| cis-Linalool oxide | −4.825 | −30.566 | −24.083 |
| Cryptone | −5.259 | −24.619 | −19.376 |
| Linalyl acetate | −5.116 | −34.935 | −29.617 |
| Nerol | −4.505 | −26.080 | −23.519 |
| Oplodiol | −6.182 | −33.777 | −25.516 |
| Spatulenol | −5.343 | −24.144 | −19.953 |
| T-Cadinol | −6.882 | −32.631 | −24.531 |
| Ascorbic acid | −6.407 | - | - |
| Compound | Binding Score (kcal/mol) | Glide Emodel (kcal/mol) | Glide Energy (kcal/mol) |
|---|---|---|---|
| 1,2-Dimethylbenzene | −4.260 | −19.110 | −15.447 |
| 4-Terpineol | −5.254 | −25.836 | −20.179 |
| α-Cadinene | −5.720 | −34.444 | −25.730 |
| α-Pinene | −3.975 | −19.073 | −15.385 |
| Bullatantriol | −4.350 | −31.223 | −25.604 |
| Camphene | −3.825 | −20.110 | −16.344 |
| cis-Linalool oxide | −3.794 | −27.360 | −23.111 |
| Cryptone | −4.537 | −25.031 | −19.913 |
| Linalyl acetate | −2.781 | −24.314 | −21.364 |
| Nerol | −2.702 | −24.865 | −21.837 |
| Oplodiol | −5.075 | −32.355 | −25.009 |
| Spatulenol | −6.190 | −31.911 | −25.145 |
| T-Cadinol | −6.300 | −35.571 | −26.388 |
| Streptokinase | −5.102 | - | - |
| Compounds | Binding Score (kcal/mol) | Glide Emodel (kcal/mol) | Glide Energy (kcal/mol) |
|---|---|---|---|
| 1,2-Dimethylbenzene | −3.658 | −11.707 | −9.535 |
| 4-Terpineol | −5.143 | −24.454 | −20.685 |
| α-Cadinene | −3.691 | −12.544 | −10.088 |
| α-Pinene | −2.928 | −12.540 | −10.571 |
| Bullatantriol | −4.098 | −23.075 | −16.971 |
| Camphene | −2.884 | −11.854 | −10.084 |
| cis-Linalool oxide | −3.512 | −25.666 | −21.378 |
| Cryptone | −3.442 | −18.344 | −14.733 |
| Linalyl acetate | −1.155 | −16.838 | −16.182 |
| Nerol | −2.924 | −24.224 | −21.507 |
| Oplodiol | - | - | - |
| Spatulenol | −2.810 | −17.092 | −14.612 |
| T-Cadinol | −2.457 | −6.109 | −5.696 |
| Diazepam | 3.140 | - | - |
| Compounds | Binding Score (kcal/mol) | Glide Emodel (kcal/mol) | Glide Energy (kcal/mol) |
|---|---|---|---|
| 1,2-Dimethylbenzene | −6.105 | −27.029 | −19.783 |
| 4-Terpineol | −5.078 | −27.507 | −20.217 |
| α-Cadinene | −7.188 | −37.235 | −27.179 |
| α-Pinene | −5.426 | −21.376 | −16.141 |
| Bullatantriol | −6.070 | −43.055 | −32.486 |
| Camphene | −5.729 | −26.921 | −19.908 |
| cis-Linalool oxide | −5.418 | −38.724 | −29.151 |
| Cryptone | −5.946 | −27.914 | −20.359 |
| Linalyl acetate | −3.648 | −31.580 | −26.453 |
| Nerol | −4.254 | −29.861 | −24.531 |
| Oplodiol | −6.948 | −39.856 | −29.137 |
| Spatulenol | −5.796 | −32.639 | −23.429 |
| T-Cadinol | −6.892 | −40.513 | −28.388 |
| Imipramine hydrochloride | 8.171 | - | - |
| Compounds | Lipinski Rules | Lipinski’s Violations ≤1 | Veber Rules | ||||
|---|---|---|---|---|---|---|---|
MW (g/mol) <500 | HBA <10 | HBD <5 | Log P ≤5 | nRB ≤10 | TPSA ≤140 | ||
| 1,2-Dimethylbenzene | 106.17 | 0 | 0 | 2.03 | No | 0 | 0.00 Å2 |
| 4-Terpineol | 154.25 | 1 | 1 | 2.51 | No | 1 | 20.23 Å2 |
| α-Cadinene | 204.35 | 0 | 0 | 3.38 | No | 1 | 0.00 Å2 |
| α-Cadinol | 222.37 | 1 | 1 | 3.15 | No | 1 | 20.23 Å2 |
| α-Pinene | 136.23 | 0 | 0 | 2.63 | No | 0 | 0.00 Å2 |
| Bullatantriol | 256.38 | 3 | 3 | 2.60 | No | 2 | 60.69 Å2 |
| Camphene | 136.23 | 0 | 0 | 2.58 | No | 0 | 0.00 Å2 |
| cis-Linalool oxide | 170.25 | 2 | 1 | 2.45 | No | 2 | 29.46 Å2 |
| Cryptone | 138.21 | 1 | 0 | 2.05 | No | 1 | 17.07 Å2 |
| Linalyl acetate | 196.29 | 2 | 0 | 3.08 | No | 6 | 26.30 Å2 |
| Nerol | 154.25 | 1 | 1 | 2.75 | No | 4 | 20.23 Å2 |
| Oplodiol | 238.37 | 2 | 2 | 2.98 | No | 1 | 40.46 Å2 |
| Spatulenol | 220.35 | 1 | 1 | 2.88 | No | 0 | 20.23 Å2 |
| T-Cadinol | 222.37 | 1 | 1 | 3.15 | No | 1 | 20.23 Å2 |
| Compounds | Parameters | ||||
|---|---|---|---|---|---|
| Ames Toxicity | Carcinogens | Acute Oral Toxicity | Rat Acute Toxicity (LD50, mol/kg) | ||
| 1,2-Dimethylbenzene | NAT | NC | III | 1.5513 | |
| 4-Terpineol | NAT | NC | III | 2.0424 | |
| α-Cadinene | AT | NC | III | 1.4298 | |
| α-Cadinol | NAT | NC | III | 2.2009 | |
| α-Pinene | NAT | NC | III | 1.5348 | |
| Bullatantriol | NAT | NC | III | 2.8543 | |
| Camphene | NAT | NC | III | 1.4664 | |
| cis-Linalool oxide | NAT | NC | III | 2.1384 | |
| Cryptone | NAT | NC | III | 1.8337 | |
| Linalyl acetate | NAT | C | IV | 1.4627 | |
| Nerol | NAT | NC | III | 1.6146 | |
| Oplodiol | NAT | NC | III | 2.9423 | |
| Spatulenol | NAT | NC | III | 2.5610 | |
| T-Cadinol | NAT | NC | III | 2.2009 | |
| Standards | Ascorbic acid | NAT | NC | IV | 1.3059 |
| Streptokinase | NAT | NC | II | 2.2785 | |
| Diazepam | NAT | NC | II | 2.5946 | |
| Imipramine hydrochloride | NAT | NC | III | 2.8931 | |
| Compounds | Human Intestinal Absorption | Human Oral Bioavailability | |
|---|---|---|---|
| 1,2-Dimethylbenzene | 0.9801 | 0.9143 | |
| 4-Terpineol | 0.9842 | 0.6286 | |
| α-Cadinene | 0.9778 | 0.7429 | |
| α-Cadinol | 0.9892 | 0.5143 | |
| α-Pinene | 0.9677 | 0.8286 | |
| Bullatantriol | 0.9841 | 0.6571 | |
| Camphene | 0.9677 | 0.6286 | |
| cis-Linalool oxide | 0.9626 | 0.5857 | |
| Cryptone | 0.9905 | 0.6857 | |
| Linalyl acetate | 0.9703 | 0.6143 | |
| Nerol | 0.9681 | 0.5429 | |
| Oplodiol | 0.9896 | 0.5857 | |
| Spatulenol | 0.9906 | 0.7330 | |
| T-Cadinol | 0.9892 | 0.5143 | |
| Standards | Ascorbic acid | 0.8150 | 0.5857 |
| Streptokinase | 0.4612 | 0.5857 | |
| Diazepam | 0.9948 | 0.9286 | |
| Imipramine hydrochloride | 0.9920 | 0.7286 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.S.; Sayem, S.A.J.; Habibullah; Quah, Y.; Lee, E.-B.; Birhanu, B.T.; Suk, K.; Park, S.-C. Investigation of Potential Antioxidant, Thrombolytic and Neuropharmacological Activities of Homalomena aromatica Leaves Using Experimental and In Silico Approaches. Molecules 2021, 26, 975. https://doi.org/10.3390/molecules26040975
Ali MS, Sayem SAJ, Habibullah, Quah Y, Lee E-B, Birhanu BT, Suk K, Park S-C. Investigation of Potential Antioxidant, Thrombolytic and Neuropharmacological Activities of Homalomena aromatica Leaves Using Experimental and In Silico Approaches. Molecules. 2021; 26(4):975. https://doi.org/10.3390/molecules26040975
Chicago/Turabian StyleAli, Md. Sekendar, Syed Al Jawad Sayem, Habibullah, Yixian Quah, Eon-Bee Lee, Biruk Tesfaye Birhanu, Kyoungho Suk, and Seung-Chun Park. 2021. "Investigation of Potential Antioxidant, Thrombolytic and Neuropharmacological Activities of Homalomena aromatica Leaves Using Experimental and In Silico Approaches" Molecules 26, no. 4: 975. https://doi.org/10.3390/molecules26040975
APA StyleAli, M. S., Sayem, S. A. J., Habibullah, Quah, Y., Lee, E.-B., Birhanu, B. T., Suk, K., & Park, S.-C. (2021). Investigation of Potential Antioxidant, Thrombolytic and Neuropharmacological Activities of Homalomena aromatica Leaves Using Experimental and In Silico Approaches. Molecules, 26(4), 975. https://doi.org/10.3390/molecules26040975