Isolation of Industrial Important Bioactive Compounds from Microalgae
Abstract
1. Introduction
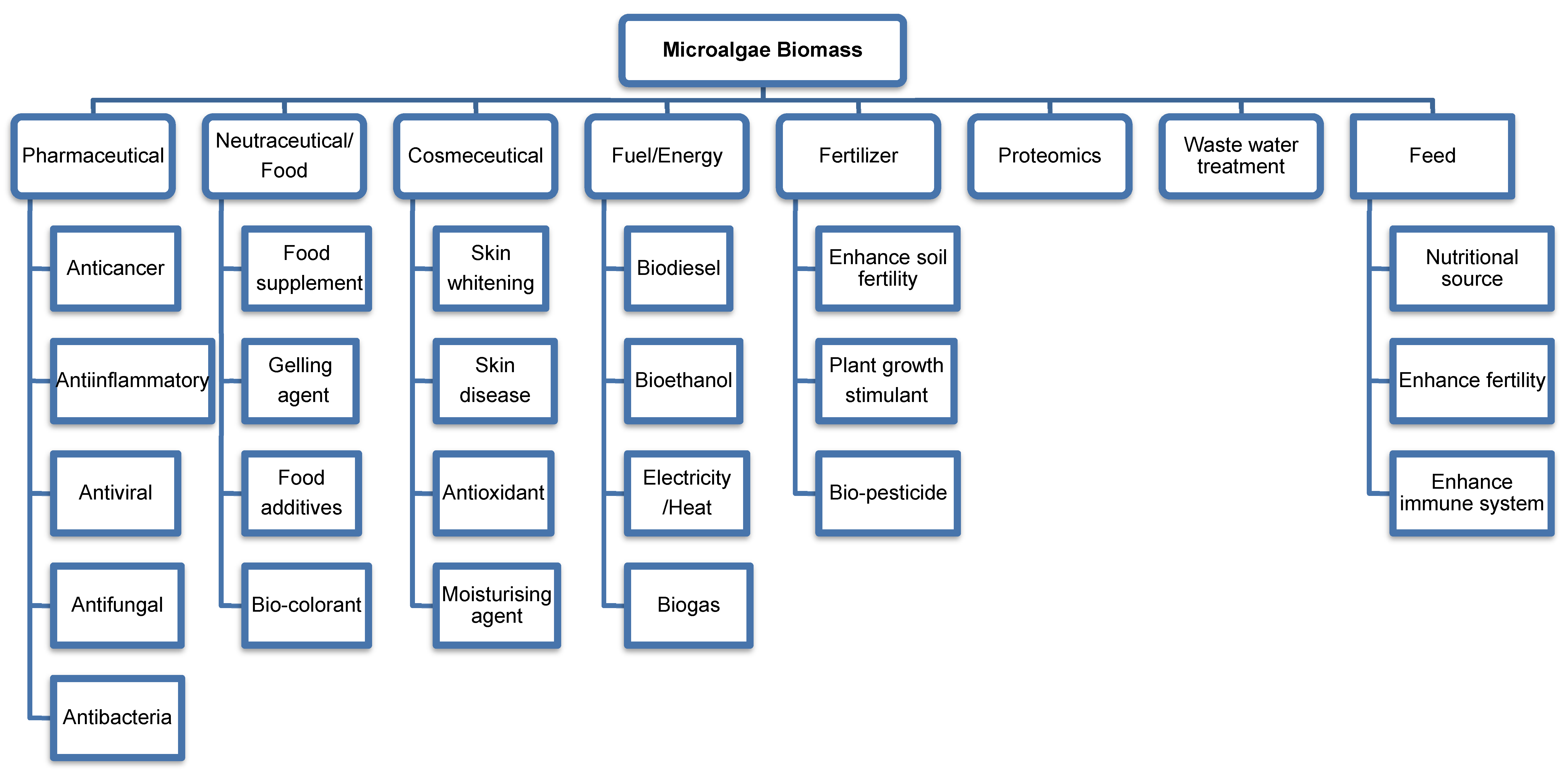
2. Microalgae Biomass
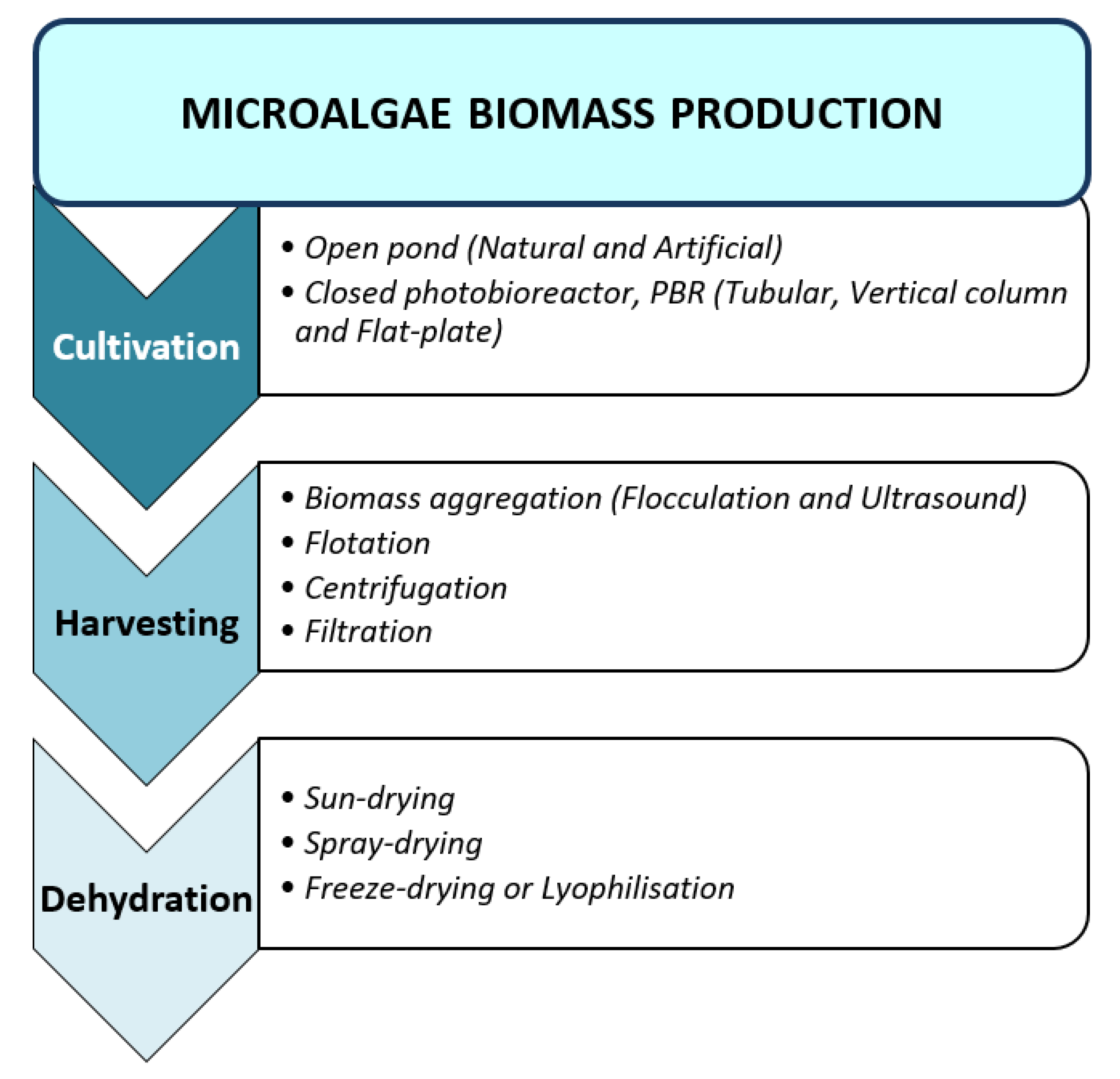
2.1. Biomass Production
2.1.1. Cultivation
2.1.2. Harvesting
2.1.3. Biomass Dehydration
2.2. Extraction of Bioactive Compound
3. Microalgae in Pharmaceuticals
3.1. Compounds with Anti-Cancer Properties
3.2. Compounds with Cardioprotective Properties
3.3. Compounds with Antiviral Properties
4. Microalgae in Nutraceuticals/Food
4.1. Algal Protein
4.2. Vitamins and Minerals
4.3. Fatty Acids
4.4. Natural Pigments
5. Microalgae in Cosmeceuticals
5.1. Anti-Ageing
5.2. Antioxidant/Stress Protection
5.3. Free Radical/Sunscreen Protection
5.4. Pigmenting Agent
5.5. Whitening Agent
5.6. Moisturising Agent
5.7. Anti-Inflammatory
5.8. Industrial Applications of Microalgae in Cosmeceuticals
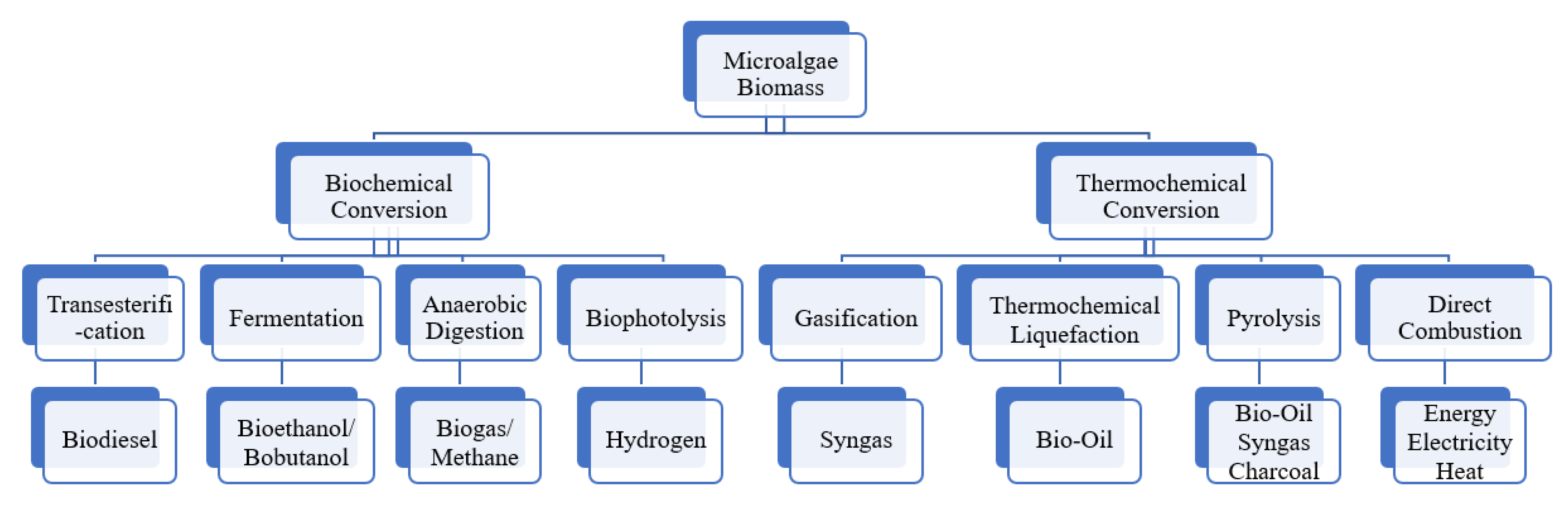
6. Microalgae in Biofuels and Energy
6.1. Biochemical Conversion
6.1.1. Transesterification-Biodiesel
6.1.2. Fermentation-Bioethanol
6.1.3. Anaerobic Digestion-Biogas/Methane
6.1.4. Biophotolysis-Hydrogen
6.2. Thermochemical Conversion
6.2.1. Gasification-Syngas
6.2.2. Thermochemical Liquefaction-Bio-Oil
6.2.3. Pyrolysis-Bio-Oil, Syngas, Charcoal
6.2.4. Direct Combustion-Energy (Heat/Electricity)
6.3. Industrial Applications of Microalgae in Biofuels
7. Microalgae in Biofertilisers
Industrial Applications of Microalgae Biofertiliser
8. Microalgae in Wastewater Treatment
9. Microalgae in Feed
10. Microalgae in Proteomics
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| ALA | α-linoleic acid |
| cGMP | current Good Manufacturing Practice |
| CPC | Chlorophytic polyculture |
| CRH | Corticotropin-releasing hormone |
| CV-N | Cyanovirin |
| DHA | Docosahexaenoic acid |
| DOE | U.S. Department of Energy |
| EAAI | Essential amino acid index |
| ECs | Emerging contaminants |
| EPA | Eicosapentaenoic acid |
| EAAI | Essential amino acid index |
| FDA | Food and Drug Administration |
| FP-PBR | Flat-plate open pond and closed photobioreactor |
| GRAS | Generally Regarded as Safe |
| HRAP | High rate algae pond |
| IC | Inhibitory concentration |
| L-DOPA | 3,4-dihydroxy-L-phenylalanine |
| m-MFC | Microalgae-microbial fuel cell |
| PBR | Open pond and closed photobioreactor |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SVN | Scytovirin |
| TPBR | Tubular open pond and closed photobioreactor |
| TAG | Triacylglycerides |
| TG | Triglyceride |
| UV | Ultraviolet |
| VCPBR | Vertical column open pond and closed photobioreactor |
| WW | Waste water |
| WWTPs | Waste water treatment plants |
References
- Pereira, A.G.; Jimenez-Lopez, C.; Fraga, M.; Lourenço-Lopes, C.; García-Oliveira, P.; Lorenzo, J.M.; Perez-Lamela, C.; Prieto, M.A.; Simal-Gandara, J. Extraction, properties, and applications of bioactive compounds obtained from microalgae. Curr. Pharm. Des. 2020, 26, 1929–1950. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.A.; Melkonian, M.; Andersen, R.A. Algal biodiversity. Phycologia 1996, 35, 308–326. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Meticulous Research. Algae Products Market by Value (Medium Value, High Value, And Low Value), Products (Hydrocolloids, Carotenoids, Omega-3 PUFA, Spirulina, Chlorella), Application (Food and Feed, Nutraceutical, Cosmetics, Chemicals)—Forecast. 2020. Available online: https://www.meticulousresearch.com/product/algae-products-market-forecast-2022 (accessed on 6 October 2020).
- Doshi, A.; Pascoe, S.; Coglan, L.; Rainey, T.J. Economic and policy issues in the production of algae-based biofuels: A review. Renew. Sust. Energy Rev. 2016, 64, 329–337. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Fact. 2018, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, J.; Zhou, Z.-G. Algae for biofuels: An emerging feedstock. In Handbook of Biofuels Production; Elsevier/Woodhead Publishing: Duxford, UK, 2016; pp. 673–698. [Google Scholar]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-based products for the food and feed sector: An outlook for Europe. In EUR—Scientific and Technical Research Reports; Vigani, M., Parisi, C., Cerezo, E.R., Eds.; Publications Office of the European Union: Luxembourg, 2014; pp. 9–18. [Google Scholar]
- Mendes, L.B.B.; Vermelho, A.B. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol. Biofuels 2013, 6, 1–14. [Google Scholar]
- Dragone, G.; Fern, B.; Vicente, A.A.; Teixeira, J.A. Third generation biofuels from microalgae. In Current Research, Technology and Educaion Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; pp. 1355–1366. [Google Scholar]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef]
- Bracharz, F.; Helmdach, D.; Aschenbrenner, I.; Funck, N.; Wibberg, D.; Winkler, A.; Bohnen, F.; Kalinowski, J.; Mehlmer, N.; Brück, T.B. Harvest of the oleaginous microalgae Scenedesmus obtusiusculus by flocculation from culture based on natural water sources. Front. Bioeng. Biotechnol. 2018, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Shobana, S.; Bakonyi, P.; Nemestóthy, N.; Xia, A.; Kumar, G. A review on chemical mechanism of microalgae flocculation via polymers. Biotechnol. Rep. 2019, 21, e00302. [Google Scholar] [CrossRef] [PubMed]
- Bosma, R.; van Spronsen, W.A.; Tramper, J.; Wijffels, R.H. Ultrasound, a new separation technique to harvest microalgae. J. Appl. Phycol. 2003, 15, 143–153. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Velan, M.; Saravanane, R. Pollution abatement and utilisation of flue gas for bioenergy production–A review. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 94–99. [Google Scholar]
- Alkarawi, M.A.; Caldwell, G.S.; Lee, J.G. Continuous harvesting of microalgae biomass using foam flotation. Algal Res. 2018, 36, 125–138. [Google Scholar] [CrossRef]
- Najjar, Y.S.; Abu-Shamleh, A. Harvesting of microalgae by centrifugation for biodiesel production: A review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Grima, E.M.; Belarbi, E.-H.; Fernández, F.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Heasman, M.; Diemar, J.; O’Connor, W.; Sushames, T.; Foulkes, L. Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs–A summary. Aquac. Res. 2000, 31, 637–659. [Google Scholar]
- Show, K.-Y.; Lee, D.-J.; Chang, J.-S. Algal biomass dehydration. Bioresour. Technol. 2013, 135, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Gultom, S.O.; Hu, B. Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies 2013, 6, 5921–5939. [Google Scholar] [CrossRef]
- De Farias Neves, F.; Demarco, M.; Tribuzi, G. Drying and quality of microalgal powders for human alimentation. In Microalgae-From Physiology to Application; Vítová, M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Mujumdar, A.S. Classification and selection of industrial dryers. In Mujumdar’s Practical Guide to Industrial Drying: Principles, Equipment and New Developments. Brossard, Canada: Exergex Corporation; Sakamon, D., Ed.; Exergex Corporation: Montreal, QC, Canada, 2000; pp. 23–77. [Google Scholar]
- Nappa, M.; Teir, S.; Sorsamäki, L.; Karinen, P. Energy Requirements of Microalgae Biomass Production; Technical Report for CCSP Deliverable D606 Espoo 2016; Research Output: Helsinki, Finland, 2016. [Google Scholar]
- Oyinloye, T.M.; Yoon, W.B. Effect of freeze-drying on quality and grinding process of food produce: A review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Ventura, S.; Nobre, B.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.; Palavra, A. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier/Woodhead Publishing: Duxford, UK, 2017; pp. 461–483. [Google Scholar]
- Günerken, E.; D’Hondt, E.; Eppink, M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Lucio, J.; Rodríguez-Jasso, R.M.; Colla, L.M.; Sáenz-Galindo, A.; Cervantes-Cisneros, D.E.; Aguilar, C.N.; Fernandes, B.D.; Ruiz, H.A. Microalgal biomass pretreatment for bioethanol production: A review. Biofuel Res. J. 2018, 5, 780–791. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Cheng, N.; Lin, L.; Zhang, C. Inhibitory effect of phycocyanin from Spirulina platensis on the growth of human leukemia K562 cells. J. Appl. Phycol. 2000, 12, 125–130. [Google Scholar] [CrossRef]
- Hao, S.; Yan, Y.; Li, S.; Zhao, L.; Zhang, C.; Liu, L.; Wang, C. The in vitro anti-tumor activity of phycocyanin against non-small cell lung cancer cells. Mar. Drugs 2018, 16, 178. [Google Scholar] [CrossRef]
- Hao, S.; Li, S.; Wang, J.; Yan, Y.; Ai, X.; Zhang, J.; Ren, Y.; Wu, T.; Liu, L.; Wang, C. Phycocyanin exerts anti-proliferative effects through down-regulating TIRAP/NF-κB activity in human non-small cell lung cancer cells. Cells 2019, 8, 588. [Google Scholar] [CrossRef]
- Deniz, I.; Ozen, M.O.; Yesil-Celiktas, O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. J. Supercrit. Fluids 2016, 108, 13–18. [Google Scholar] [CrossRef]
- Gantar, M.; Dhandayuthapani, S.; Rathinavelu, A. Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line LNCaP. J. Med. Food 2012, 15, 1091–1095. [Google Scholar] [CrossRef]
- Safaei, M.; Maleki, H.; Soleimanpour, H.; Norouzy, A.; Zahiri, H.; Vali, H.; Noghabi, K. Development of a novel method for the purification of C-phycocyanin pigment from a local cyanobacterial strain Limnothrix sp. NS01 and evaluation of its anticancer properties. Sci. Rep. 2019, 9, 9474. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Abecassis, R.; Relevi, N.; Levi, Z.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. Prevention of atherosclerosis progression by 9-cis-β-carotene rich alga Dunaliella in apoE-deficient mice. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Briskey, D.; Nalley, J.O.; Ganuza, E. Omega-3 eicosapentaenoic acid (EPA) rich extract from the microalga nannochloropsis decreases cholesterol in healthy individuals: A double-blind, randomized, placebo-controlled, three-month supplementation study. Nutrients 2020, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef]
- Bokesch, H.R.; O’Keefe, B.R.; McKee, T.C.; Pannell, L.K.; Patterson, G.M.; Gardella, R.S.; Sowder, R.C.; Turpin, J.; Watson, K.; Buckheit, R.W. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry 2003, 42, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, E.N.; Mentel, R.; Wray, V.; Jansen, R.; Nimtz, M.; Lalk, M.; Mundt, S. Cyclic depsipeptides, ichthyopeptins A and B, from Microcystis ichthyoblabe. J. Nat. Prod. 2007, 70, 1084–1088. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; Junior, R.G.D.O.; Fuentes-Grünewald, C.; Picot, L. Analysis of scientific research driving microalgae market opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.J.; Ben-Amotz, A. Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Gilroy, D.J.; Kauffman, K.W.; Hall, R.A.; Huang, X.; Chu, F.S. Assessing potential health risks from microcystin toxins in blue-green algae dietary supplements. Environ. Health Perspect. 2000, 108, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Martínez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann. Nutr. Metab. 2008, 52, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.; Troisi, F.; Thomas, L. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- FEBICO. ApoX Surface Anti-Viral Spray. Available online: https://www.febico.com/en/product/Spray.html (accessed on 8 October 2020).
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A Review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; de Morais, A.M.M.B. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef]
- Winwood, R.J. Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. OCL 2013, 20, D604. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Sowder, R.C.; Henderson, L.E.; Cardellina, J.H.; McMahon, J.B.; Rajamani, U.; Pannell, L.K.; Boyd, M.R. Isolation, primary sequence determination, and disulfide bond structure of cyanovirin-N, an anti-HIV (human immunodeficiency virus) protein from the CyanobacteriumNostoc ellipsosporum. Biochem. Biophys. Res. Commun. 1997, 238, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Schols, D. Algal lectins as potential HIV microbicide candidates. Mar. Drugs 2012, 10, 1476–1497. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.; Zubeck, H. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J. Nutr. Food Sci. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Bioeconomy BW. Microalgae Can Produce More than Just Fuel. Available online: https://www.biooekonomie-bw.de/en/articles/news/microalgae-can-produce-more-than-just-fuel (accessed on 10 September 2020).
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Kumudha, A.; Selvakumar, S.; Dilshad, P.; Vaidyanathan, G.; Thakur, M.S.; Sarada, R. Methylcobalamin–A form of vitamin B12 identified and characterised in Chlorella vulgaris. Food Chem. 2015, 170, 316–320. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognised as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Denery, J.R.; Dragull, K.; Tang, C.; Li, Q.X. Pressurized fluid extraction of carotenoids from Haematococcus pluvialis and Dunaliella salina and kavalactones from Piper methysticum. Anal. Chim. Acta 2004, 501, 175–181. [Google Scholar] [CrossRef]
- Haque, F.; Dutta, A.; Thimmanagari, M.; Chiang, Y.W. Intensified green production of astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 1–11. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Tech. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; da Silva, T.L. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Kagan, M.L.; Levy, A.; Leikin-Frenkel, A. Comparative study of tissue deposition of omega-3 fatty acids from polar-lipid rich oil of the microalgae Nannochloropsis oculata with krill oil in rats. Food Funct. 2015, 6, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kagan, M.L.; West, A.L.; Zante, C.; Calder, P.C. Acute appearance of fatty acids in human plasma–a comparative study between polar-lipid rich oil from the microalgae Nannochloropsis oculata and krill oil in healthy young males. Lipids Health Dis. 2013, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef] [PubMed]
- Marcati, A.; Ursu, A.V.; Laroche, C.; Soanen, N.; Marchal, L.; Jubeau, S.; Djelveh, G.; Michaud, P. Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res. 2014, 5, 258–263. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Ahuja, K.; Singh, S. Algae Protein Market. Size by Source; Global Market Insights, Inc.: Selbyville, DE, USA, 2020. [Google Scholar]
- Capelli, B.; Cysewski, G.R. Potential health benefits of spirulina microalgae. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- CHITOSE Group. Tavelmout; A Japanese Algae-Based Protein Start-Up; Raised Total 1.7 Billion Japanese Yen. Preparing for World Wide Increasing Protein Demand. Available online: https://chitose-bio.com/news/792/ (accessed on 10 September 2020).
- Siva Kiran, R.; Madhu, G.; Satyanarayana, S. Spirulina in combating protein energy malnutrition (PEM) and protein energy wasting (PEW)—A review. J. Nutr. Res. 2015, 3, 62–79. [Google Scholar]
- Ak, B.; Avsaroglu, E.; Isik, O.; Özyurt, G.; Kafkas, E.; Etyemez, M. Nutritional and physicochemical characteristics of bread enriched with microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 6, 30–38. [Google Scholar]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L. Development of new microalgae-based sourdough “crostini”: Functional effects of Arthrospira platensis (spirulina) addition. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grahl, S.; Strack, M.; Weinrich, R.; Mörlein, D. Consumer-oriented product development: The conceptualization of novel food products based on spirulina (arthrospira platensis) and resulting consumer expectations. J. Food Qual. 2018, 2018. [Google Scholar] [CrossRef]
- Stanic-Vucinic, D.; Minic, S.; Nikolic, M.R.; Velickovic, T.C. Spirulina phycobiliproteins as food components and complements. In Microalgal Biotechnology; IntechOpen: London, UK, 2018. [Google Scholar]
- Chiong, T.; Acquah, C.; Lau, S.; Khor, E.; Danquah, M. Microalgal-based protein by-products: Extraction, purification, and applications. In Protein Byproducts; Elsevier/Academic Press: London, UK, 2016; pp. 213–234. [Google Scholar]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.R.; Chew, K.W.; Mohd Zaid, H.F.; Chu, D.-T.; Tao, Y.; Show, P.L. Microalgal protein extraction from Chlorella vulgaris FSP-E using triphasic partitioning technique with sonication. Front. Bioeng. Biotechnol. 2019, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Energy and Protein Requirement; Report of a Joint FAO/WHO ad hoc Expert Committee; World Health Organization (WHO): Geneva, Switzerland, 1973. [Google Scholar]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional composition, nutritional properties, and biological activities of Moroccan Spirulina microalga. J. Food Qual. 2019, 2019. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Annapurna, V.; Deosthale, Y.; Bamji, M.S. Spirulina as a source of vitamin A. Plant Foods Hum. Nutr. 1991, 41, 125–134. [Google Scholar] [CrossRef]
- Annapurna, V.; Shah, N.; Bhaskaram, P.; Bamji, M.S.; Reddy, V. Bioavailability of spirulina carotenes in preschool children. J. Clin. Biochem. Nutr. 1991, 10, 145–151. [Google Scholar] [CrossRef]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Fujita, T.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Pseudovitamin B12 is the predominant cobamide of an algal health food, spirulina tablets. J. Agric. Food Chem. 1999, 47, 4736–4741. [Google Scholar] [CrossRef] [PubMed]
- Madhubalaji, C.K.; Rashmi, V.; Chauhan, V.S.; Shylaja, M.D.; Sarada, R. Improvement of vitamin B12 status with Spirulina supplementation in Wistar rats validated through functional and circulatory markers. J. Food Biochem. 2019, 43, e13038. [Google Scholar] [CrossRef]
- Puyfoulhoux, G.; Rouanet, J.-M.; Besançon, P.; Baroux, B.; Baccou, J.-C.; Caporiccio, B. Iron availability from iron-fortified spirulina by an in vitro digestion/Caco-2 cell culture model. J. Agric. Food Chem. 2001, 49, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Pinteus, S.; Reboleira, J.; Pedrosa, R.; Bernardino, S. Chapter 3.10—Chlorella. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Elsevier/Academic Press: London, UK, 2019; pp. 187–193. [Google Scholar] [CrossRef]
- Merchant, R.E.; Phillips, T.W.; Udani, J. Nutritional supplementation with Chlorella pyrenoidosa lowers serum methylmalonic acid in vegans and vegetarians with a suspected vitamin B12 deficiency. J. Med. Food 2015, 18, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Takekoshi, H.; Nakano, M. Chlorella pyrenoidosa supplementation reduces the risk of anemia, proteinuria and edema in pregnant women. Plant Foods Hum. Nutr. 2010, 65, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Dal Bosco, C.; di Caprio, F.; Prosini, L.; Gentili, A.; Pagnanelli, F.; Palocci, C. Extraction of carotenoids and fat-soluble vitamins from Tetradesmus Obliquus microalgae: An optimized approach by using supercritical CO2. Molecules 2019, 24, 2581. [Google Scholar] [CrossRef]
- Kumudha, A.; Sarada, R. Effect of different extraction methods on vitamin B12 from blue green algae, Spirulina platensis. Pharm. Anal. Acta 2015, 6. [Google Scholar] [CrossRef]
- Tomdio, A.; Ritchie, M.; Miller, A.C. Omega-3 fatty acids and cardiovascular disease prevention. Am. Fam. Physician 2019, 100, 209–210. [Google Scholar]
- Shahidi, F. Omega-3 oils: Sources, applications, and health effects. In Marine Nutraceuticals and Functional Foods; Shahidi, F., Barrow, C., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2008; pp. 23–61. [Google Scholar]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of total lipid yield by nitrogen, carbon, and iron supplementation in isolated microalgae. J. Phycol. 2017, 53, 855–868. [Google Scholar] [CrossRef]
- Goncalves, E.C.; Wilkie, A.C.; Kirst, M.; Rathinasabapathi, B. Metabolic regulation of triacylglycerol accumulation in the green algae: Identification of potential targets for engineering to improve oil yield. Plant Biotechnol. J. 2016, 14, 1649–1660. [Google Scholar] [CrossRef]
- Wang, X.; Fosse, H.K.; Li, K.; Chauton, M.S.; Vadstein, O.; Reitan, K.I. Influence of nitrogen limitation on lipid accumulation and EPA and DHA content in four marine microalgae for possible use in aquafeed. Front. Mar. Sci. 2019, 6, 95. [Google Scholar] [CrossRef]
- Hoffmann, M.; Marxen, K.; Schulz, R.; Vanselow, K.H. TFA and EPA productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar. Drugs 2010, 8, 2526–2545. [Google Scholar] [CrossRef] [PubMed]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Sato, N.; Tsuzuki, M.; Kawaguchi, A. Glycerolipid synthesis in Chlorella kessleri 11 h: II. Effect of the CO2 concentration during growth. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1633, 35–42. [Google Scholar] [CrossRef]
- Tatsuzawa, H.; Takizawa, E.; Wada, M.; Yamamoto, Y. Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. 1. J. Phycol. 1996, 32, 598–601. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Lorente, E.; Hapońska, M.; Clavero, E.; Torras, C.; Salvadó, J. Steam explosion and vibrating membrane filtration to improve the processing cost of microalgae cell disruption and fractionation. Processes 2018, 6, 28. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef]
- Coward, T.; Fuentes-Grünewald, C.; Silkina, A.; Oatley-Radcliffe, D.L.; Llewellyn, G.; Lovitt, R.W. Utilising light-emitting diodes of specific narrow wavelengths for the optimization and co-production of multiple high-value compounds in Porphyridium purpureum. Bioresour. Technol. 2016, 221, 607–615. [Google Scholar] [CrossRef]
- Gao, B.; Chen, A.; Zhang, W.; Li, A.; Zhang, C. Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J. Ocean Univ. China 2017, 16, 916–924. [Google Scholar] [CrossRef]
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the health implications of synthetic and natural food colourants—A critical review. UK J. Pharm. Biosci. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Schultz, H. NutraIngredients USA: Chinese Suppliers Angling to Snare Bigger Share of Natural Astaxanthin Market. Available online: https://www.nutraingredients-usa.com/Article/2015/04/13/Chinese-suppliers-angling-to-snare-bigger-share-of-natural-astaxanthin-market (accessed on 5 September 2020).
- Chacón-Lee, T.; González-Mariño, G. Microalgae for “Healthy” Foods—Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Rabie, M. Algal and Algal Extract Dietary Supplement Composition. U.S. Patent Application WO 2007/062274 A1, 31 May 2007. [Google Scholar]
- Silva, S.C.; Ferreira, I.C.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.N. Algae as a source of phycocyanin and other industrially important pigments. In Algal Biorefinery: An Integrated Approach; Springer International Publishing and Capital Publishing Company: New Delhi, India, 2015; pp. 253–276. [Google Scholar]
- Chen, C.-Y.; Kao, P.-C.; Tan, C.H.; Show, P.L.; Cheah, W.Y.; Lee, W.-L.; Ling, T.C.; Chang, J.-S. Using an innovative pH-stat CO2 feeding strategy to enhance cell growth and C-phycocyanin production from Spirulina platensis. Biochem. Eng. J. 2016, 112, 78–85. [Google Scholar] [CrossRef]
- Bryant, D.A.; Guglielmi, G.; de Marsac, N.T.; Castets, A.-M.; Cohen-Bazire, G. The structure of cyanobacterial phycobilisomes: A model. Arch. Microbiol. 1979, 123, 113–127. [Google Scholar] [CrossRef]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S. Use of phycobiliproteins from atacama cyanobacteria as food colorants in a dairy beverage prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef]
- USFDA. Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. Available online: https://www.fda.gov/industry/color-additive-inventories/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices (accessed on 17 September 2020).
- Hejazi, M.A.; Wijffels, R.H. Milking of microalgae. Trends Biotechnol. 2004, 22, 189–194. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Jiang, J.-G. Continuous cultivation of Dunaliella salina in photobioreactor for the production of β-carotene. Eur. Food Res. Technol. 2008, 227, 953–959. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Golzary, A. Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol. 2020, 101771. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P.J. Carotenoid production by Dunaliella salina under red light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Ruane, M. Extraction of Caroteniferous Materials from Algae. Australian Patent No. 7,239,574, 1977. [Google Scholar]
- Pirwitz, K.; Flassig, R.J.; Rihko-Struckmann, L.K.; Sundmacher, K. Energy and operating cost assessment of competing harvesting methods for D. salina in a β-carotene production process. Algal Res. 2015, 12, 161–169. [Google Scholar] [CrossRef]
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1. 1 under optimized culture conditions. PLoS ONE 2019, 14, e0221930. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Singh, Y.; Khattar, J.; Singh, D. Phycobiliprotein production by a novel cold desert cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1. J. Appl. Phycol. 2017, 29, 1819–1827. [Google Scholar] [CrossRef]
- Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. J. Nutr. Biochem. 2007, 18, 287–296. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef]
- Pangestuti, R.; Suryaningtyas, I.T.; Siahaan, E.A.; Kim, S.-K. Cosmetics and cosmeceutical applications of microalgae pigments. In Pigments from Microalgae Handbook; Springer Nature: Cham, Switzerland, 2020; pp. 611–633. [Google Scholar]
- Sawant, S.S.; Mane, V.K. Correlating the anti-aging activity with the bioactive profile of Chlorella emersonii KJ725233; its Toxicological Studies for a Potential use in Cosmeceuticals. Pharmacogn. Commun. 2017, 7, 152–157. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Recovery of functional pigments from four different species of microalgae. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 26–30. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Ahmed, I.; Iqbal, H.M. High-value compounds from microalgae with industrial exploitability—A review. Front. Biosci. 2017, 9, 319–342. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Arvela, P.; Hachemi, I.; Murzin, D.Y. Comparative study of the extraction methods for recovery of carotenoids from algae: Extraction kinetics and effect of different extraction parameters. J. Chem. Technol. Biotechnol. 2014, 89, 1607–1626. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L.; Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Ryu, B.; Himaya, S.; Kim, S.-K. Applications of microalgae-derived active ingredients as cosmeceuticals. In Handbook of Marine Microalgae; Elsevier/Academic Press: London, UK, 2015; pp. 309–316. [Google Scholar]
- Kim, H.M.; Jung, J.H.; Kim, J.Y.; Heo, J.; Cho, D.H.; Kim, H.S.; An, S.; An, I.S.; Bae, S. The protective effect of violaxanthin from Nannochloropsis oceanica against ultraviolet B-induced damage in normal human dermal fibroblasts. Photochem. Photobiol. 2019, 95, 595–604. [Google Scholar] [CrossRef]
- Matsui, M.; Tanaka, K.; Higashiguchi, N.; Okawa, H.; Yamada, Y.; Tanaka, K.; Taira, S.; Aoyama, T.; Takanishi, M.; Natsume, C. Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci. 2016, 132, 55–64. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilisation of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Chakdar, H.; Pabbi, S. Algal pigments for human health and cosmeceuticals. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–188. [Google Scholar]
- Biba, E. Protection: The sunscreen pill. Nature 2014, 515, S124–S125. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-T.; Chen, P.-Y.; Wu, J.-J.; Lee, T.-M.; Hsu, S.-L.; Chang, C.-M.J.; Young, C.-C.; Shieh, C.-J. Purification of algal anti-tyrosinase zeaxanthin from Nannochloropsis oculata using supercritical anti-solvent precipitation. J. Supercrit. Fluids 2011, 55, 955–962. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Bonté, F. Skin moisturization mechanisms: New data. Ann. Pharm. Fr. 2011, 69, 135–141. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Zanella, L.; Alam, M.A. Extracts and bioactives from microalgae (sensu stricto): Opportunities and challenges for a new generation of cosmetics. In Microalgae Biotechnology for Food, Health and High Value Products; Springer Nature: Singapore, 2020; pp. 295–349. [Google Scholar]
- Zmijewski, M.A.; Slominski, A.T. Neuroendocrinology of the skin: An overview and selective analysis. Dermatoendocrinology 2011, 3, 3–10. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Chrousos, G.P. Stress-related skin disorders. Rev. Endocr. Metab. Disord. 2016, 17, 295–304. [Google Scholar] [CrossRef]
- Truzzi, F.; Marconi, A.; Pincelli, C. Neurotrophins in healthy and diseased skin. Dermatoendocrinology 2011, 3, 32–36. [Google Scholar] [CrossRef]
- Borroni, R.; Truzzi, F.; Pincelli, C. The skin neurotrophic network in health and disease. Actas Dermo-Sifiliogr. 2009, 100, 70–74. [Google Scholar] [CrossRef]
- Grewe, M.; Vogelsang, K.; Ruzicka, T.; Stege, H.; Krutmann, J. Neurotrophin-4 production by human epidermal keratinocytes: Increased expression in atopic dermatitis. J. Investig. Dermatol. 2000, 114, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, S.; Daniltchenko, M.; Tobin, D.J.; Hagen, E.; Hunt, S.P.; Klapp, B.F.; Arck, P.C.; Peters, E.M. Further exploring the brain–skin connection: Stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J. Investig. Dermatol. 2008, 128, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Kinkelin, I.; Bröcker, E.-B.; Koltzenburg, M.; Carlton, S.M. Localization of ionotropic glutamate receptors in peripheral axons of human skin. Neurosci. Lett. 2000, 283, 149–152. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidve, P.; Prajapati, N.; Kalia, K.; Tiwari, V. Astaxanthin ameliorates behavioral and biochemical alterations in in-vitro and in-vivo model of neuropathic pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Tredici, M.R.; Rodolfi, L.; Biondi, N.; Bassi, N.; Sampietro, G. Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant. Algal Res. 2016, 19, 253–263. [Google Scholar] [CrossRef]
- Fernandez, F.G.A.; Sevilla, J.M.F.; Grima, E.M. Microalgae: The basis of mankind sustainability. In Case Study of Innovative Projects-Successful Real Cases; Llamas, B., Ed.; Intech Open: London, UK, 2017; pp. 123–140. [Google Scholar]
- Algae World News. Josie Maran Argan Beta Retinoid Pink Algae Serum is Unlike Anything Else. Available online: https://news.algaeworld.org/2020/01/josie-maran-argan-beta-retinoid-pink-algae-serum-is-unlike-anything-else/#more-41347 (accessed on 18 September 2020).
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Morvan, P.; Vallee, R. Effects of Chlorella Extract on Skin—Personal Care Magazine. Available online: https://www.kako-shujsati.com/hujsanje-trebuh/images/chlorella-extract-on-skin.pdf (accessed on 19 September 2020).
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Maiz, D. The underwater world: A source of inexhaustible inspiration. Parf. Cosmet. Actual 2007, 194, 136–160. [Google Scholar]
- Campiche, R.; Sandau, P.; Kurth, E.; Massironi, M.; Imfeld, D.; Schuetz, R. Protective effects of an extract of the freshwater microalga Scenedesmus rubescens on UV-irradiated skin cells. Int. J. Cosmet. Sci. 2018, 40, 187–192. [Google Scholar] [CrossRef]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269. [Google Scholar]
- Hussian, A. The role of microalgae in renewable energy production: Challenges and opportunities. In Marine Ecology-Biotic and Abiotic Interactions; Türkoğlu, M., Önal, U., Ismen, A., Eds.; Intech Open: London, UK, 2018; pp. 257–283. [Google Scholar] [CrossRef]
- Musa, M.; Ayoko, G.A.; Ward, A.; Rösch, C.; Brown, R.J.; Rainey, T.J. Factors affecting microalgae production for biofuels and the potentials of chemometric methods in assessing and optimizing productivity. Cells 2019, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Bayro-Kaiser, V.; Nelson, N. Microalgal hydrogen production: Prospects of an essential technology for a clean and sustainable energy economy. Photosynth. Res. 2017, 133, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Cruz, Y.R.; Aranda, D.A.; Seidl, P.R.; Diaz, G.C.; Carliz, R.G.; Fortes, M.M.; da Ponte, D.; de Paula, R.C. Cultivation systems of microalgae for the production of biofuels. In Biofuels-State of Development; Biernat, K., Ed.; Intech Open: London, UK, 2018; pp. 199–218. [Google Scholar]
- Abo, B.O.; Odey, E.A.; Bakayoko, M.; Kalakodio, L. Microalgae to biofuels production: A review on cultivation, application and renewable energy. Rev. Environ. Health 2019, 34, 91–99. [Google Scholar] [CrossRef]
- USDOE. Biomass Conversion: From Feedstocks to Final Products. Available online: https://www.energy.gov/sites/prod/files/2016/07/f33/conversion_factsheet.pdf (accessed on 1 October 2020).
- Nasreen, S.; Nafees, M.; Qureshi, L.A.; Asad, M.S.; Sadiq, A.; Ali, S.D. Review of catalytic transesterification methods for biodiesel production. In Biofuels: State of Development; Biernat, K., Ed.; Intech Open: London, UK, 2018; pp. 93–119. [Google Scholar]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M.M. A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzyme Res. 2011, 2011. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef]
- Sheehan, J.; Camobreco, V.; Duffield, J.; Graboski, M.; Shapouri, H. An Overview of Biodiesel and Petroleum Diesel Life Cycles; National Renewable Energy Lab. (NREL): Golden, CO, USA; US Department of Energy (DOE): Oak Ridge, TN, USA, 1998.
- Wang, H.; Ji, C.; Bi, S.; Zhou, P.; Chen, L.; Liu, T. Joint production of biodiesel and bioethanol from filamentous oleaginous microalgae Tribonema sp. Bioresour. Technol. 2014, 172, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Rempel, A.; Machado, T.; Treichel, H.; Colla, E.; Margarites, A.C.; Colla, L.M. Saccharification of Spirulina platensis biomass using free and immobilized amylolytic enzymes. Bioresour. Technol. 2018, 263, 163–171. [Google Scholar] [CrossRef] [PubMed]
- De Farias Silva, C.E.; Bertucco, A. Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- Astolfi, A.L.; Rempel, A.; Cavanhi, V.A.F.; Alves, M.; Deamici, K.M.; Colla, L.M.; Costa, J.A.V. Simultaneous saccharification and fermentation of Spirulina sp. and corn starch for the production of bioethanol and obtaining biopeptides with high antioxidant activity. Bioresour. Technol. 2020, 301, 122698. [Google Scholar] [CrossRef]
- Patidar, J.; Sharma, R.; Yadav, M.; Tiwari, A. Microalgae as sustainable renewable energy feedstock for bioethanol production. Sch. Acad. J. Biosci. 2017, 5, 536–542. [Google Scholar] [CrossRef]
- Kim, H.M.; Oh, C.H.; Bae, H.-J. Comparison of red microalgae (Porphyridium cruentum) culture conditions for bioethanol production. Bioresour. Technol. 2017, 233, 44–50. [Google Scholar] [CrossRef]
- Chandra, N.; Shukla, P.; Mallick, N. Role of cultural variables in augmenting carbohydrate accumulation in the green microalga Scenedesmus acuminatus for bioethanol production. Biocatal. Agric. Biotechnol. 2020, 26, 101632. [Google Scholar] [CrossRef]
- Harun, R.; Jason, W.; Cherrington, T.; Danquah, M.K. Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl. Energy 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Guo, H.; Daroch, M.; Liu, L.; Qiu, G.; Geng, S.; Wang, G. Biochemical features and bioethanol production of microalgae from coastal waters of Pearl River Delta. Bioresour. Technol. 2013, 127, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Li, P.-J.; Liu, C.-C.; Chang, J.-S. Bioprocess development on microalgae-based CO2 fixation and bioethanol production using Scenedesmus obliquus CNW-N. Bioresour. Technol. 2013, 145, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rizza, L.S.; Smachetti, M.E.S.; Do Nascimento, M.; Salerno, G.L.; Curatti, L. Bioprospecting for native microalgae as an alternative source of sugars for the production of bioethanol. Algal Res. 2017, 22, 140–147. [Google Scholar] [CrossRef]
- Wu, N.; Moreira, C.M.; Zhang, Y.; Doan, N.; Yang, S.; Phlips, E.J.; Svoronos, S.A.; Pullammanappallil, P.C. Techno-economic analysis of biogas production from microalgae through anaerobic digestion. In Anaerobic Digestion; IntechOpen: London, UK, 2019. [Google Scholar]
- Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A brief review of anaerobic digestion of algae for bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef]
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from microalgae: Review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal pretreatment of microalgae for biomethane production: Experimental studies, kinetics and energy analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Yu, J.; Takahashi, P. Biophotolysis-based hydrogen production by cyanobacteria and green microalgae. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2007; Volume 1, pp. 79–89. [Google Scholar]
- Nagarajan, D.; Lee, D.-J.; Kondo, A.; Chang, J.-S. Recent insights into biohydrogen production by microalgae–From biophotolysis to dark fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Maneeruttanarungroj, C.; Lindblad, P.; Incharoensakdi, A. A newly isolated green alga, Tetraspora sp. CU2551, from Thailand with efficient hydrogen production. Int. J. Hydrogen Energy 2010, 35, 13193–13199. [Google Scholar] [CrossRef]
- Eroglu, E.; Melis, A. Photobiological hydrogen production: Recent advances and state of the art. Bioresour. Technol. 2011, 102, 8403–8413. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Kim, H.-C.; Choi, J.-A.; Abou-Shanab, R.; Dempsey, B.A.; Regan, J.M.; Kim, J.R.; Song, H.; Nam, I.-H.; Kim, S.-N. Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat. Commun. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Giannelli, L. Advances in the biotechnology of hydrogen production with the microalga Chlamydomonas reinhardtii. Crit. Rev. Biotechnol. 2015, 35, 485–496. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 2010, 9, 486–501. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef]
- Adnan, M.A.; Hossain, M.M. CO2 gasification of microalgae (N. Oculata)–A thermodynamic study. MATEC Web Conf. 2018, 154, 01002. [Google Scholar] [CrossRef]
- USDOE. Biofuel Basics. Available online: https://www.energy.gov/eere/bioenergy/biofuels-basics (accessed on 1 October 2020).
- Xu, Y.-P.; Duan, P.-G.; Wang, F.; Guan, Q.-Q. Liquid fuel generation from algal biomass via a two-step process: Effect of feedstocks. Biotechnol. Biofuels 2018, 11, 83. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Wang, X.; Zhang, B.; Tian, W.; Zhang, J. Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Bioresour. Technol. 2018, 250, 474–480. [Google Scholar] [CrossRef]
- Adamczyk, M.; Sajdak, M. Pyrolysis behaviours of microalgae Nannochloropsis gaditana. Waste Biomass Valorization 2018, 9, 2221–2235. [Google Scholar] [CrossRef]
- International Energy Agency (IEA) Bioenergy. Task 34: Biomass Pyrolysis; Bioenergy Research Group, Aston University: Birmingham, UK, 2007; pp. 1–20. [Google Scholar]
- Chye, J.T.T.; Jun, L.Y.; Yon, L.S.; Pan, S.; Danquah, M.K. Biofuel production from algal biomass. In Bioenergy and Biofuels, 1st ed.; Konur, O., Ed.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2018; p. 621. [Google Scholar]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.B.; Abdelaziz, A.E.; Hallenbeck, P.C. Algal biofuels: Challenges and opportunities. Bioresour. Technol. 2013, 145, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z. Algae for Biofuel Production. Available online: https://farm-energy.extension.org/algae-for-biofuel-production (accessed on 15 September 2020).
- Biofuelwatch. Microalgae Biofuels Myths and Risks. Available online: https://haseloff.plantsci.cam.ac.uk/resources/SynBio_reports/Microalgae-Biofuels-Myths-and-Risks-2017.pdf (accessed on 1 October 2020).
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Rasheeq, A.A.; Arumugam, A.; Nambi, K.N.; Sampathkumar, P. Marine microalgal extracts on cultivable crops as a considerable bio-fertilizer: A Review. Indian J. Tradit. Knowl. 2019, 18, 849–854. [Google Scholar]
- Pereira, I.; Ortega, R.; Barrientos, L.; Moya, M.; Reyes, G.; Kramm, V. Development of a biofertiliser based on filamentous nitrogen-fixing cyanobacteria for rice crops in Chile. J. Appl. Phycol. 2009, 21, 135–144. [Google Scholar] [CrossRef]
- Osman, M.E.H.; El-Sheekh, M.M.; El-Naggar, A.H.; Gheda, S.F. Effect of two species of cyanobacteria as biofertilisers on some metabolic activities, growth, and yield of pea plant. Biol. Fertil. Soils 2010, 46, 861–875. [Google Scholar] [CrossRef]
- Chittapun, S.; Limbipichai, S.; Amnuaysin, N.; Boonkerd, R.; Charoensook, M. Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: A pot experiment. J. Appl. Phycol. 2018, 30, 79–85. [Google Scholar] [CrossRef]
- Agwa, O.; Ogugbue, C.; Williams, E. Field evidence of Chlorella vulgaris potentials as a biofertiliser for Hibiscus esculentus. Int. J. Agric. Res. 2017, 12, 181–189. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertiliser and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The impact of using microalgae as biofertiliser in maize (Zea mays L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Jochum, M.; Moncayo, L.P.; Jo, Y.-K. Microalgal cultivation for biofertilization in rice plants using a vertical semi-closed airlift photobioreactor. PLoS ONE 2018, 13, e0203456. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Subramanian, J.; Arumugam, A.; Rasheeq, A.A.; Sampathkumar, P. Exploring the microalgae biofertiliser effect on onion cultivation by field experiment. Waste Biomass Valorization 2020, 11, 77–87. [Google Scholar] [CrossRef]
- Bumandalai, O.; Tserennadmid, R. Effect of Chlorella vulgaris as a biofertiliser on germination of tomato and cucumber seeds. Int. J. Aquat. Biol. 2019, 7, 95–99. [Google Scholar]
- Nayak, M.; Swain, D.K.; Sen, R. Strategic valorization of de-oiled microalgal biomass waste as biofertiliser for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci. Total Environ. 2019, 682, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Bocchi, S.; Malgioglio, A. Azolla-Anabaena as a biofertiliser for rice paddy fields in the Po Valley, a temperate rice area in Northern Italy. Int. J. Agron. 2010, 2010. [Google Scholar] [CrossRef]
- Sholkamy, E.N.; El-Komy, H.; Al-Arfaj, A.A.; Abdel-Megeed, A.; Mostafa, A.A. Potential role of Nostoc muscorum and Nostoc rivulare as biofertilisers for the enhancement of maize growth under different doses of n-fertilizer. Afr. J. Microbiol. Res. 2012, 6, 7435–7448. [Google Scholar]
- Karthikeyan, N.; Prasanna, R.; Nain, L.; Kaushik, B.D. Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur. J. Soil Biol. 2007, 43, 23–30. [Google Scholar] [CrossRef]
- Uysal, O.; Uysal, F.O.; Ekinci, K. Evaluation of microalgae as microbial fertilizer. Eur. J. Sustain. Dev. 2015, 4, 77–82. [Google Scholar] [CrossRef]
- Prasanna, R.; Kanchan, A.; Kaur, S.; Ramakrishnan, B.; Ranjan, K.; Singh, M.C.; Hasan, M.; Saxena, A.K.; Shivay, Y.S. Chrysanthemum growth gains from beneficial microbial interactions and fertility improvements in soil under protected cultivation. Hortic. Plant J. 2016, 2, 229–239. [Google Scholar] [CrossRef]
- Prasanna, R.; Kanchan, A.; Ramakrishnan, B.; Ranjan, K.; Venkatachalam, S.; Hossain, F.; Shivay, Y.S.; Krishnan, P.; Nain, L. Cyanobacteria-based bioinoculants influence growth and yields by modulating the microbial communities favourably in the rhizospheres of maize hybrids. Eur. J. Soil Biol. 2016, 75, 15–23. [Google Scholar] [CrossRef]
- Suleiman, A.K.A.; Lourenço, K.S.; Clark, C.; Luz, R.L.; da Silva, G.H.R.; Vet, L.E.M.; Cantarella, H.; Fernandes, T.V.; Kuramae, E.E. From toilet to agriculture: Fertilization with microalgal biomass from wastewater impacts the soil and rhizosphere active microbiomes, greenhouse gas emissions and plant growth. Resour. Conserv. Recycl. 2020, 161, 104924. [Google Scholar] [CrossRef]
- Babu, S.; Prasanna, R.; Bidyarani, N.; Singh, R. Analysing the colonisation of inoculated cyanobacteria in wheat plants using biochemical and molecular tools. J. Appl. Phycol. 2015, 27, 327–338. [Google Scholar] [CrossRef]
- Bidyarani, N.; Prasanna, R.; Chawla, G.; Babu, S.; Singh, R. Deciphering the factors associated with the colonization of rice plants by cyanobacteria. J. Basic Microbiol. 2015, 55, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Bidyarani, N.; Prasanna, R.; Babu, S.; Hossain, F.; Saxena, A.K. Enhancement of plant growth and yields in Chickpea (Cicer arietinum L.) through novel cyanobacterial and biofilmed inoculants. Microbiol. Res. 2016, 188, 97–105. [Google Scholar] [CrossRef]
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2015, 171, 78–89. [Google Scholar] [CrossRef]
- Prasanna, R.; Ramakrishnan, B.; Simranjit, K.; Ranjan, K.; Kanchan, A.; Hossain, F.; Nain, L. Cyanobacterial and rhizobial inoculation modulates the plant physiological attributes and nodule microbial communities of chickpea. Arch. Microbiol. 2017, 199, 1311–1323. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Kaur, S.; Prasanna, R.; Ranjan, K.; Kanchan, A.; Hossain, F.; Shivay, Y.S.; Nain, L. Microbial inoculation of seeds characteristically shapes the rhizosphere microbiome in desi and kabuli chickpea types. J. Soils Sediments 2017, 17, 2040–2053. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Årstøl, E.; Hohmann-Marriott, M.F. Cyanobacterial siderophores—Physiology, structure, biosynthesis, and applications. Mar. Drugs 2019, 17, 281. [Google Scholar] [CrossRef]
- Mazur, H.; Konop, A.; Synak, R. Indole-3-acetic acid in the culture medium of two axenic green microalgae. J. Appl. Phycol. 2001, 13, 35–42. [Google Scholar] [CrossRef]
- Sergeeva, E.; Liaimer, A.; Bergman, B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 2002, 215, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, E.; Kosakovskaya, I.; Romanenko, P. Phytohormones of microalgae: Biological role and involvement in the regulation of physiological processes. Pt I. auxins, abscisic acid, ethylene. Int. J. Algae 2015, 17, 275–289. [Google Scholar] [CrossRef]
- Stirk, W.; Ördög, V.; van Staden, J.; Jäger, K. Cytokinin-and auxin-like activity in Cyanophyta and microalgae. J. Appl. Phycol. 2002, 14, 215–221. [Google Scholar] [CrossRef]
- Hussain, A.; Hasnain, S. Phytostimulation and biofertilization in wheat by cyanobacteria. J. Ind. Microbiol. Biotechnol. 2011, 38, 85–92. [Google Scholar] [CrossRef]
- Kumar, M.; Prasanna, R.; Bidyarani, N.; Babu, S.; Mishra, B.K.; Kumar, A.; Adak, A.; Jauhari, S.; Yadav, K.; Singh, R. Evaluating the plant growth promoting ability of thermotolerant bacteria and cyanobacteria and their interactions with seed spice crops. Sci. Hortic. 2013, 164, 94–101. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.; Kalaji, H. Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 2017, 55, 510–521. [Google Scholar] [CrossRef]
- Babu, S.; Bidyarani, N.; Chopra, P.; Monga, D.; Kumar, R.; Prasanna, R.; Kranthi, S.; Saxena, A.K. Evaluating microbe-plant interactions and varietal differences for enhancing biocontrol efficacy in root rot disease challenged cotton crop. Eur. J. Plant Pathol. 2015, 142, 345–362. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Ahluwalia, A.S.; Bansal, R.; Babu, S.; Singh, R.; Shivay, Y.S.; Nain, L. Exploring the efficacy of wastewater-grown microalgal biomass as a biofertiliser for wheat. Environ. Sci. Pollut. Res. 2016, 23, 6608–6620. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Bansal, R.; Bidyarani, N.; Singh, R.; Shivay, Y.S.; Nain, L.; Ahluwalia, A.S. Wastewater grown microalgal biomass as inoculants for improving micronutrient availability in wheat. Rhizosphere 2017, 3, 150–159. [Google Scholar] [CrossRef]
- Chandini; Kumar, R.; Kumar, R.; Prakash, O. The impact of chemical fertilizers on our environment and ecosystem. In Research Trends in Environmental Sciences; Akinik Publications: New Delhi, India, 2019; pp. 69–86. [Google Scholar]
- Mahapatra, D.M.; Chanakya, H.; Joshi, N.; Ramachandra, T.; Murthy, G. Algae-based biofertilisers: A biorefinery approach. In Microorganisms for Green Revolution; Springer Nature: Singapore, 2018; pp. 177–196. [Google Scholar]
- Rojas Crespo, E.; Iglesias Hernandez, D.; Acien Fernandez, F.G.; Pozo Dengra, J. Method for Obtaining Concentrates of Biofertilisers and Biostimulants for Agricultural Use from Biomass of Microalgae, Including Cyanobacteria. U.S. Patent Application 6/575,483, 1 April 2020. [Google Scholar]
- Ecological Pruducts Industries. Blue Green Algae Biofertiliser. Available online: https://ecologicalproduct.tradeindia.com/blue-green-algae-bio-fertilizer.html (accessed on 9 October 2020).
- June Pharmaceutical Comp. Ltd. Spirulina Superfertilizers: Shwe Awzar®. Available online: http://www.junespirulina.com/products/Superfertilizes (accessed on 9 October 2020).
- Biorizon Biotech. Spirulina: Algafert®. Available online: http://www.biorizon.es/bio-booster/hydrolyzed/algafert/?lang=en (accessed on 9 October 2020).
- MikroAlg Food Agric Ind Inc. Terradoc® Biofertiliser. Available online: http://mikroalg.com/urun-etiketi/terradoc-10 (accessed on 9 October 2020).
- Accelergy Corp. TerraSyncTM Biofertiliser. Available online: http://algaebiomass.org/wp-content/gallery/2012-algae-biomass-summit/2010/06/Allen-Mark-Accelergy-Corporation-Carbon-Capture-and-Utilisation-Challenges-and-Opportunities.pdf (accessed on 9 October 2020).
- MCT Tarim Ltd. Sti. Available online: https://mcttarim.en.ecplaza.net/products/emek-microbial-fertilizer_2956105 (accessed on 9 October 2020).
- Emparan, Q.; Harun, R.; Danquah, M. Role of phycoremediation for nutrient removal from wastewaters: A review. Appl. Ecol. Environ. Res. 2019, 17, 889–915. [Google Scholar] [CrossRef]
- Canizares, R.; Domínguez, A. Growth of Spirulina maxima on swine waste. Bioresour. Technol. 1993, 45, 73–75. [Google Scholar] [CrossRef]
- Cañizares, R.; Rivas, L.; Montes, C.; Dominguez, A.; Travieso, L.; Benitez, F. Aerated swine-wastewater treatment with K-carrageenan-immobilized Spirulina maxima. Bioresour. Technol. 1994, 47, 89–91. [Google Scholar] [CrossRef]
- Lim, S.-L.; Chu, W.-L.; Phang, S.-M. Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour. Technol. 2010, 101, 7314–7322. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; He, N.; Zheng, Y.; Li, H.; Wang, H.; Wang, Y.; Lu, Y.; Li, Q.; Peng, Y. Nitrogen and phosphorus removal from anaerobically digested wastewater by microalgae cultured in a novel membrane photobioreactor. Biotechnol. Biofuels 2018, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Cañizares-Villanueva, R.; Martínez-Roldán, A.; Perales-Vela, H.; Vázquez-Hernández, M.; Melchy-Antonio, O. Bioremediation of Copper and Other Heavy Metals Using Microbial Biomass. In Handbook of Metal-Microbe Interactions and Bioremediation; Das, S., Dash, H.R., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 585–602. [Google Scholar] [CrossRef]
- Dwivedi, S. Bioremediation of heavy metal by algae: Current and future perspective. J. Adv. Lab. Res. Biol. 2012, 3, 195–199. [Google Scholar]
- Hernández-Zamora, M.; Perales-Vela, H.V.; Flores-Ortíz, C.M.; Cañizares-Villanueva, R.O. Physiological and biochemical responses of Chlorella vulgaris to Congo Red. Ecotoxicol. Environ. Saf. 2014, 108, 72–77. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.-S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Tolboom, S.N.; Carrillo-Nieves, D.; de Jesús Rostro-Alanis, M.; de la Cruz Quiroz, R.; Barceló, D.; Iqbal, H.M.; Parra-Saldivar, R. Algal-based removal strategies for hazardous contaminants from the environment—A review. Sci. Total Environ. 2019, 665, 358–366. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Wu, J.; Luo, Y. Phycoremediation of coastal waters contaminated with bisphenol A by green tidal algae Ulva prolifera. Sci. Total Environ. 2019, 661, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Dayana Priyadharshini, S.; Bakthavatsalam, A.K. A comparative study on growth and degradation behavior of C. pyrenoidosa on synthetic phenol and phenolic wastewater of a coal gasification plant. J. Environ. Chem. Eng. 2019, 7, 103079. [Google Scholar] [CrossRef]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.-Q.; Ying, G.-G.; Yang, B.; Liu, S.; Lai, H.-J.; Liu, Y.-S.; Chen, Z.-F.; Zhou, G.-J. Biotransformation of progesterone and norgestrel by two freshwater microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): Transformation kinetics and products identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.; Sharma, N.; Rautela, I.; Nanda, M.; Arora, N.; Singh, A.; Chauhan, P. Microalgae fuel cell for wastewater treatment: Recent advances and challenges. J. Water Process Eng. 2020, 38, 101549. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Dong, C.D.; Chang, J.S. Microalgae-microbial fuel cell (mMFC): An integrated process for electricity generation, wastewater treatment, CO2 sequestration and biomass production. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem. 2005, 40, 1347–1361. [Google Scholar] [CrossRef]
- Daneshvar, N.; Ayazloo, M.; Khataee, A.; Pourhassan, M. Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp. Bioresour. Technol. 2007, 98, 1176–1182. [Google Scholar] [CrossRef]
- Mona, S.; Kaushik, A.; Kaushik, C. Biosorption of reactive dye by waste biomass of Nostoc linckia. Ecol. Eng. 2011, 37, 1589–1594. [Google Scholar] [CrossRef]
- Mohan, S.V.; Ramanaiah, S.; Sarma, P. Biosorption of direct azo dye from aqueous phase onto Spirogyra sp. I02: Evaluation of kinetics and mechanistic aspects. Biochem. Eng. J. 2008, 38, 61–69. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Removal of trimethoprim, sulfamethoxazole, and triclosan by the green alga Nannochloris sp. J. Hazard. Mater. 2016, 315, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Norvill, Z.N.; Blánquez, P.; Vicent, T.; Guieysse, B. Ciprofloxacin removal during secondary domestic wastewater treatment in high rate algal ponds. Chemosphere 2017, 180, 33–41. [Google Scholar] [CrossRef]
- Villar-Navarro, E.; Baena-Nogueras, R.M.; Paniw, M.; Perales, J.A.; Lara-Martín, P.A. Removal of pharmaceuticals in urban wastewater: High rate algae pond (HRAP) based technologies as an alternative to activated sludge based processes. Water Res. 2018, 139, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Guo, B.; Wang, H.; Huo, H.; Zhang, A.; Niu, S. Growth behavior, glucose consumption and phenol removal efficiency of Chlorella vulgaris under the synergistic effects of glucose and phenol. Ecotoxicol. Environ. Saf. 2019, 186, 109762. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Raja, R.; Coelho, A.; Hemaiswarya, S.; Kumar, P.; Carvalho, I.S.; Alagarsamy, A. Applications of microalgal paste and powder as food and feed: An update using text mining tool. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 740–747. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997, 15, 401–409. [Google Scholar] [CrossRef]
- Pratiwy, F.M.; Pratiwi, D.Y. The potentiality of microalgae as a source of DHA and EPA for aquaculture feed: A review. Int. J. Fish. Aquat. Stud. 2020, 8, 39–41. [Google Scholar]
- Martinez-Porchas, M.; Martinez-Cordova, L.R.; Lopez-Elias, J.A.; Porchas-Cornejo, M.A. Bioremediation of aquaculture effluents. In Microbial Biodegradation and Bioremediation; Elsevier: London, UK, 2014; pp. 539–553. [Google Scholar]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.d.R.; Leite, M.d.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2020, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Vaudel, M.; Verheggen, K.; Csordas, A.; Ræder, H.; Berven, F.S.; Martens, L.; Vizcaíno, J.A.; Barsnes, H. Exploring the potential of public proteomics data. Proteomics 2016, 16, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Acero, F.J.; Amil-Ruiz, F.; Durán-Peña, M.J.; Carrasco, R.; Fajardo, C.; Guarnizo, P.; Fuentes-Almagro, C.; Vallejo, R.A. Valorisation of the microalgae Nannochloropsis gaditana biomass by proteomic approach in the context of circular economy. J. Proteom. 2019, 193, 239–242. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as platforms for production of recombinant proteins and valuable compounds: Progress and prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Shamriz, S.; Ofoghi, H. Expression of recombinant PfCelTOS antigen in the chloroplast of Chlamydomonas reinhardtii and its potential use in detection of malaria. Mol. Biotechnol. 2019, 61, 102–110. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Peptide Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Zheng, L.-H.; Wang, Y.-J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.-K.; Sun, M. Antitumor peptides from marine organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.S.; Panjaitan, F.C.A.; Chang, Y.-W. Prediction of Bioactive Peptides from Chlorella sorokiniana Proteins Using Proteomic Techniques in Combination with Bioinformatics Analyses. Int. J. Mol. Sci. 2019, 20, 1786. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-P.; Williams, E.; Wang, D.-Z.; Xie, Z.-X.; Hsia, R.-C.; Jenck, A.; Halden, R.; Li, J.; Chen, F.; Place, A.R. Responses of Nannochloropsis oceanica IMET1 to long-term nitrogen starvation and recovery. Plant Physiol. 2013, 162, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.-A.T.; Padula, M.P.; Evenhuis, C.R.; Commault, A.S.; Ralph, P.J.; Tamburic, B. Proteomic and biophysical analyses reveal a metabolic shift in nitrogen deprived Nannochloropsis oculata. Algal Res. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Anand, V.; Singh, P.K.; Banerjee, C.; Shukla, P. Proteomic approaches in microalgae: Perspectives and applications. 3 Biotech. 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine natural products from microalgae: An-omics overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef] [PubMed]


| Compound | Microalgae Species | Isolation/Extraction Method | Cell/Virus/Animal Model/Clinical Patient | Effect | References |
|---|---|---|---|---|---|
| Anticancer Agents | |||||
| Lutein | Chlorella vulgaris | Ethanol extraction and partitioned with hexane | Colon cancer (HCT116) cells | Antiproliferative effect IC50 = 40.31 ± 4.43 µg/mL | [31] |
| Violaxanthin | Dunaliella tertiolecta | Dichloromethane extract | Human mammary carcinoma cell lines (MCF-7) and human prostatic carcinoma (LNCaP) cells | Potent inhibition of MCF-7 and LNCaP cells at growth inhibition (GI50) of 56.1 and 60.9 µg/mL, respectively | [32] |
| Fucoxanthin | Phaeodactylum tricornutum UTEX 640 | ressurized liquid extraction | Human liver cancer cell lines (Hep-G2), Human colon cancer (Caco-2) cells and HeLa cell line | An inhibitory effect of up to 58% was measured in Hep-G2 cells. In HeLa and Caco-2 cells, the effect was stronger than that of the positive control with a final concentration of 5% DMSO. | [33] |
| Phycocyanin | Spirulina platensis | Freeze-thawing followed by solvent extraction | Liver cancer (Hep-G2) cancer cells, Non-small cell lung cancer (NSCLC) | Inhibited liver cancer cells and leukemia cells. Induce apoptosis in H460 cells reaching 3.72 ± 0.98% and suppress growth of NSCLC cells | [34,35,36,37] |
| Phycocyanin | Spirulina platensis | Supercritical fluid extraction | Human lung cancer cells (A549) | IC50 = 26.82 µg/mL | [38] |
| Phycocyanin | Limnothrix sp. 37-2-1 | Fractional precipitation and purification with activated charcoal and chitosan | Prostate Cell Line LNCaP | C-PC alone (250 and 500 µg/mL) killed 65% and 70% of cells while C-PC (500 µg/mL) combined with Topetencan (TPT) (1 µM) killed 80% of cells due to additive effect | [39] |
| Phycocyanin | Limnothrix sp. NS01 | Four-step purification procedure including the adsorption of impurities with chitosan, activated charcoal, ammonium sulfate precipitation, and ion-exchange chromatography | Human breast cancer cell line (MCF-7) | IC50 for 24, 48 and 72 h exposure to C-PC were 5.92, 5.66 and 4.52 µg/µL | [40] |
| Cardioprotective agents | |||||
| cis β-carotene | Dunaliella bardawil (containing a mixture of cis and trans-isomers) | Enriched extract | Mice fed a high-fat diet | In old mice with established atherosclerotic lesion, Dunaliella inhibited significantly plasma cholesterol elevation and atherosclerosis progression | [41] |
| Eicosapentanoic acid (EPA) | Nannochloropsis | Enriched EPA oil | Healthy subjects | Lower cholesterol levels | [42] |
| Antiviral agent | |||||
| Cyanovirin | Nostoc ellpsosporum | Aqueous extraction | HIV-1 laboratory strains; HIV-1 primary isolates; HIV-2; SIV; | HIV-1 laboratory strains (EC50 0.1–5.8 nM), HIV-1 primary isolates (EC50 1.5–36.8 nM), HIV-2 (EC50 2.3–7.6 nM), SIV (EC50 11 nM) | [43] |
| Scytovirin | Scytonema varium | Aqueous extraction | HIV-1 laboratory strain (HIV-1RF); HIV-1 primary isolates (ROJO) in peripheral blood mononuclear cell (PBMC); HIV-1 primary isolates (Ba-L and ADA) in macrophages | HIV-1RF (EC50 0.3 nM), HIV-1 primary isolates (ROJO) in PBMC (EC50 7 nM), HIV-1 primary isolates (Ba-L) (EC50 22 nM), HIV-1 primary isolates (ADA) (EC50 17 nM) | [44] |
| Mixture of Icthypeptins A and Icthypeptins | Microcystic ichthyoblabe | Methanol extract | Influenza A | IC50 12.5 µg/mL | [45] |
| Calcium spirulan | Spirulina platensis | Aqueous extraction | HIV-1; Herpes simplex virus type 1 (HSV-1) | HIV-1 (IC50 9.3 µg/mL), Herpes simplex virus type 1 (HSV-1) (IC50 9.0 µg/mL). | [46] |
| Sulfated exopolysaccharide | Porphyridium cruentum | Aqueous extraction | Herpes simplex virus type 1 (HSV-1); Herpes simplex virus type 2 (HSV-2); Vericella virus (VZV) | HSV-1 (CPE50 protection 1 µg/mL), HSV-2 (CPE50 protection 5 µg/mL), VZV (CPE50 protection 0.7 µg/mL). | [47] |
| Microalgae | Bioactive Component | Product | Pharmaceutical Applications | References |
|---|---|---|---|---|
| Haematococcus pluvialis | Astaxanthin | Spirulina (Earth Spirulina Group, ES Co, Seoul, Korea) | Lipid lowering | [53] |
| Schizochytrium limacinum | Docosahexaenoic acid (DHA) | Maris DHA oil (IOI, Hamburg, Germany) | Rheumatoid arthritis | [54] |
| Nannochloropsis | Eicosapentaenoic acid (EPA) | Almega®PL (iWi Life) (Qualitas Health, Houston, US) | Cholesterol lowering | [42] |
| Arthrospira FEM-101 | Allophycocyanin | ApoX surface antiviral spray (FEBICO, Taiwan) | Antiviral | [55] |
| Biomass | Extracted Component | Isolation/Extraction Method | Application in Industry | Company | References |
|---|---|---|---|---|---|
| Spirulina platensis | Algae protein; Biomass | Enzymatic hydrolysis, Physical processes, Chemical extraction, Ultrasound-assisted extraction, Pulsed electric field, and Microwave-assisted extraction | Health supplement, Health food, Infant formula | Saxony-Anhalt (Germany); Parry Nutraceuticals (India); Japan Spirulina Co., Ltd. (Japan); Siam Alga Co., Ltd. (Thailand) | [3,64,65] |
| Spirulina platensis | Vitamin 12 | Water extraction method | Health supplement | Myanmar Spirulina Factory (Myanmar) | [65,66] |
| Chlorella vulgaris Chorella sp. | Biomass, pigments | Microwave-assisted extraction | Health food, food supplement | Nikken Sohonsha Corp. (Japan); Chlorella manufacturing and Co. (Taiwan); Klötze (Germany); Ocean Nutrition (Canada) | [3,65] |
| Haematococcus pluvialis | Astaxanthin | Mechanical treatments, chemical treatments using solvents, pressurized extraction, ultrasounds and microwaves | Food supplement, bio-colorant | Algae Health Science (China); Cyanotech Corporation (USA); Aquasearch Algatechnologies (Israel) | [65,67,68,69,70] |
| Dunaliella salina | Beta-carotene | Solvent extraction; Microwave- assisted extraction | Health food, Dietary supplement, bio-colourant | Cyanotech (USA); Earthrise Nutritionals (USA); Nature Beta Technologies Cognis (Israel); Betadene (Australia); Nature Beta Technologies Cognis (Australia) | [65,71] |
| Spirulina platensis | Phycocyanin | Microwave-assisted extraction | Bio-colourant, Health supplement | Panmol/Madaus (Austria); Yunnan Green A Biological Project Co., Ltd. (China). | [65] |
| Ulkenia sp. | DHA | Solvent extraction and microwave | Dietary supplement; | Nutrinova (Germany) | [3] |
| Schizochytrium sp. | DHA | Cellular hydrolysis | Dietary supplement; Health food | OmegaTech (USA) | [3] |
| Crypthecodinium cohnii | DHA | Microwave | Infant formula | Martek (USA) | [72] |
| Nannochloropsis oculata | Omega-3 PUFA | Solvent extraction; Microwave | Omega-3 supplements | Qualitas (USA); Cleanalgae SL (Spain); Astaxa (Germany) | [73,74] |
| Porphyridium spp. | Polysaccharides | Ultrafiltration | Food additives, Nutrition | InnovalG (France) | [65,75,76] |
| Euglena gracilis | Biomass | Flocculation | Health food | Euglena (Japan) | [77] |
| Odontella aurita | Fatty acids | Health supplement | InnovalG (France) | [65] |
| Skin Cells | Secreted Compounds | Reference |
|---|---|---|
| Keratinocytes, melanocytes | Corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), catecholamines | [162,163,164] |
| Dermal fibroblasts | ACTH, cortisol, prolactin | |
| Skin nerve endings | Adrenaline, noradrenaline, substance P | |
| Sebaceous glands | CRH, prolactin | |
| Cutaneous nerve endings and almost all skin cells | Produces and responds to special cytokines (neurotrophins) | [162,165,166] |
| Microalgae | Commercial Name/Company | Application | Reference |
|---|---|---|---|
| Dunaliella salina | Blue Retinol™ | Stimulates skin cell growth and proliferation | [175] |
| Nannochloropsis oculata | Pentapharm | Promotes excellent skin-tightening | [142,175] |
| Chlorella vulgaris | Dermochlorella/CODIF Recherche and Nature | Stimulates collagen synthesis, supports tissue regeneration, reduces wrinkle formation, anti-ageing | [3,176] |
| Arthospira sp. | Protulines, Exsymol | Assists in skin-tightening and repairs signs of ageing skin | [144] |
| Phaeodactylum tricornutum | Givaudan | Prevents premature ageing | [142] |
| Argan Beta-Retinoid Pink Algae Serum | Josie Maran | Eliminate fine lines, wrinkles, dark spots, dullness, dry and flaky skin | [174] |
| Porphyridium sp. | Alguard®/Frutarom | Anti-wrinkle, protection from UV damage | [151,177] |
| Porphyridium cruentum | SILIDINE®/Greentech | Improves skin aspect, decrease redness, vascular toning | [142] |
| Cicatrol®/Greensea | Anti-ageing, antioxidant | [178] | |
| Tetraselmis suecica | O+ Gold Microalgae Extract®/Greensea | Anti-inflammatory | [178] |
| Scenedesmus rubescens | Pepha®-Age/DSM Nutritional Products | Protects from photo-ageing, pro-collagen | [179] |
| Nannochloropsis oculata | Pepha®-Tight/DSM Nutritional Products (Pentapharm) | Protects from oxidative stress, simulates collagen synthesis and skin tightening | [180] |
| Dunaliella salina | Pepha®-Ctive/DSM Nutritional Products (Pentapharm) | Stimulates collagen synthesis, cell proliferation, energy metabolism | |
| Dunaliella salina, Haematococcus pluvialis | REVEAL Color Correcting Eye Serum Brightener/Algenist | Treats uneven and dull skin, dark circles under eye, gives brighter complexion | [181] |
| Chlorella vulgaris | Phytomer/Phytomer | Protects skin, neutralises inflammation | [151] |
| Chlorella sp. | Golden ChlorellaTM/Terravia Holdings | Hydrates skin and hair | [142] |
| Microalgae | Ethanol Yield (g Ethanol/g Substrate) | Reference |
|---|---|---|
| Chlorococcum infusionum | 0.26 | [203] |
| Chlamydomonas reinhardtii | 0.24 | [204] |
| Chlorococcum humicola | 0.52 | [205] |
| Scenedesmus abundans PKU AC 12 | 0.103 | [206] |
| Chlorella vulgaris FSP-E | 11.66 (87.59%) | [207] |
| Scenedesmus obliquus CNW-N | 8.55 (99.8%) | [208] |
| Desmodesmus sp. FG strain SP2-3 (unidentified green microalga) | 0.24 | [209] |
| Scenedesmus acuminatus | 0.12 | [202] |
| Microalgae | Crops | Reference |
|---|---|---|
| Acutodesmus dimorphus | Tomato | [240] |
| Spirulina platensis, Chlorella vulgaris | Maize | [241] |
| Chlorella vulgaris | Hibiscus esculentus | [239] |
| Chlorella vulgaris (UTEX 2714), Scenedesmus dimorphus (UTEX 1237) | Rice | [242] |
| Spirulina platensis, Chlorella vulgaris | Onion | [243] |
| Chlorella vulgaris | Tomato and Cucumber seeds | [244] |
| Scenedesmus sp. | Rice | [245] |
| Anabaena azolla | Rice | [246] |
| Nostoc muscorum Nostoc rivulare | Maize | [247] |
| Microalgae | Commercial Name | Company | Country | Reference |
|---|---|---|---|---|
| Cyanobacteria (Blue-green algae) | Skipper Khad® and Power Play-90 WSG | Ecological Products Industries | India | [274] |
| Spirulina sp. | Shwe Awzar® | June Industry Limited | Myanmar | [275] |
| Spirulina sp. | Algafert® | Biorizon Biotech | Spain | [276] |
| Chlorella vulgaris | Terradoc® | Mikroalg Food and Agriculture Industries | Turkey | [277] |
| Cyanobacteria | TerraSync™ | Accelergy Corporation | USA | [278] |
| Chlorella sp. | EMEK | MCT Tarim Ltd. | Turkey | [279] |
| Pollutants | Microalgae Bioremediation | References |
|---|---|---|
| Nitrogen & Phosporus | Phormidium sp., Spirulina maxima, Chlorella vulgaris, Scenedesmus dimorphus, Scenedesmus quadricauda, Chlorella sorokiniana, Chlorella vulgaris ESP-6 | [281,282,283,284] |
| Heavy Metals | Chlorella sp., Tetradesmus sp., Scenedesmus sp., Porphyridium sp., Chaetoceros sp., Oscillatoria sp., Sprogyra sp., Scenedesmus sp., Anacytis sp., Chlamydomonas sp., Anabaena sp., Ceratium sp., Scenedesmus sp., Calothrix sp., Arthrospira platensis | [285,298,299] |
| Colorants | Chlorella vulgaris, Cosmarium sp., Nostoc linckia, Spirogyra sp. | [300,301,302,303] |
| Emerging Pollutants | ||
| Antibiotics Phenol | Nannochloris sp., Mixture of algae-bacteria consortia in pilot high rate algae pond (HRAP), Mixture of algae-bacteria consortia (dominated by Coelastrum sp.) in HRAP, Mixture of algae-bacteria consortia in 1-L HRAP S. obliquus, C. vulgaris, Chlorella sp., Scenedesmus sp., Chlamydomonas mexicana, Chlorella sorokiniana Chlorella vulgaris Chlorella pyrenoidosa | [304,305,306,307,308] [293,309] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasubramaniam, V.; Gunasegavan, R.D.-N.; Mustar, S.; Lee, J.C.; Mohd Noh, M.F. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules 2021, 26, 943. https://doi.org/10.3390/molecules26040943
Balasubramaniam V, Gunasegavan RD-N, Mustar S, Lee JC, Mohd Noh MF. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules. 2021; 26(4):943. https://doi.org/10.3390/molecules26040943
Chicago/Turabian StyleBalasubramaniam, Vimala, Rathi Devi-Nair Gunasegavan, Suraiami Mustar, June Chelyn Lee, and Mohd Fairulnizal Mohd Noh. 2021. "Isolation of Industrial Important Bioactive Compounds from Microalgae" Molecules 26, no. 4: 943. https://doi.org/10.3390/molecules26040943
APA StyleBalasubramaniam, V., Gunasegavan, R. D.-N., Mustar, S., Lee, J. C., & Mohd Noh, M. F. (2021). Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules, 26(4), 943. https://doi.org/10.3390/molecules26040943