Phytochemical Screening and Evaluation of Antioxidant Properties and Antimicrobial Activity against Xanthomonas axonopodis of Euphorbia tirucalli Extracts in Binh Thuan Province, Vietnam
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening
2.2. The Content of Polyphenol and Flavonoids
2.3. Antioxidant Activity Basing on DPPH Assay
2.4. Antibacterial Test by Well Diffusion Method
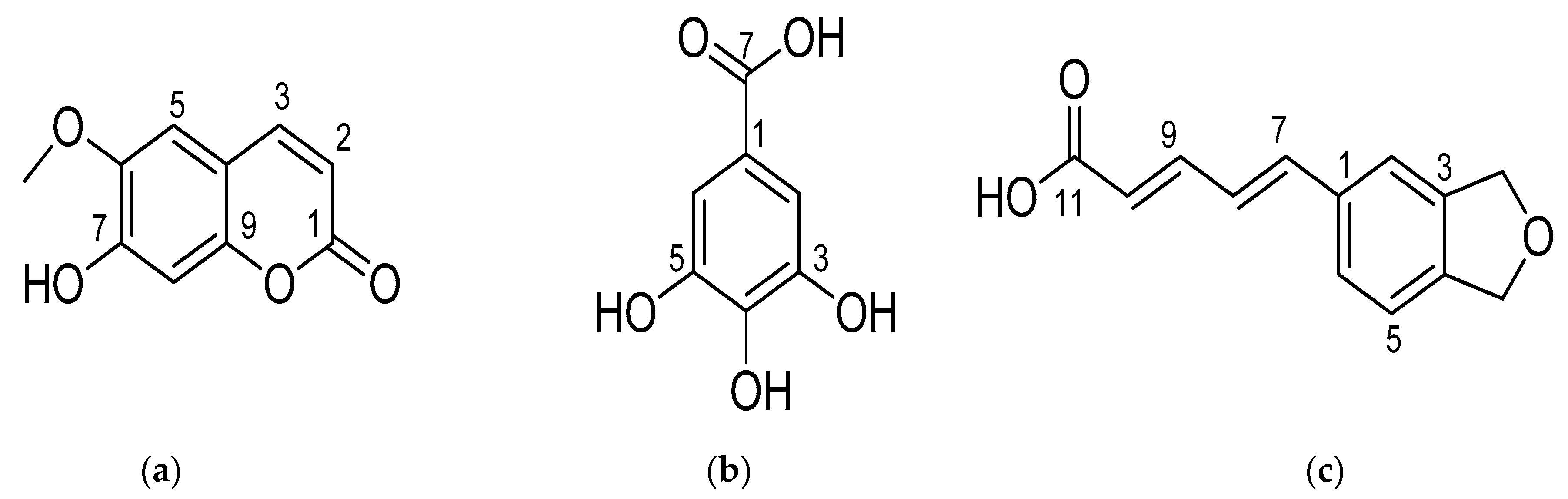
2.5. Isolation of Substances from Ethyl Acetate Fraction
3. Discussion
4. Materials and Methods
4.1. Source of the Plant Material
4.2. Preparation of Plant Extracts
4.3. Standard Bacteria
4.4. Phytochemical Screening
4.4.1. Alkaloids
4.4.2. Flavonoids
4.4.3. Saponins
4.4.4. Tannin
4.4.5. Anthraquinones
4.4.6. Terpenoids
4.5. Quantification of Polyphenol Content
4.6. Quantification of Flavonoids Content
4.7. Chromatographic Separation and Isolation of Constituents
4.8. Structure Elucidation of the Compounds
4.9. DPPH Free Radical Scavenging Assay
4.10. Antibacterial Activity Assay
4.11. MIC Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability: Samples of the compounds are available from the authors. |
References
- Silva, A.C.; de Faria, D.E.; Borges, N.B.; de Souza, I.A.; Peters, V.M.; Guerra, M.d.O. Toxicological screening of Euphorbia tirucalli L.: Developmental toxicity studies in rats. J. Ethnopharmacol. 2007, 110, 154–159. [Google Scholar] [CrossRef]
- Jalan, N.; Kumar, D.; Andrade, M.O.; Yu, F.; Jones, F.B.; Graham, J.H.; White, F.F.; Setubal, J.C.; Wang, N. Comparative genomic and transcriptome analyses of pathotypes of Xanthomonas citri subsp. citri provide insights into mechanisms of bacterial virulence and host range. BMC Genomics 2013, 14, 1–45. [Google Scholar] [CrossRef]
- Brain, K.R.; Turner, T.D. The Practical Evaluation of Phytopharmaceuticals, 1st ed.; Wrightscience Technical: Bristol, UK, 1975; p. 144. [Google Scholar]
- Evans, W.C. Trease Evans Pharmacognosy, 14th ed.; WB Saunders Ltd.: London, UK, 1966; pp. 119–159. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Jadhav, D.M.; Gawai, D.U.; Khillare, E.M. Evaluation if antibacterial and antifungal activity of Euphorbia tirucalli L. Bionano Front. 2010, 3, 332–334. [Google Scholar]
- Upadhyay, B.; Singh, K.; Kumar, A. Ethnomedicinal, phytochemical and antimicrobial studies of Euphorbia tirucalli L. J. Phytol. 2010, 2, 65–77. [Google Scholar]
- Le, T.K.D.; Bùi, X.H.; Nguyễn, T.A.T.; Phạm, N.K.T.; Dương, T.H. Chemical constituents of Euphorbia tirucalli L. Sci. Technol. Dev. J. Nat. Sci. 2019, 2, 76–82. [Google Scholar] [CrossRef]
- Le, N.T.M.; Minh, T.T.L.; Oanh, V.T.T. Isolation and characterization of Xanthomonas axonopodis causing canker disease of lime tree and evaluation of ability of extraction from stem of Euphorbia tirucalli against X. axonopodis. J. Technol. Sci. Food 2018, 16, 11–20. [Google Scholar]
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Sechler, A.; Agarkova, I.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.K. Emended classification of xanthomonad pathogens on citrus, systematic and applied. Microbiology 2006, 29, 690–695. [Google Scholar]
- Brunings, A.M.; Gabriel, D.W. Xanthomonas citri: Breaking the surface. Mol. Plant Pathol. 2003, 4, 141–157. [Google Scholar] [CrossRef]
- Shankara, S.R.; Rangarajana, R.; Saradab, D.V.L.; Kumar, C.S. Evaluation of antibacterial activity and phytochemical screening of Wrightia tinctoria L. Pharm. J. 2010, 2, 19–22. [Google Scholar] [CrossRef]
- Younes, A.B.; Salem, M.B.; Abed, H.E.; Jarraya, R. Phytochemical screening and antidiabetic, antihyperlipidemic, and antioxidant properties of Anthyllis henoniana (Coss.) Flowers extracts in an alloxan-induced rats model of diabetes. Evid. Based Complement. Altern. Med. 2018, 3, 1–14. [Google Scholar] [CrossRef]
- Orlanda, J.F.F.; Vale, V.V. Phytochemical analysis and photoprotective activity of the ethanolic extract of Euphorbia tirucalli Linneau (Euphorbiaceae). Rev. Bras. Plants Med. 2015, 17, 730–736. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yoon, W.B.; Lee, O.-H.; Cha, S.J.; Kim, J.D. Radical-scavening-linked antioxidant activities of extracts from black chokeberry and blueberry cultivated in Korea. Food Chem. 2014, 146, 71–77. [Google Scholar] [CrossRef]
- El, H.M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar]
- Krishnaiah, D.; Bono, A.; Sarbatly, R.; Anisuzzaman, S.M. Antioxidant activity and total phenolic content of an isolated Morinda citrifolia L. methanolic extract from Polyethersulphone (PES) membrane separator. J. King Saud Univ. Eng. Sci. 2015, 27, 63–67. [Google Scholar]
- Shariffar, F.; Dehghn-Nudeh, G.; Mirtajaldini, M. Major favonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Miceli, N.; Buongiorno, L.P.; Celi, M.G. Role of the favonoid-rich fraction in the antioxidant and cytotoxic activities of. Nat. Prod. Res. 2015, 30, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rao, N.R.; Vijeepallam, K.; Pandy, V. Phytochemical, pharmacological and biological profiles of Tragia species (Family: Euphorbiaceae). Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 105–112. [Google Scholar] [CrossRef]
- Melo, J.G.; Santos, A.G.; Amorim, E.L.C.; Nascimento, S.C.; Albuquerque, U.P. Medicinal plants used as antitumor agents in Brazil: An ethnobotanical approach. Evid. Based Complement. Altern. Med. 2011, 2011, 1–14. [Google Scholar] [CrossRef]
- Sultan, S.; Kimaro, C.C.; Amri, E. Antifungal activity and phytochemical screening of different solvent extracts of Euphorbia tirucalli Linn. J. Adv. Biol. Biotechnol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Samuel, K.; Naresh, K.; Padmaja, G. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep. 2015, 1, 520–529. [Google Scholar]
- Bhatt, M.K.; Dholwani, K.K.; Saluja, A.K. Isolation and structure elucidation of Scopoletin from Ipomoea reniformis (Convolvulaceae). J. Appl. Pharm. Sci. 2011, 1, 138–144. [Google Scholar]
- Akhmad, D.; Soleh, K.; Leonardus, B.S.K.; Yana, M.S. Scopoletin, a coumarin derivative compound isolated from Macaranga gigantifolia Merr. J. Appl. Pharm. Sci. 2012, 2, 175–177. [Google Scholar]
- Yasir, A.; Jahangir, M.; Aziz, u.R.; Ishtiaq, S.; Shahid, M. Antimicrobial, hemolytic and thrombolytic activities of some new N-substituted-2-({5-[(1E,3E)F-4-(1,3-benzodioxol5-yl)-1,3-butadienyl]-1,3,4-oxadiazol-2-yl}sulfanyl) propanamides. Trop. J. Pharm. Res. 2017, 16, 1973–1981. [Google Scholar] [CrossRef][Green Version]
- Zihan, R.K.; Fatema, M.; Suriya, S.; Muhammad, A.A.-M.; Abdul, G.; Obaidur, R.; Farhana, A. Isolation of bulk amount of piperine as active pharmaceutical ingredient (API) from black pepper and white pepper (Piper nigrum L.). Pharmacol. Pharm. 2017, 8, 253–262. [Google Scholar]
- Takao, K.; Miyashiro, T.; Sugita, Y. Synthesis and biological evaluation of piperic acid amides as free radical scavengers and α-Glucosidase Inhibitors. Chem. Pharm. Bull. 2015, 63, 326–333. [Google Scholar] [CrossRef][Green Version]
- Xiang, Y.; Li, F.; Dong, N.; Tian, S.; Zhang, H.; Du, X.; Zhou, X.; Xu, X.; Yang, H.; Xie, J.; et al. Investigation of a Salmonellosis outbreak caused by multidrug resistant Salmonella typhimurium in China. Front. Microbiol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Tiwtawat, N.; Markus, B.; Henrik, B.; Kwankamol, T.; Wichai, S.; Srunya, V. Scopoletin from Lasianthus lucidus Blume (Rubiaceae): A potential antimicrobial against multidrug-resistant Pseudomonas aeruginosa. J. Appl. Pharm. Sci. 2018, 8, 1–6. [Google Scholar]
- Garland, M.; Namrita, L.; Ahmed, H.; Thilivhali, E.T. Antimicrobial constituents of Artemisia afra Jacq. ex willd. against periodontal pathogens. Evid. Based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Mayela, G.S.; Ana, M.R.E.; Raul, R.H.; Sonia, A.L.S.; Cristobal, N.A.G.; Alejandro, Z.C.; Tanya, B.S.V.; Jesus, A.M.C. Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp. Ther. Med. 2016, 11, 619–624. [Google Scholar]
- Silva, I.C.; Regasini, L.O.; Petrônio, M.S.; Silva, D.H.S.; Bolzani, V.S.; Belasque, J., Jr.; Sacramento, L.V.S.; Ferreira, H. Antibacterial activity of alkyl gallates against Xanthomonas citri subsp. citri. J. Bacteriol. 2013, 195, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lu, W.; Zhou, X. Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: Persimmon, guava, and sweetsop. Biomed. Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Anabela, B.; Carla, F.; Maria, J.S.; Manuel, S. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar]
- Zarai, Z.; Emna, B.; Nadia, B.S.; Youssef, G.; Adel, S. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT-Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Toda, M.; Okubo, S.; Ohnishi, R.; Shimamura, T. Antibacterial and bactericidal activities of Japanese green tea. Jpn. J. Bacteriol. 1989, 44, 669–672. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically, 6th ed.; Approved Standard M7-A6; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003.
- Mazzanti, G.; Mascellino, M.T.; Battinelli, L.; Coluccia, D.; Manganaro, M.; Saso, L. Antimicrobial investigation of semipurified fractions of Ginkgo biloba leaves. J. Ethnopharmacol. 2000, 71, 83–88. [Google Scholar] [CrossRef]
- Devienne, K.F.; Raddi, M.S.G. Screening for antimicrobial activity of natural products using a microplate photometer. Braz. J. Microbiol. 2002, 33, 166–168. [Google Scholar] [CrossRef]
| Solvent | Alkaloids | Tannin | Saponin | Flavonoids | Anthraquinon | Terpenoids |
|---|---|---|---|---|---|---|
| Concentrated ethanol extract | ++ | + | - | ++ | - | +++ |
| n-hexane | - | - | - | + | - | + |
| Ethyl acetate | ++ | + | - | ++ | - | +++ |
| Butanol | + | + | - | + | - | - |
| Samples | Polyphenol Content (mg GAE/g) | Flavonoid Content (μg QE/g) |
|---|---|---|
| Concentrated ethanol extract | 185.28 ± 5.37 | 824.15 ± 25.55 |
| n-hexane | 16.65 ± 0.45 | 97.97 ± 0.79 |
| Ethyl acetate | 106.32 ± 0.49 | 450.83 ± 0.93 |
| Butanol | 61.87 ± 0.82 | 273.34 ± 0.46 |
| Samples | Concentration% RSA | |||||
|---|---|---|---|---|---|---|
| 3.125 (µg DM/mL) | 6.25 (µg DM/mL) | 12.5 (µg DM/mL) | 25 (µg DM/mL) | 50 (µg DM/mL) | SC50 (µg DM/mL) | |
| Concentrated ethanol extract | 10.94 ± 2.40 | 19.85 ± 1.75 | 34.95 ± 1.89 | 46.48 ± 0.61 | 85.56 ± 0.13 | 14.93 ± 0.85 a |
| n-hexane | 0.53 ± 1.33 | 0.96 ± 1.33 | 1.40 ± 1.83 | 2.52 ± 1.06 | 4.33 ± 1.92 | 528.33 ± 25.15 c |
| Ethyl acetate | 13.93 ± 2.40 | 20.38 ± 1.75 | 38.11 ± 1.89 | 77.08 ± 0.61 | 96.23 ± 0.13 | 12.91 ± 0.70 a |
| Butanol | 2.93 ± 1.33 | 5.09 ± 1.31 | 8.24 ± 1.20 | 9.32 ± 0.31 | 23.37 ± 1.01 | 55.56 ± 0.51 b |
| The control (Trolox) | 16.5 ± 1.06 | 32.23 ± 2.40 | 68.53 ± 1.54 | 132.5 ± 2.48 | 235 ± 0.15 | 8.23 ± 0.23 a |
| Concentration (mg/mL) | Zone of Inhibition (mm) | ||
|---|---|---|---|
| n-Hexane | Ethyl Acetate | Butanol | |
| 1.25 | - | 10.57 ± 0.80 b | 3.70 ± 0.30 b |
| 2.5 | - | 12.30 ± 0.79 c | 7.63 ± 0.65 c |
| 5.0 | 6.10 ± 0.65 b | 14.33 ± 0.76 d | 10.03 ± 0.55 d |
| 7.5 | 8.67 ± 0.76 c | 17.67 ± 0.57 e | 11.03 ± 0.35 e |
| Streptomycin (0.01 mg/mL) | 20.13 ± 0.61 d | 20.13 ± 0.61 f | 20.13 ± 0.61 f |
| DMSO 10% | - | - | - |
| Position | Substance 1 (Scopoletin) | Substance 2 (Gallic Acid) | Substance 3 (Piperic Acid) | |||
|---|---|---|---|---|---|---|
| δH, J (Hz) | δC | δH, J (Hz) | δC | δH, J (Hz) | δC | |
| 1 | 122.2 | 131.4 | ||||
| 2 | 7.14 (1H, s) | 110.4 | 7.11, d, 1.5 | 108.3 | ||
| 3 | 6.17, d, 9.5 | 112.4 | 146.4 | 148.1 | ||
| 4 | 7.84, d, 9.5 | 143.7 | 139.6 | 148.4 | ||
| 5 | 7.19, s | 109.1 | 146.4 | 6.85, d, 9.0 | 105.4 | |
| 6 | 7.14 (1H, s) | 110.4 | 7.00, dd, 9.0, 1.5 | 120.9 | ||
| 7 | 154.1 | 170.5 | 6.86, d, 15.5 | 137.5 | ||
| 8 | 6.80, s | 102.8 | 6.97, dd, 15.5, 10.5 | 122.4 | ||
| 9 | 7.31, dd, 14.5, 10.5 | 141.8 | ||||
| 10 | 111.2 | 6.86, d, 14.5 | 125.8 | |||
| 11 | 164.4 | |||||
| 3-/5/6-OCH3 | 3.90 | 55.7 | ||||
| O-CH2-O | 6.03 | 101.4 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, N.T.M.; Cuong, D.X.; Thinh, P.V.; Minh, T.N.; Manh, T.D.; Duong, T.-H.; Minh, T.T.L.; Oanh, V.T.T. Phytochemical Screening and Evaluation of Antioxidant Properties and Antimicrobial Activity against Xanthomonas axonopodis of Euphorbia tirucalli Extracts in Binh Thuan Province, Vietnam. Molecules 2021, 26, 941. https://doi.org/10.3390/molecules26040941
Le NTM, Cuong DX, Thinh PV, Minh TN, Manh TD, Duong T-H, Minh TTL, Oanh VTT. Phytochemical Screening and Evaluation of Antioxidant Properties and Antimicrobial Activity against Xanthomonas axonopodis of Euphorbia tirucalli Extracts in Binh Thuan Province, Vietnam. Molecules. 2021; 26(4):941. https://doi.org/10.3390/molecules26040941
Chicago/Turabian StyleLe, Nguyen Thị My, Dang Xuan Cuong, Pham Van Thinh, Truong Ngoc Minh, Tran Dinh Manh, Thuc-Huy Duong, Tran Thi Le Minh, and Vo Thi Thu Oanh. 2021. "Phytochemical Screening and Evaluation of Antioxidant Properties and Antimicrobial Activity against Xanthomonas axonopodis of Euphorbia tirucalli Extracts in Binh Thuan Province, Vietnam" Molecules 26, no. 4: 941. https://doi.org/10.3390/molecules26040941
APA StyleLe, N. T. M., Cuong, D. X., Thinh, P. V., Minh, T. N., Manh, T. D., Duong, T.-H., Minh, T. T. L., & Oanh, V. T. T. (2021). Phytochemical Screening and Evaluation of Antioxidant Properties and Antimicrobial Activity against Xanthomonas axonopodis of Euphorbia tirucalli Extracts in Binh Thuan Province, Vietnam. Molecules, 26(4), 941. https://doi.org/10.3390/molecules26040941







