The Azide-Allene Dipolar Cycloaddition: Is DFT Able to Predict Site- and Regio-Selectivity?
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental
2.2. Computational
2.2.1. Global DFT Reactivity Indices
2.2.2. Local DFT Reactivity Indices
2.2.3. Transition States and Energetic Analysis
3. Materials and Methods
3.1. Cycloaddition Between Allene 1b and 4-Substituted-Phenyl Azides 3b,c
3.2. Reaction Between Allene 1b and 4-Methoxyphenylazide 3b in Carbon Tetrachloride
3.3. Reaction Between Allene 1b and 4-Methoxyphenylazide 3b in CDCl3
3.4. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-Dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide−alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.S.; Jardim, G.A.M.; de Carvalho, R.L.; Araujo, M.H.; da Silva Júnior, E.N. Beyond copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition: Synthesis and mechanism insights. Tetrahedron 2019, 75, 3697–3712. [Google Scholar] [CrossRef]
- Ayouchia, H.B.E.; Bahsis, L.; Anane, H.; Domingo, L.R.; Stiriba, S.-E. Understanding the mechanism and regioselectivity of the copper(I) catalyzed [3 + 2] cycloaddition reaction between azide and alkyne: A systematic DFT study. RSC Adv. 2018, 8, 7670–7678. [Google Scholar] [CrossRef]
- Wang, C.; Ikhlef, D.; Khalal, S.; Saillard, J.-Y.; Astruc, D. Metal-catalyzed azide-alkyne “Click” reactions: Mechanistic overview and recent trends. Coord. Chem. Rev. 2016, 316, 1–20. [Google Scholar] [CrossRef]
- Lopez, S.A.; Munk, M.E.; Houk, K.N. Mechanisms and transition states of 1,3-dipolar cycloadditions of phenyl azide with enamines: A computational analysis. J. Org. Chem. 2013, 78, 1576–1582. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. Arylazide cycloaddition to methyl propiolate: DFT-based quantitative prediction of regioselectivity. Chem. Eur. J. 2003, 9, 2770–2774. [Google Scholar] [CrossRef]
- Jones, G.O.; Houk, K.N. Predictions of substituent effects in thermal azide 1,3-dipolar cycloadditions: Implications for dynamic combinatorial (reversible) and click (irreversible) chemistry. J. Org. Chem. 2008, 73, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Molecular Orbitals and Organic Chemical Reactions; Wiley: Hoboken, NJ, USA, 2010; Chapter 6; pp. 253–368. ISBN 978-0-470-74658-5. [Google Scholar]
- Houk, K.N.; Sims, J.; Duke, R.E.; Strozier, R.W.; George, J.K. Frontier molecular orbitals of 1,3 dipoles and dipolarophiles. J. Am. Chem. Soc. 1973, 95, 7287–7301. [Google Scholar] [CrossRef]
- Houk, K.N.; Sims, J.; Watts, C.R.; Luskus, L.J. Origin of reactivity, regioselectivity, and periselectivity in 1,3-dipolar cycloadditions. J. Am. Chem. Soc. 1973, 95, 7301–7315. [Google Scholar] [CrossRef]
- Murakami, M.; Matsuda, T. Cycloadditions of allenes. In Modern Allene Chemistry; WILEY-VCH Verlag: Weinheim, Germany, 2004; Volume 2, Chapter 12.3; pp. 750–759. ISBN 978-3-527-30671-8. [Google Scholar]
- Hayakawa, K.; Nishiyama, H.; Kanematsu, K. Reagent design and study of allene as a promising class of reagents (synthons) for cycloaddition. The Site-selective and regioselective diels-alder reactions of (phenylsulfonyl)propadiene and alkylation of the adducts. J. Org. Chem. 1985, 50, 512–517. [Google Scholar] [CrossRef]
- Bruché, L.; Gelmi, M.L.; Zecchi, G. 1,3-dipolar cycloadditions of 3,5-dichloro-2,4,6-trimethylbenzonitrile oxide to (phenylsulfonyl) allenes. J. Org. Chem. 1985, 50, 3206–3208. [Google Scholar] [CrossRef]
- Battioni, P.; Vo-Quang, L.; Vo-Quang, P. 1,3-Dipolar cycloaddition to monofunctional allenes. 2. Nitrilimines, nitriloxides and azides. Bull. Soc. Chim. Fr. 1978, 415–427. [Google Scholar]
- Soriano, E.; Fernández, I. Allenes and computational chemistry: From bonding situations to reaction mechanisms. Chem. Soc. Rev. 2014, 43, 3041–3105. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, A.; Bagatti, M.; Gandolfi, R. Theoretical and experimental study of the regioselectivity of the reaction of diazomethane with allene. Tetrahedron 1994, 50, 5561–5568. [Google Scholar] [CrossRef]
- Rastelli, A.; Bagatti, M.; Gandolfi, R. Ab initio study of concerted cycloadditions of allene, monofluoroallene, and 1,1-difluoroallene with diazomethane, formonitrile oxide, cyclopentadiene, and furan. J. Am. Chem. Soc. 1995, 117, 4965–4975. [Google Scholar] [CrossRef]
- Kavitha, K.; Venuvanalingam, P. 1,3-dipolar additions involving allenes: A density functional study of concerted and stepwise mechanisms. J. Chem. Soc. Perkin Trans. 2 2002, 2130–2139. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Wang, X.-R.; Zhang, J.-H.; Fang, D.-C. An Experimental and theoretical study on the interaction of N-heterocyclic carbene-derived 1,3-dipoles with methoxycarbonylallenes: Highly regio- and stereoselective [3+2]-cycloadditions controlled by the structures of n-heterocycles of 1,3-dipoles. J. Org. Chem. 2009, 74, 2357–2367. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. Site- and regioselectivity of nitrile oxide–allene cycloadditions: DFT-Based semiquantitative predictions. J. Org. Chem. 2017, 82, 10710–10714. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Yang, W.; Parr, R.G. Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. USA 1985, 82, 6723–6726. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Szentpály, L. v.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in diels–alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Ponti, A. DFT-based regioselectivity criteria for cycloaddition reactions. J. Phys. Chem. A 2000, 104, 8843–8846. [Google Scholar] [CrossRef]
- Ponti, A.; Molteni, G. DFT-based quantitative prediction of regioselectivity: Cycloaddition of nitrilimines to methyl propiolate. J. Org. Chem. 2001, 66, 5252–5255. [Google Scholar] [CrossRef]
- Ponti, A.; Molteni, G. Uncommon aqueous media for nitrilimine cycloadditions. II. Computational study of the effect of water on reaction rate. New J. Chem. 2002, 26, 1346–1351. [Google Scholar] [CrossRef]
- Ponti, A.; Molteni, G. DFT-HSAB prediction of regioselectivity in 1,3-dipolar cycloadditions: Behavior of (4-substituted) benzonitrile oxides towards methyl propiolate. Chem. Eur. J. 2006, 12, 1156–1161. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. The nitrilimine–alkene cycloaddition regioselectivity rationalized by density functional theory reactivity indices. Molecules 2017, 22, 202. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. Assessment of mechanistic hypotheses of 1,3-dipolar cycloaddition of (arylsulfonyl)allene to nitrilimines by DFT reactivity indices. Tetrahedron 2003, 59, 5225–5229. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. Regioselectivity of aryl azide cycloaddition to methyl propiolate in aqueous media: Experimental evidence versus local DFT HSAB principle. Arkivoc 2006, 2006, 49–56. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Cremer, D.; Kraka, E. Characterizing chemical similarity with vibrational spectroscopy: New insights into the substituent effects in monosubstituted benzenes. J. Phys. Chem. A 2017, 121, 8086–8096. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zou, W.; Cremer, D.; Kraka, E. Correlating the vibrational spectra of structurally related molecules: A spectroscopic measure of similarity. J. Comput. Chem. 2018, 39, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Zou, W.; Tao, Y. Decoding chemical information from vibrational spectroscopy data: Local vibrational mode theory. Wires Comput. Mol. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Bleiholder, R.; Shechter, H. Addition of electronegatively substituted azides to allenes. J. Am. Chem. Soc. 1968, 90, 2131–2137. [Google Scholar] [CrossRef]
- Blackwell, G.B.; Haszeldine, R.N.; Taylor, D.R. Polyhalogeno-allenes and -acetylenes. Part 15. Dipolar cycloadditions of n-phenylsydnone and aryl azides to perfluoropropadiene and perfluoropropyne. J. Chem. Soc. Perkin 1 1982, 2207–2210. [Google Scholar] [CrossRef]
- Broggini, G.; Molteni, G.; Zecchi, G. New mechanistic evidence on the reaction between sulfonylallenes and nitrile oxides. J. Org. Chem. 1994, 59, 8271–8274. [Google Scholar] [CrossRef]
- Karton, A.; Rabinovich, E.; Martin, J.M.L.; Ruscic, B. W4 Theory for computational thermochemistry. In pursuit of confident sub-kj/mol predictions. J. Chem. Phys. 2006, 125, 144108. [Google Scholar] [CrossRef]
- Karton, A.; Martin, J.M.L. Explicitly correlated Wn theory: W1-F12 and W2-F12. J. Chem. Phys. 2012, 136, 124114. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2008, 4, 1849–1868. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Ess, D.H.; Houk, K.N. Theory of 1,3-dipolar cycloadditions: Distortion/interaction and frontier molecular orbital models. J. Am. Chem. Soc. 2008, 130, 10187–10198. [Google Scholar] [CrossRef]
- Børresen, S.; Crandall, J.K. Reaction of picryl azide with aryloxyallenes. J. Org. Chem. 1976, 41, 678–681. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. How does the global electron density transfer diminish activation energies in polar cycloaddition reactions? A molecular electron density theory study. Tetrahedron 2017, 73, 1718–1724. [Google Scholar] [CrossRef]
- Roy, R.K.; Krishnamurti, S.; Geerlings, P.; Pal, S. Local softness and hardness based reactivity descriptors for predicting intra- and intermolecular reactivity sequences: Carbonyl compounds. J. Phys. Chem. A 1998, 102, 3746–3755. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the question of zwitterionic intermediates in the [3+2] cycloaddition reactions: A critical review. Organics 2020, 1, 5. [Google Scholar] [CrossRef]
- Lee, T.J.; Rice, J.E.; Scuseria, G.E.; Schaefer, H.F. Theoretical investigations of molecules composed only of fluorine, oxygen and nitrogen: Determination of the equilibrium structures of FOOF, (NO) 2 and FNNF and the transition state structure for FNNF cis-trans isomerization. Chim. Acta 1989, 75, 81–98. [Google Scholar] [CrossRef]
- Janssen, C.L.; Nielsen, I.M.B. New diagnostics for coupled-cluster and Møller–Plesset perturbation theory. Chem. Phys. Lett. 1998, 290, 423–430. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Cheeseman, J.R.; Scalmani, G.; Caricato, M.; Hratchian, H.P.; Li, X.; Barone, V.; Bloino, J.; Zheng, G.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Jensen, F. Unifying general and segmented contracted basis sets. Segmented polarization consistent basis sets. J. Chem. Theory Comput. 2014, 10, 1074–1085. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis set exchange: A community database for computational sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Mortier, W.J. The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef] [PubMed]
- Chirlian, L.E.; Francl, M.M. Atomic charges derived from electrostatic potentials: A detailed study. J. Comput. Chem. 1987, 8, 894–905. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]

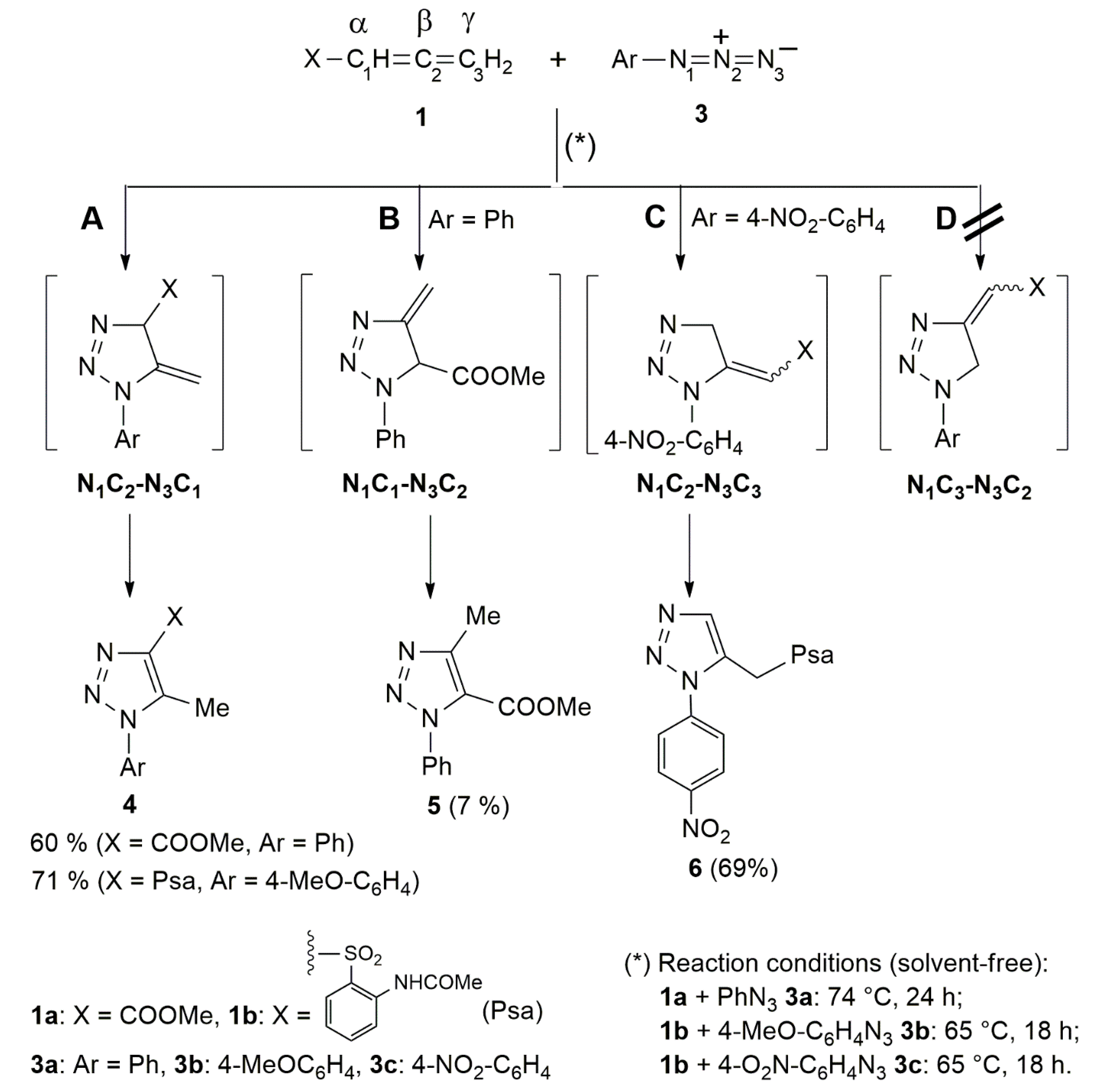
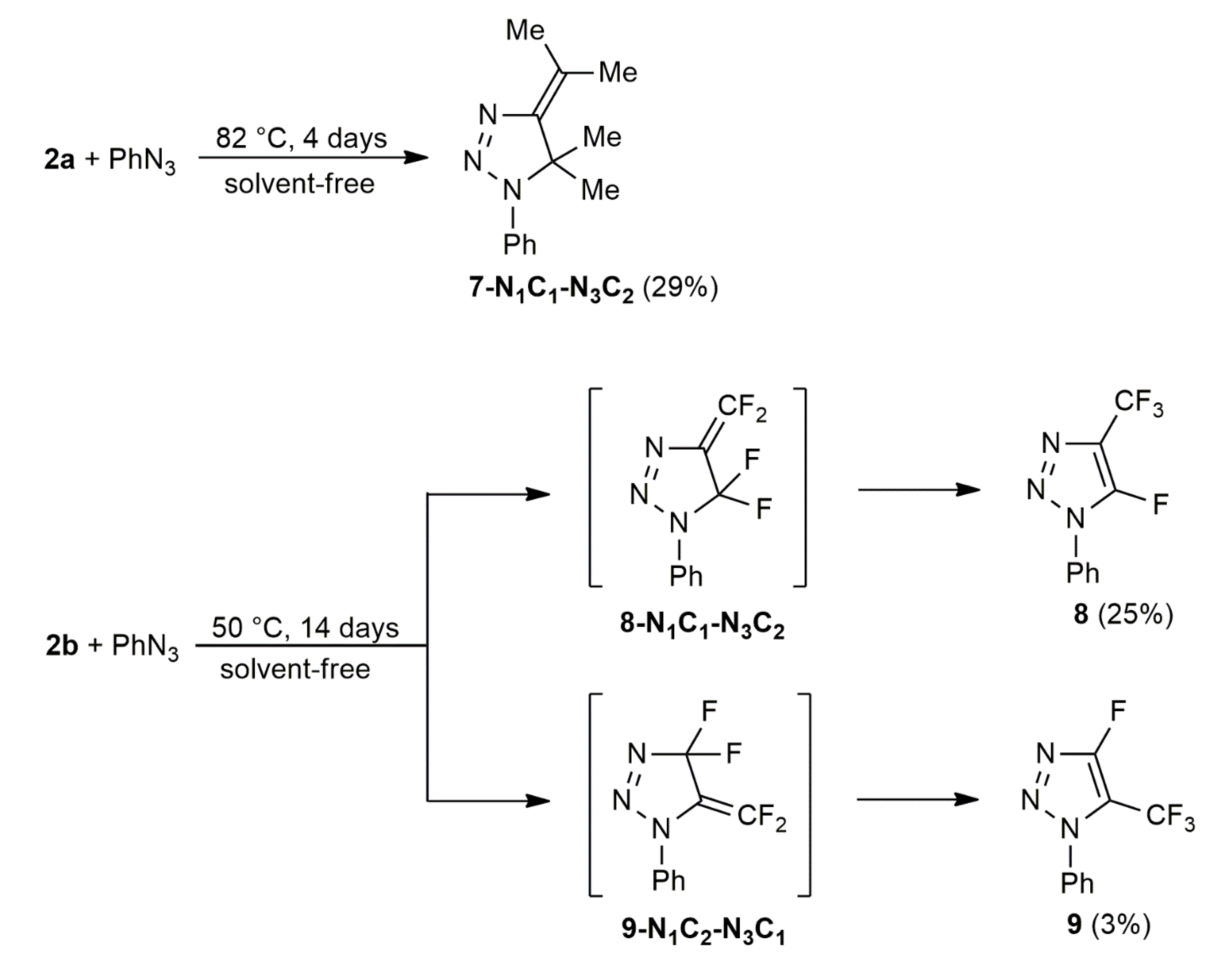
| Entry | Reaction | Overall Yield | Product Ratio | |
|---|---|---|---|---|
| (%) | 4:5 | 8:9 | ||
| 1 | 1a+3a | 67 | 90:10 | - |
| 2 | 1b+3b | 71 | 100:0 | - |
| 3 | 1b+3c | 69 a | - | - |
| 4 | 2a+3a | 29 b | - | - |
| 5 | 2b+3a | 28 | - | 90:10 |
| B3LYP/6-31G(d,p) | M08-HX/pcseg-3 //M08-HX/pcseg-2 | ωB97X-D/pcseg-3 //ωB97X-D/pcseg-2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µ | S | ω | Na | µ | S | ω | N | µ | S | ω | N | |||
| 2a | −2.55 | 0.137 | 0.44 | 2.91 | −3.73 | 0.098 | 0.68 | 3.14 | −3.42 | 0.098 | 0.57 | 3.13 | ||
| 2b | −4.18 | 0.136 | 1.19 | 1.27 | −5.02 | 0.079 | 1.00 | 0.66 | −4.62 | 0.080 | 0.85 | 0.77 | ||
| 1a | −4.13 | 0.163 | 1.39 | 1.92 | −4.91 | 0.091 | 1.10 | 1.59 | −4.65 | 0.093 | 1.00 | 1.60 | ||
| 1b | −4.08 | 0.199 | 1.66 | 2.54 | −4.77 | 0.116 | 1.32 | 2.90 | −4.53 | 0.116 | 1.19 | 2.78 | ||
| 3a | −3.63 | 0.193 | 1.27 | 2.92 | −3.89 | 0.103 | 0.78 | 3.23 | −3.66 | 0.103 | 0.69 | 3.13 | ||
| 3b | −3.29 | 0.210 | 1.13 | 3.46 | −3.66 | 0.112 | 0.75 | 3.85 | −3.46 | 0.113 | 0.68 | 3.76 | ||
| 3c | −4.82 | 0.229 | 2.66 | 2.12 | −5.24 | 0.119 | 1.64 | 2.54 | −5.03 | 0.121 | 1.53 | 2.47 | ||
| Compound | Atom | 1000 s (eV−1) |
|---|---|---|
| 2a | C2 | 23.1 (s–) |
| 2b | C2 | 50.5 (s+) |
| 1a | C1 | 20.9 (s+) |
| 1b | C2 | 5.3 (s+) |
| 1b | C3 | 1.3 (s–) |
| 3a | N3 | 20.9 (s+) |
| 3a | N3 | 24.4 (s–) |
| 3b | N1 | 23.1 (s–) |
| 3c | N3 | 20.2 (s+) |
| ΔΩ (kJ/mol) | ||||
|---|---|---|---|---|
| Reaction | N1C2-N3C1 | N1C2-N3C1 | N1C2-N3C3 | N1C3-N3C2 |
| 2a+3a | −0.22 (−0.05, −0.17) | −0.05 (−0.05,0.00) | – | – |
| 2b+3a | −0.91 (−0.20, −0.71) | −0.05 (−0.17,0.12) | – | – |
| 1a+3a | −0.51 (−0.48, −0.04) | −0.60 (−0.04, −0.56) | 0.30 (−0.04,0.34) | 0.34 (0.38, −0.04) |
| 1b+3b | −0.34 (-0.10, −0.24) | −0.35 (−0.26, −0.10) | −0.29 (−0.26, −0.04) | −0.28 (−0.04, −0.24) |
| 1b+3c | −0.019(−0.007, −0.012) | −0.018(−0.011, −0.007) | −0.025 (−0.011, −0.013) | −0.024 (−0.012, −0.012) |
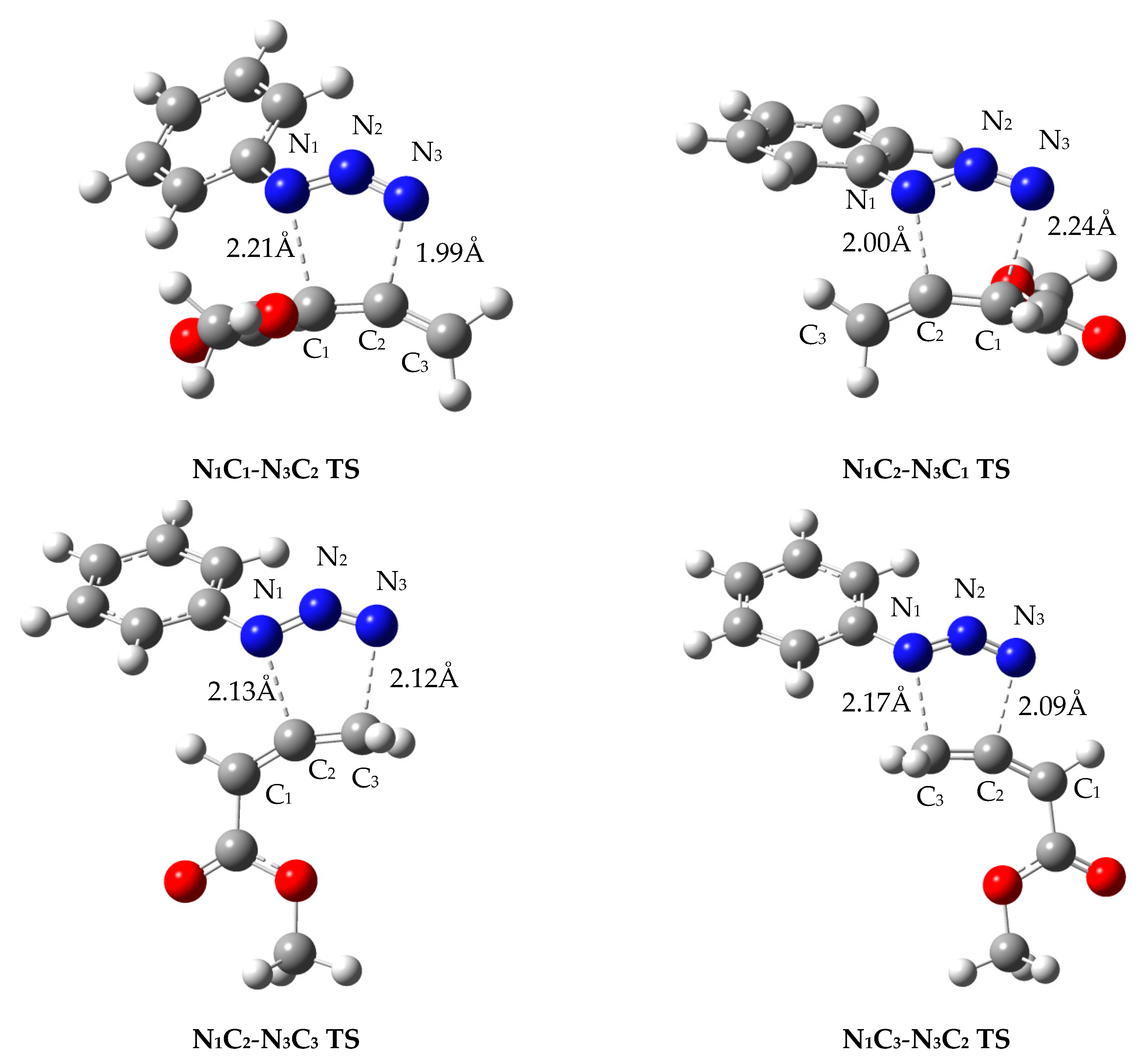
| Reaction | Isomer | R1a (Å) | R3b (Å) | <R> c (Å) | Δ d (Å) | N1-N̂2-N3 (°) | C1-Ĉ2-C3 (°) |
|---|---|---|---|---|---|---|---|
| 2a+3a | N1C1-N3C2 | 2.33 | 2.12 | 2.22 | 0.21 | 139.9 | 147.3 |
| N1C2-N3C1 | 2.17 | 2.20 | 2.18 | –0.03 | 137.1 | 151.5 | |
| 2b+3a | N1C1-N3C2 | 2.12 | 2.15 | 2.14 | –0.03 | 141.0 | 146.3 |
| N1C2-N3C1 | 2.12 | 2.06 | 2.09 | 0.07 | 140.2 | 145.4 | |
| 1a+3a | N1C1-N3C2 | 2.21 | 1.99 | 2.10 | 0.22 | 139.2 | 151.8 |
| N1C2-N3C1 | 2.00 | 2.24 | 2.12 | –0.24 | 140.9 | 149.8 | |
| N1C2-N3C3 | 2.13 | 2.12 | 2.13 | 0.01 | 139.2 | 154.9 | |
| N1C3-N3C2 | 2.17 | 2.09 | 2.13 | 0.08 | 139.5 | 154.4 | |
| 1b+3b | N1C1-N3C2 | 2.14 | 2.09 | 2.11 | 0.05 | 140.1 | 150.4 |
| N1C2-N3C1 | 2.15 | 2.13 | 2.14 | 0.03 | 143.2 | 146.3 | |
| N1C2-N3C3 | 2.23 | 1.94 | 2.09 | 0.29 | 140.2 | 152.3 | |
| N1C3-N3C2 | 1.92 | 2.33 | 2.12 | –0.41 | 140.5 | 153.9 | |
| 1b+3c | N1C1-N3C2 | 2.13 | 2.13 | 2.13 | 0.00 | 140.3 | 153.9 |
| N1C2-N3C1 | 2.21 | 1.95 | 2.08 | 0.26 | 138.7 | 151.0 | |
| N1C2-N3C3 | 1.95 | 2.27 | 2.11 | –0.33 | 141.7 | 147.3 | |
| N1C3-N3C2 | 2.14 | 2.09 | 2.11 | 0.05 | 139.2 | 152.0 |
| δEdist (kJ/mol) | |||||
|---|---|---|---|---|---|
| Reaction | Isomer | δEint (kJ/mol) | Total | Azide | Allene |
| 2a+3a | N1C1-N3C2 | 0 | 0 | 0 | 0 |
| N1C2-N3C1 | 4.4 | 4.8 | 11.6 | –6.8 | |
| 2b+3a | N1C1-N3C2 | 0 | 0 | 0 | 0 |
| N1C2-N3C1 | –2.7 | 27.7 | 22.5 | 5.3 | |
| 1a+3a | N1C1-N3C2 | –1.1 | 2.5 | 9.8 | –7.3 |
| N1C2-N3C1 | 0 | 0 | 0 | 0 | |
| N1C2-N3C3 | 11.9 | –8.1 | 3.8 | –11.9 | |
| N1C3-N3C2 | 15.6 | –10.2 | 3.8 | –13.9 | |
| 1b+3b | N1C1-N3C2 | 13.4 | 1.2 | 18.0 | –16.8 |
| N1C2-N3C1 | 0 | 0 | 0 | 0 | |
| N1C2-N3C3 | 25.4 | –22.4 | 12.7 | –35.1 | |
| N1C3-N3C2 | 43.0 | –39.0 | 9.9 | –49.0 | |
| 1b+3c | N1C1-N3C2 | 15.5 | –1.2 | 13.8 | –15.0 |
| N1C2-N3C1 | 0 | 0 | 0 | 0 | |
| N1C2-N3C3 | 24.9 | –23.0 | 7.2 | –30.2 | |
| N1C3-N3C2 | 46.1 | –44.9 | 2.5 | –47.3 | |
| M08-HX | ωB97X-D | B3LYP | Exp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction | Isomer | δΔH‡ (kJ/mol) | Y (%) | δΔH‡ (kJ/mol) | Y (%) | δΔH‡ (kJ/mol) | Y (%) | Y (%) | ||||
| 2a+3a | N1C1-N3C2 | 0 | 96 | 0 | 97 | 0.0 | 99 | 100 | ||||
| N1C2-N3C1 | 9.5 | 4 | 10.0 | 3 | 13.7 | 1 | 0 | |||||
| 2b+3a | N1C1-N3C2 | 0 | 100 | 0.0 | 100 | 0.0 | 100 | 89 | ||||
| N1C2-N3C1 | 24.9 | 0 | 22.9 | 0 | 22.3 | 0 | 11 | |||||
| 1a+3a | N1C1-N3C2 | 2.0 | 27 | 2.4 | 24 | 1.6 | 24 | 10 | ||||
| N1C2-N3C1 | 0.0 | 54 | 0 | 56 | 3.6 | 12 | 90 | |||||
| N1C2-N3C3 | 4.1 | 13 | 4.1 | 13 | 2.0 | 21 | 0 | |||||
| N1C3-N3C2 | 6.1 | 7 | 6.5 | 6 | 0 | 43 | 0 | |||||
| 1b+3b | N1C1-N3C2 | 15.7 | 0 | 19.5 | 0 | 19.7 | 0 | 0 | ||||
| N1C2-N3C1 | 0 | 66 | 3.9 | 17 | 19.4 | 0 | 100 | |||||
| N1C2-N3C3 | 2.8 | 25 | 0 | 69 | 6.0 | 10 | 0 | |||||
| N1C3-N3C2 | 5.6 | 9 | 4.4 | 14 | 0 | 90 | 0 | |||||
| 1b+3c | N1C1-N3C2 | 15.4 | 0 | 19.6 | 0 | 23.5 | 0 | 0 | ||||
| N1C2-N3C1 | 0 | 51 | 5.4 | 8 | 28.1 | 0 | 0 | |||||
| N1C2-N3C3 | 1.6 | 28 | 0 | 58 | 8.8 | 4 | 100 | |||||
| N1C3-N3C2 | 2.6 | 20 | 1.5 | 34 | 0 | 96 | 0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molteni, G.; Ponti, A. The Azide-Allene Dipolar Cycloaddition: Is DFT Able to Predict Site- and Regio-Selectivity? Molecules 2021, 26, 928. https://doi.org/10.3390/molecules26040928
Molteni G, Ponti A. The Azide-Allene Dipolar Cycloaddition: Is DFT Able to Predict Site- and Regio-Selectivity? Molecules. 2021; 26(4):928. https://doi.org/10.3390/molecules26040928
Chicago/Turabian StyleMolteni, Giorgio, and Alessandro Ponti. 2021. "The Azide-Allene Dipolar Cycloaddition: Is DFT Able to Predict Site- and Regio-Selectivity?" Molecules 26, no. 4: 928. https://doi.org/10.3390/molecules26040928
APA StyleMolteni, G., & Ponti, A. (2021). The Azide-Allene Dipolar Cycloaddition: Is DFT Able to Predict Site- and Regio-Selectivity? Molecules, 26(4), 928. https://doi.org/10.3390/molecules26040928








