Applications of Vibrational Spectroscopy for Analysis of Connective Tissues
Abstract
:1. Overview
2. Connective Tissues
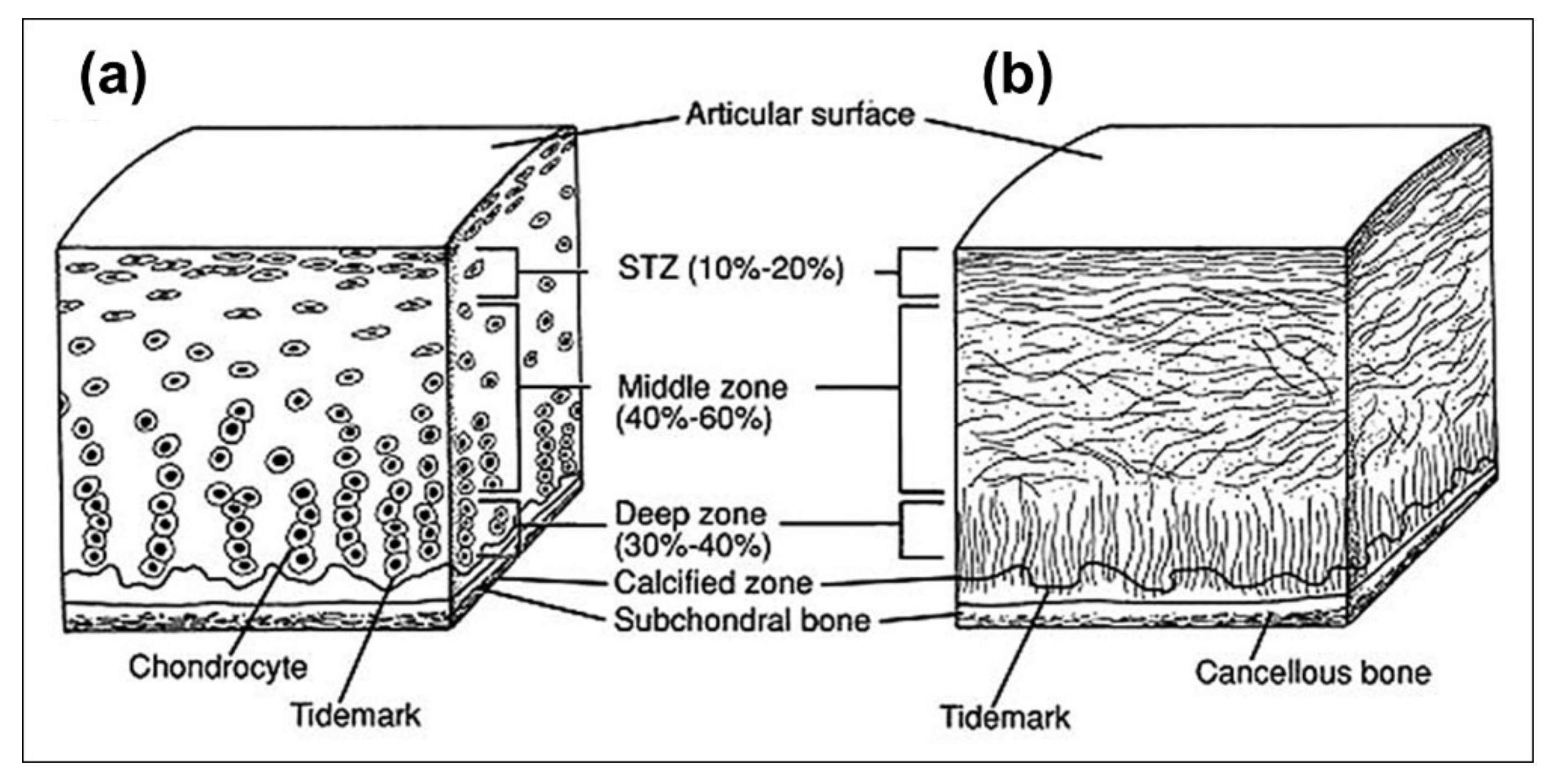
2.1. Cartilage
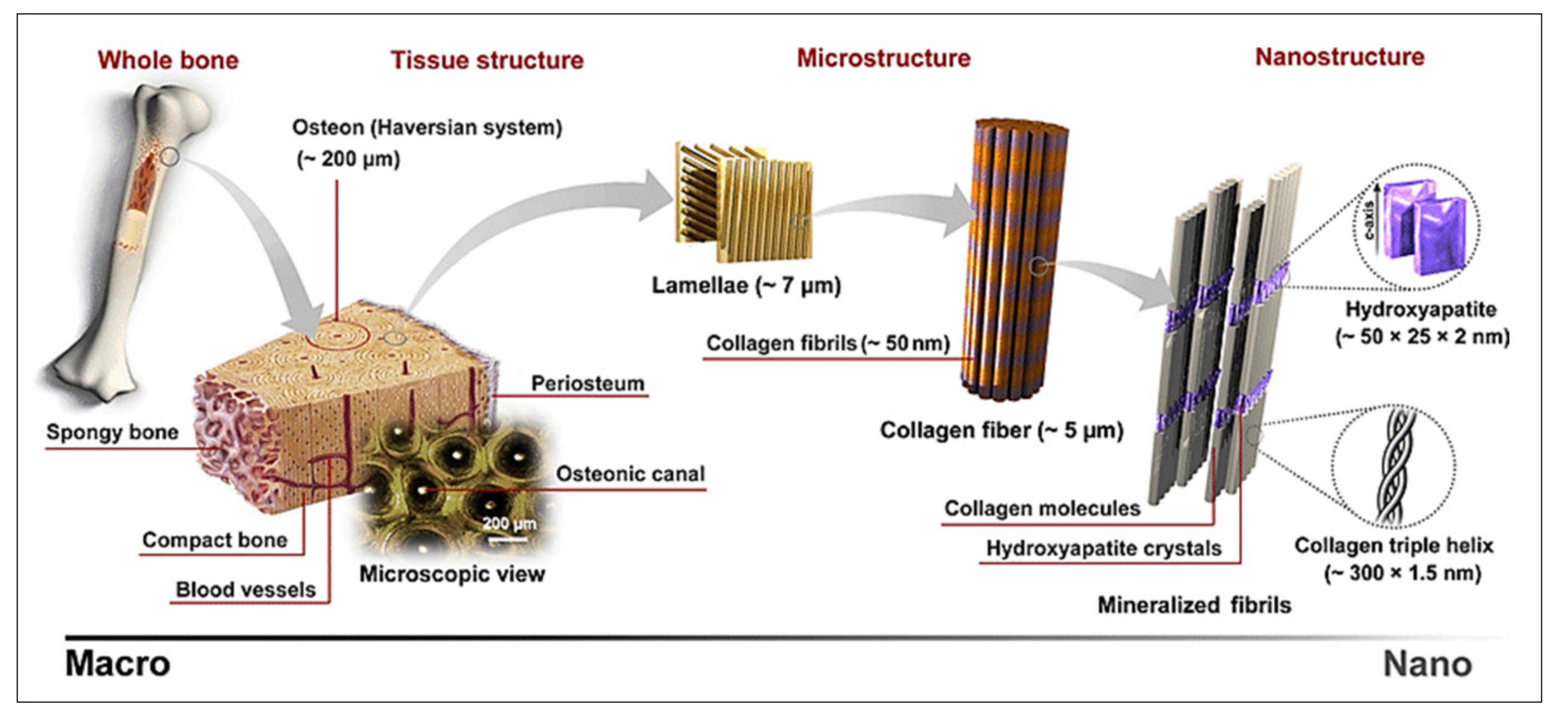
2.2. Bone
3. Vibrational Spectroscopy
3.1. Vibrational Spectroscopy Modalities
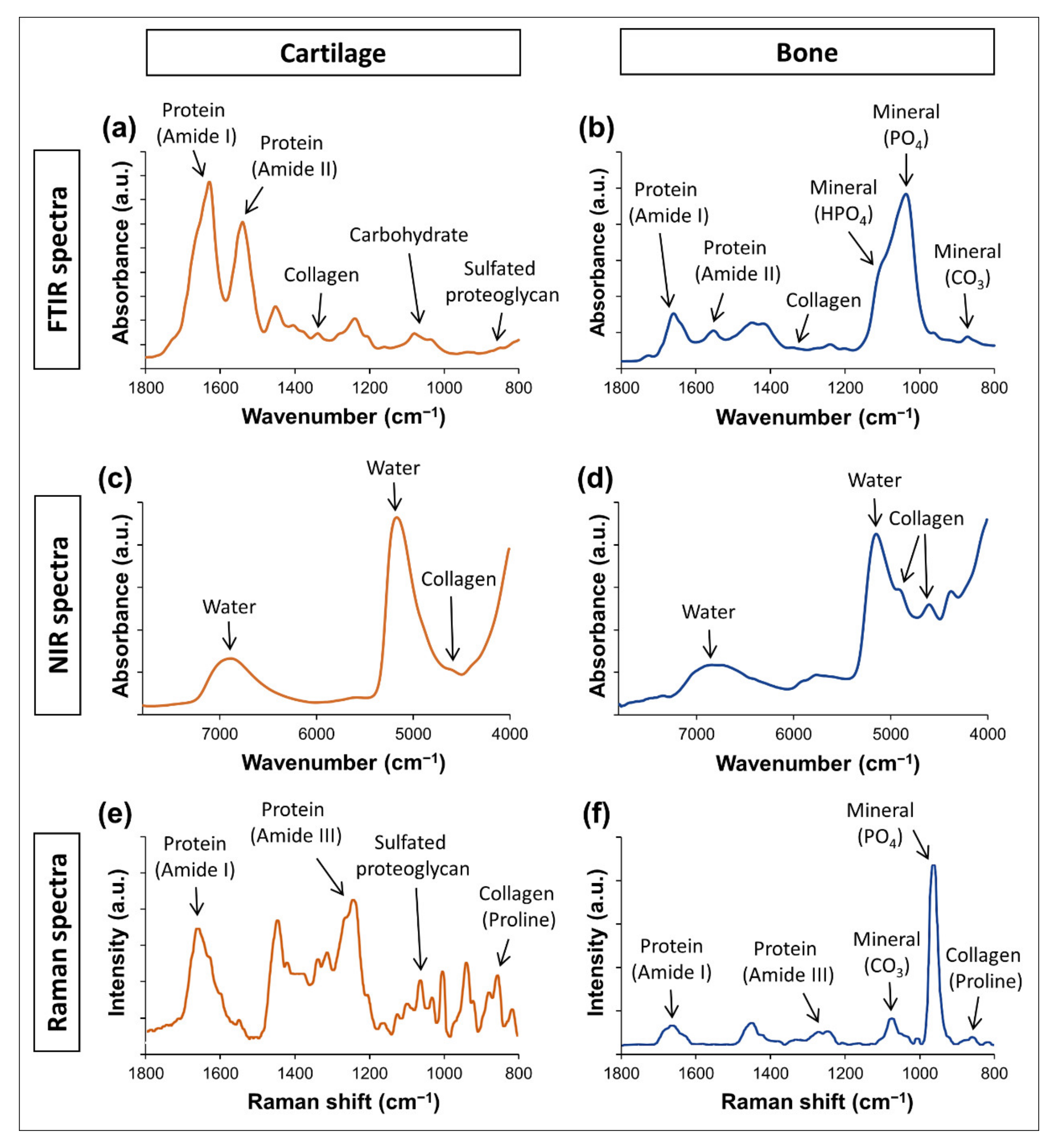
3.1.1. Mid Infrared (FTIR) Spectroscopy
3.1.2. Near Infrared Spectroscopy
3.1.3. Raman Spectroscopy
3.2. Advanced Vibrational Spectroscopy Techniques
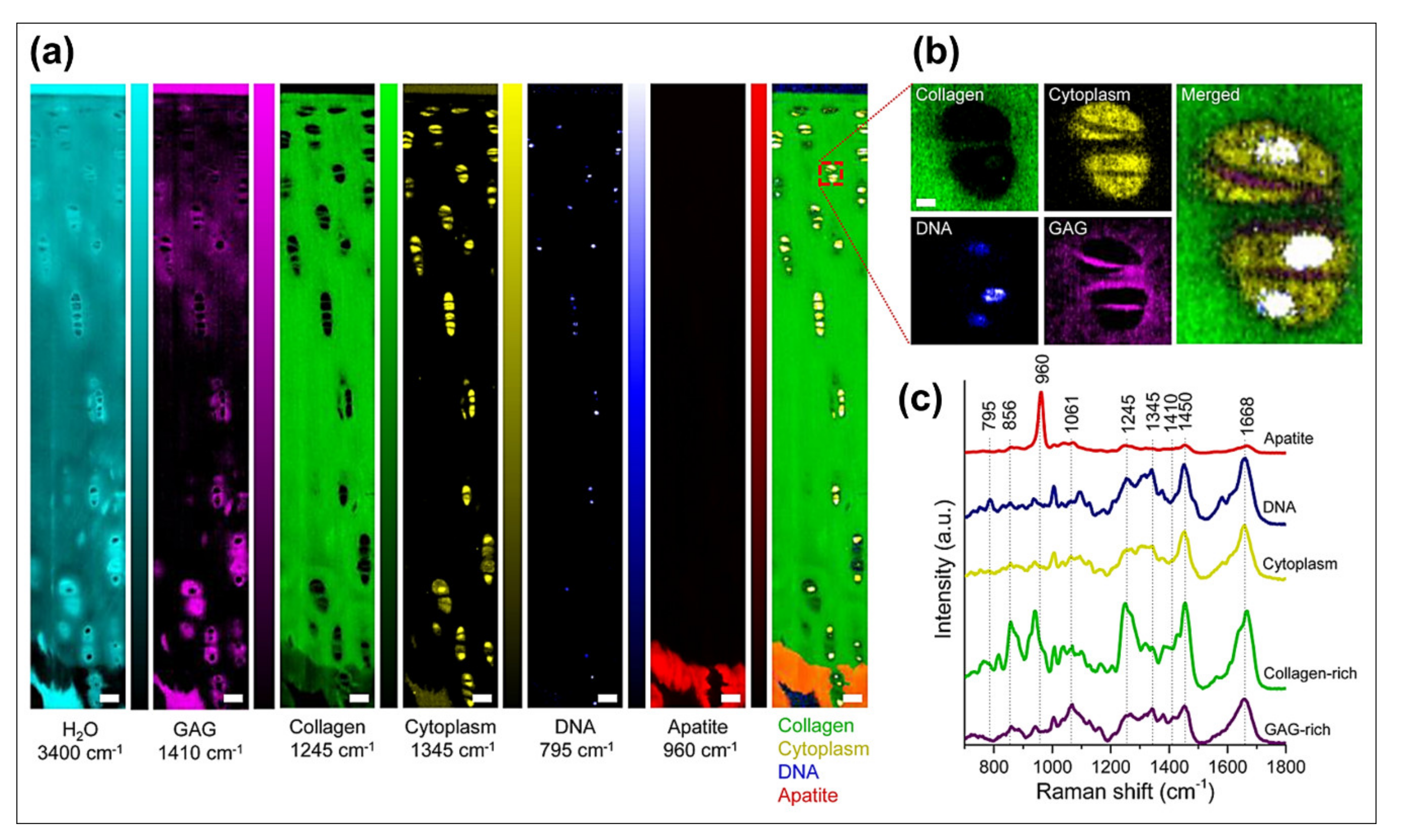
3.2.1. Spectral Imaging
3.2.2. Fiber Optic Probes
3.3. Spectral Data Analysis
3.3.1. Pre-Processing
3.3.2. Post-Processing
4. Application of Vibrational Spectroscopy for Connective Tissue Analysis
4.1. Applications for Cartilage Assessment
4.1.1. Mid Infrared Spectral Analysis of Cartilage
4.1.2. Near Infrared Spectral Analysis of Cartilage
4.1.3. Raman Spectral Analysis of Cartilage
4.2. Applications for Bone Assessment
4.2.1. Mid Infrared Spectral Analysis of Bone
4.2.2. Near Infrared Spectral Analysis of Bone
4.2.3. Raman Spectral Analysis of Bone
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boskey, A.; Camacho, N.P. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 2007, 28, 2465–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, E.A.; Donnelly, E. Raman and Fourier transform infrared imaging for characterization of bone material properties. Bone 2020, 139, 115490. [Google Scholar] [CrossRef] [PubMed]
- Rieppo, L.; Toyras, J.; Saarakkala, S. Vibrational spectroscopy of articular cartilage. Appl. Spectrosc. Rev. 2017, 52, 249–266. [Google Scholar] [CrossRef]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Rep. 2013, 2, 447. [Google Scholar] [CrossRef] [Green Version]
- Boskey, A.L.; Imbert, L. Bone quality changes associated with aging and disease: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 93–106. [Google Scholar] [CrossRef]
- Hunt, H.B.; Donnelly, E. Bone quality assessment techniques: Geometric, compositional, and mechanical characterization from macroscale to nanoscale. Clin. Rev. Bone Miner. Metab. 2016, 14, 133–149. [Google Scholar] [CrossRef] [Green Version]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentili, C.; Cancedda, R. Cartilage and bone extracellular matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S.; Koyama, E.; Pacifici, M. Articular Cartilage: Structural and Developmental Intricacies and Questions. Curr. Osteoporos. Rep. 2015, 13, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.-K.; Scherping, S.; Mardi, T.; Richard Steadman, J.; Woo, S.L.Y. Basic science of articular cartilage injury and repair. Oper. Tech. Sports Med. 1995, 3, 78–86. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mow, V.C.; Ratcliffe, A. Restoration of Injured or Degenerated Articular Cartilage. JAAOS—J. Am. Acad. Orthop. Surg. 1994, 2, 192–201. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef]
- Boskey, A.L. Mineralization of bones and teeth. Elements 2007, 3, 385–391. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Valsami-Jones, E. Bone and tooth mineralization: Why apatite? Elements 2008, 4, 97–104. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Gamsjaeger, S.; Klaushofer, K. Vibrational spectroscopic techniques to assess bone quality. Osteoporos. Int. 2017, 28, 2275–2291. [Google Scholar] [CrossRef]
- Mandair, G.S.; Morris, M.D. Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy Rep. 2015, 4, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.D.; Mandair, G.S. Raman Assessment of Bone Quality. Clin. Orthop. Relat. Res. 2011, 469, 2160–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Diem, M.; Romeo, M.; Boydston-White, S.; Miljkovic, M.; Matthaus, C. A decade of vibrational micro-spectroscopy of human cells and tissue (1994–2004). Analyst 2004, 129, 880–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.M.; Dumas, P. From structure to cellular mechanism with infrared microspectroscopy. Curr. Opin. Struct. Biol. 2010, 20, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.L.A.; Kazarian, S.G. Attenuated total reflection Fourier-transform infrared (ATR-FTIR) imaging of tissues and live cells. Chem. Soc. Rev. 2016, 45, 1850–1864. [Google Scholar] [CrossRef] [PubMed]
- Rieppo, L.; Saarakkala, S.; Narhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelsohn, R.; Paschalis, E.P.; Boskey, A.L. Infrared spectroscopy, microscopy, and microscopic imaging of mineralizing tissues: Spectra-structure correlations from human iliac crest biopsies. J. Biomed. Opt. 1999, 4, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turker-Kaya, S.; Huck, C.W. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef] [Green Version]
- Balan, V.; Mihai, C.T.; Cojocaru, F.D.; Uritu, C.M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational Spectroscopy Fingerprinting in Medicine: From Molecular to Clinical Practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.J.; Byrne, H.J.; Chalmers, J.; Gardner, P.; Goodacre, R.; Henderson, A.; Kazarian, S.G.; Martin, F.L.; Moger, J.; Stone, N.; et al. Clinical applications of infrared and Raman spectroscopy: State of play and future challenges. Analyst 2018, 143, 1735–1757. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C. Introduction to Infrared and Raman-Based Biomedical Molecular Imaging and Comparison with Other Modalities. Molecules 2020, 25, 5547. [Google Scholar] [CrossRef] [PubMed]
- Bunaciu, A.A.; Hoang, V.D.; Aboul-Enein, H.Y. Vibrational Micro-Spectroscopy of Human Tissues Analysis: Review. Crit. Rev. Anal. Chem. 2017, 47, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Afara, I.O.; Shaikh, R.; Nippolainen, E.; Querido, W.; Torniainen, J.; Sarin, J.K.; Kandel, S.; Pleshko, N.; Töyräs, J. Characterization of connective tissues using near-infrared spectroscopy and imaging. Nat. Protoc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumelis, N.; Tzaphlidou, M. Spectroscopic assessment of normal cortical bone: Differences in relation to bone site and sex. Sci. World J. 2010, 10, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef]
- Querido, W.; Ailavajhala, R.; Padalkar, M.; Pleshko, N. Validated Approaches for Quantification of Bone Mineral Crystallinity Using Transmission Fourier Transform Infrared (FT-IR), Attenuated Total Reflection (ATR) FT-IR, and Raman Spectroscopy. Appl. Spectrosc. 2018, 72, 1581–1593. [Google Scholar] [CrossRef]
- Hanifi, A.; McGoverin, C.; Ou, Y.T.; Safadi, F.; Spencer, R.G.; Pleshko, N. Differences in infrared spectroscopic data of connective tissues in transflectance and transmittance modes. Anal. Chim. Acta 2013, 779, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilling, M.J.; Bassan, P.; Gardner, P. Comparison of transmission and transflectance mode FTIR imaging of biological tissue. Analyst 2015, 140, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22. [Google Scholar] [CrossRef]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Aenugu, H.P.R.; Kumar, D.S.; Srisudharson, N.P.; Ghosh, S.; Banji, D. Near Infra Red Spectroscopy—An Overview. Int. J. ChemTech. Res. 2011, 3, 825–836. [Google Scholar]
- Palukuru, U.P.; McGoverin, C.M.; Pleshko, N. Assessment of hyaline cartilage matrix composition using near infrared spectroscopy. Matrix Biol. 2014, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Palukuru, U.P.; Hanifi, A.; McGoverin, C.M.; Devlin, S.; Lelkes, P.I.; Pleshko, N. Near infrared spectroscopic imaging assessment of cartilage composition: Validation with mid infrared imaging spectroscopy. Anal. Chim. Acta 2016, 926, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Padalkar, M.V.; Spencer, R.G.; Pleshko, N. Near Infrared Spectroscopic Evaluation of Water in Hyaline Cartilage. Ann. Biomed. Eng. 2013, 41, 2426–2436. [Google Scholar] [CrossRef] [Green Version]
- Padalkar, M.V.; Pleshko, N. Wavelength-dependent penetration depth of near infrared radiation into cartilage. Analyst 2015, 140, 2093–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karchner, J.P.; Yousefi, F.; Bitman, S.R.; Darvish, K.; Pleshko, N. Non-Destructive Spectroscopic Assessment of High and Low Weight Bearing Articular Cartilage Correlates with Mechanical Properties. Cartilage 2019, 10, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Ailavajhala, R.; Oswald, J.; Rajapakse, C.S.; Pleshko, N. Environmentally-Controlled Near Infrared Spectroscopic Imaging of Bone Water. Sci. Rep. 2019, 9, 10199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ailavajhala, R.; Querido, W.; Rajapakse, C.S.; Pleshko, N. Near infrared spectroscopic assessment of loosely and tightly bound cortical bone water. Analyst 2020, 145, 3713–3724. [Google Scholar] [CrossRef]
- McGoverin, C.M.; Lewis, K.; Yang, X.; Bostrom, M.P.; Pleshko, N. The contribution of bone and cartilage to the near-infrared spectrum of osteochondral tissue. Appl. Spectrosc. 2014, 68, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Rajapakse, C.S.; Padalkar, M.V.; Yang, H.J.; Ispiryan, M.; Pleshko, N. Non-destructive NIR spectral imaging assessment of bone water: Comparison to MRI measurements. Bone 2017, 103, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Granke, M.; Does, M.D.; Nyman, J.S. The Role of Water Compartments in the Material Properties of Cortical Bone. Calcif. Tissue Int. 2015, 97, 292–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarin, J.K.; Te Moller, N.C.R.; Mancini, I.A.D.; Brommer, H.; Visser, J.; Malda, J.; van Weeren, P.R.; Afara, I.O.; Töyräs, J. Arthroscopic near infrared spectroscopy enables simultaneous quantitative evaluation of articular cartilage and subchondral bone in vivo. Sci. Rep. 2018, 8, 13409. [Google Scholar] [CrossRef] [PubMed]
- Shanas, N.; Querido, W.; Dumont, A.; Yonko, E.; Carter, E.; Ok, J.; Karchner, J.P.; Barbe, M.F.; Ali, S.; Patil, C.; et al. Clinical application of near infrared fiber optic spectroscopy for noninvasive bone assessment. J. Biophotonics 2020, 13, e201960172. [Google Scholar] [CrossRef] [PubMed]
- Baykal, D.; Irrechukwu, O.; Lin, P.C.; Fritton, K.; Spencer, R.G.; Pleshko, N. Nondestructive Assessment of Engineered Cartilage Constructs Using Near-Infrared Spectroscopy. Appl. Spectrosc. 2010, 64, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandel, S.; Querido, W.; Falcon, J.M.; Reiners, D.J.; Pleshko, N. Approaches for In Situ Monitoring of Matrix Development in Hydrogel-Based Engineered Cartilage. Tissue Eng. Part C Methods 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- McGoverin, C.M.; Hanifi, A.; Palukuru, U.P.; Yousefi, F.; Glenn, P.B.M.; Shockley, M.; Spencer, R.G.; Pleshko, N. Nondestructive Assessment of Engineered Cartilage Composition by Near Infrared Spectroscopy. Ann. Biomed. Eng. 2016, 44, 680–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi, F.; Kim, M.; Nahri, S.Y.; Mauck, R.L.; Pleshko, N. Near-Infrared Spectroscopy Predicts Compositional and Mechanical Properties of Hyaluronic Acid-Based Engineered Cartilage Constructs. Tissue Eng. Part A 2018, 24, 106–116. [Google Scholar] [CrossRef]
- Yücel, M.A.; Selb, J.J.; Huppert, T.J.; Franceschini, M.A.; Boas, D.A. Functional Near Infrared Spectroscopy: Enabling Routine Functional Brain Imaging. Curr. Opin. Biomed. Eng. 2017, 4, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Hong, A.L.; Ispiryan, M.; Padalkar, M.V.; Jones, B.C.; Batzdorf, A.S.; Shetye, S.S.; Pleshko, N.; Rajapakse, C.S. MRI-derived bone porosity index correlates to bone composition and mechanical stiffness. Bone Rep. 2019, 11, 100213. [Google Scholar] [CrossRef]
- Karchner, J.P.; Querido, W.; Kandel, S.; Pleshko, N. Spatial correlation of native and engineered cartilage components at micron resolution. Ann. N. Y. Acad. Sci. 2019, 1442, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front. Bioeng. Biotechnol. 2019, 7, 303. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef] [Green Version]
- Albro, M.B.; Bergholt, M.S.; St-Pierre, J.P.; Vinals Guitart, A.; Zlotnick, H.M.; Evita, E.G.; Stevens, M.M. Raman spectroscopic imaging for quantification of depth-dependent and local heterogeneities in native and engineered cartilage. Regen. Med. 2018, 3, 3. [Google Scholar] [CrossRef]
- Mansfield, J.; Yu, J.; Attenburrow, D.; Moger, J.; Tirlapur, U.; Urban, J.; Cui, Z.F.; Winlove, P. The elastin network: Its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy. J. Anat. 2009, 215, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Akiva, A.; Malkinson, G.; Masic, A.; Kerschnitzki, M.; Bennet, M.; Fratzl, P.; Addadi, L.; Weiner, S.; Yaniv, K. On the pathway of mineral deposition in larval zebrafish caudal fin bone. Bone 2015, 75, 192–200. [Google Scholar] [CrossRef]
- Bennet, M.; Akiva, A.; Faivre, D.; Malkinson, G.; Yaniv, K.; Abdelilah-Seyfried, S.; Fratzl, P.; Masic, A. Simultaneous Raman microspectroscopy and fluorescence imaging of bone mineralization in living zebrafish larvae. Biophys. J. 2014, 106, L17–L19. [Google Scholar] [CrossRef] [Green Version]
- Bergholt, M.S.; St-Pierre, J.P.; Offeddu, G.S.; Parmar, P.A.; Albro, M.B.; Puetzer, J.L.; Oyen, M.L.; Stevens, M.M. Raman Spectroscopy Reveals New Insights into the Zonal Organization of Native and Tissue-Engineered Articular Cartilage. ACS Cent. Sci. 2016, 2, 885–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, R. Infrared spectroscopic imaging: The next generation. Appl. Spectrosc. 2012, 66, 1091–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, X.; Li, G.; Doty, S.B.; Camacho, N.P. A novel method for determination of collagen orientation in cartilage by Fourier transform infrared imaging spectroscopy (FT-IRIS). Osteoarthr. Cartil. 2005, 13, 1050–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifacio, A.; Beleites, C.; Vittur, F.; Marsich, E.; Semeraro, S.; Paoletti, S.; Sergo, V. Chemical imaging of articular cartilage sections with Raman mapping, employing uni- and multi-variate methods for data analysis. Analyst 2010, 135, 3193–3204. [Google Scholar] [CrossRef] [Green Version]
- Gourion-Arsiquaud, S.; Faibish, D.; Myers, E.; Spevak, L.; Compston, J.; Hodsman, A.; Shane, E.; Recker, R.R.; Boskey, E.R.; Boskey, A.L. Use of FTIR Spectroscopic Imaging to Identify Parameters Associated With Fragility Fracture. J. Bone Miner. Res. 2009, 24, 1565–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourion-Arsiquaud, S.; Lukashova, L.; Power, J.; Loveridge, N.; Reeve, J.; Boskey, A.L. Fourier Transform Infrared Imaging of Femoral Neck Bone: Reduced Heterogeneity of Mineral-to-Matrix and Carbonate-to-Phosphate and More Variable Crystallinity in Treatment-Naive Fracture Cases Compared With Fracture-Free Controls. J. Bone Miner. Res. 2013, 28, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Høgset, H.; Horgan, C.C.; Armstrong, J.P.K.; Bergholt, M.S.; Torraca, V.; Chen, Q.; Keane, T.J.; Bugeon, L.; Dallman, M.J.; Mostowy, S.; et al. In vivo biomolecular imaging of zebrafish embryos using confocal Raman spectroscopy. Nat. Commun. 2020, 11, 6172. [Google Scholar] [CrossRef]
- Imbert, L.; Gourion-Arsiquaud, S.; Villarreal-Ramirez, E.; Spevak, L.; Taleb, H.; van der Meulen, M.C.H.; Mendelsohn, R.; Boskey, A.L. Dynamic structure and composition of bone investigated by nanoscale infrared spectroscopy. PLoS ONE 2018, 13, e0202833. [Google Scholar] [CrossRef]
- Khanarian, N.T.; Boushell, M.K.; Spalazzi, J.P.; Pleshko, N.; Boskey, A.L.; Lu, H.H. FTIR-I compositional mapping of the cartilage-to-bone interface as a function of tissue region and age. J. Bone Miner. Res. 2014, 29, 2643–2652. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Bi, X.H.; Horton, W.E.; Spencer, R.G.; Camacho, N.P. Fourier transform infrared imaging spectroscopic analysis of tissue engineered cartilage: Histologic and biochemical correlations. J. Biomed. Opt. 2005, 10, 031105. [Google Scholar] [CrossRef] [PubMed]
- Masci, M.; Wang, M.; Imbert, L.; Barnes, A.M.; Spevak, L.; Lukashova, L.; Huang, Y.; Ma, Y.; Marini, J.C.; Jacobsen, C.M.; et al. Bone mineral properties in growing Col1a2(+/G610C) mice, an animal model of osteogenesis imperfecta. Bone 2016, 87, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querido, W.; Shanas, N.; Bookbinder, S.; Oliveira-Nunes, M.C.; Krynska, B.; Pleshko, N. Fourier transform infrared spectroscopy of developing bone mineral: From amorphous precursor to mature crystal. Analyst 2020, 145, 764–776. [Google Scholar] [CrossRef]
- Rieppo, L.; Rieppo, J.; Jurvelin, J.S.; Saarakkala, S. Fourier transform infrared spectroscopic imaging and multivariate regression for prediction of proteoglycan content of articular cartilage. PLoS ONE 2012, 7, e32344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.X.; Lloyd, A.A.; Burket, J.C.; Gourion-Arsiquaud, S.; Donnelly, E. Altered distributions of bone tissue mineral and collagen properties in women with fragility fractures. Bone 2016, 84, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanifi, A.; Bi, X.H.; Yang, X.; Kavukcuoglu, B.; Lin, P.C.; DiCarlo, E.; Spencer, R.G.; Bostrom, M.P.G.; Pleshko, N. Infrared Fiber Optic Probe Evaluation of Degenerative Cartilage Correlates to Histological Grading. Am. J. Sports Med. 2012, 40, 2853–2861. [Google Scholar] [CrossRef] [Green Version]
- Hanifi, A.; McCarthy, H.; Roberts, S.; Pleshko, N. Fourier Transform Infrared Imaging and Infrared Fiber Optic Probe Spectroscopy Identify Collagen Type in Connective Tissues. PLoS ONE 2013, 8, e64822. [Google Scholar] [CrossRef] [PubMed]
- Hanifi, A.; Palukuru, U.; McGoverin, C.; Shockley, M.; Frank, E.; Grodzinsky, A.; Spencer, R.G.; Pleshko, N. Near infrared spectroscopic assessment of developing engineered tissues: Correlations with compositional and mechanical properties. Analyst 2017, 142, 1320–1332. [Google Scholar] [CrossRef] [Green Version]
- Afara, I.; Singh, S.; Oloyede, A. Application of near infrared (NIR) spectroscopy for determining the thickness of articular cartilage. Med. Eng. Phys. 2013, 35, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Afara, I.O.; Prasadam, I.; Crawford, R.; Xiao, Y.; Oloyede, A. Near infrared (NIR) absorption spectra correlates with subchondral bone micro-CT parameters in osteoarthritic rat models. Bone 2013, 53, 350–357. [Google Scholar] [CrossRef]
- Afara, I.O.; Florea, C.; Olumegbon, I.A.; Eneh, C.T.; Malo, M.K.H.; Korhonen, R.K.; Töyräs, J. Characterizing human subchondral bone properties using near-infrared (NIR) spectroscopy. Sci. Rep. 2018, 8, 9733. [Google Scholar] [CrossRef] [Green Version]
- Buckley, K.; Kerns, J.G.; Parker, A.W.; Goodship, A.E.; Matousek, P. Decomposition of in vivo spatially offset Raman spectroscopy data using multivariate analysis techniques. J. Raman Spectrosc. 2014, 45, 188–192. [Google Scholar] [CrossRef]
- Buckley, K.; Kerns, J.G.; Vinton, J.; Gikas, P.D.; Smith, C.; Parker, A.W.; Matousek, P.; Goodship, A.E. Towards the in vivo prediction of fragility fractures with Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 610–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Z.Y.; Sinjab, F.; Nommeots-Nomm, A.; Jones, J.; Ruiz-Cantu, L.; Yang, J.; Rose, F.; Notingher, I. Feasibility of Spatially Offset Raman Spectroscopy for in Vitro and in Vivo Monitoring Mineralization of Bone Tissue Engineering Scaffolds. Anal. Chem. 2017, 89, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Bergholt, M.S.; Albro, M.B.; Stevens, M.M. Online quantitative monitoring of live cell engineered cartilage growth using diffuse fiber-optic Raman spectroscopy. Biomaterials 2017, 140, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Stevens, O.; Petterson, I.E.I.; Day, J.C.C.; Stone, N. Developing fibre optic Raman probes for applications in clinical spectroscopy. Chem. Soc. Rev. 2016, 45, 1919–1934. [Google Scholar] [CrossRef]
- Gomes da Costa, S.; Richter, A.; Schmidt, U.; Breuninger, S.; Hollricher, O. Confocal Raman microscopy in life sciences. Morphologie 2019, 103, 11–16. [Google Scholar] [CrossRef]
- Zhang, D.; Li, C.; Zhang, C.; Slipchenko, M.N.; Eakins, G.; Cheng, J.X. Depth-resolved mid-infrared photothermal imaging of living cells and organisms with submicrometer spatial resolution. Sci. Adv. 2016, 2, e1600521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klementieva, O.; Sandt, C.; Martinsson, I.; Kansiz, M.; Gouras, G.K.; Borondics, F. Super-Resolution Infrared Imaging of Polymorphic Amyloid Aggregates Directly in Neurons. Adv. Sci. (Weinh.) 2020, 7, 1903004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakir, G.; Girouard, B.E.; Wiens, R.; Mastel, S.; Dillon, E.; Kansiz, M.; Gough, K.M. Orientation Matters: Polarization Dependent IR Spectroscopy of Collagen from Intact Tendon Down to the Single Fibril Level. Molecules 2020, 25, 4295. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.E.; Xiao, Y.; Lei, Z.; Ault, A.P. Simultaneous Optical Photothermal Infrared (O-PTIR) and Raman Spectroscopy of Submicrometer Atmospheric Particles. Anal. Chem. 2020, 92, 9932–9939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Weckhuysen, B.M.; Wain, A.J.; Pollard, A.J. Nanoscale chemical imaging using tip-enhanced Raman spectroscopy. Nat. Protoc. 2019, 14, 1169–1193. [Google Scholar] [CrossRef]
- Cooney, T.F.; Skinner, H.T.; Angel, S.M. Comparative Study of Some Fiber-Optic Remote Raman Probe Designs. Part I: Model for Liquids and Transparent Solids. Appl. Spectrosc. 1996, 50, 836–848. [Google Scholar] [CrossRef]
- Santos, L.F.; Wolthuis, R.; Koljenović, S.; Almeida, R.M.; Puppels, G.J. Fiber-optic probes for in vivo Raman spectroscopy in the high-wavenumber region. Anal. Chem. 2005, 77, 6747–6752. [Google Scholar] [CrossRef]
- Yousefi, F.; Kandel, S.; Pleshko, N. Infrared Spectroscopic Quantification of Methacrylation of Hyaluronic Acid: A Scaffold for Tissue Engineering Applications. Appl. Spectrosc. 2018, 72, 1455–1466. [Google Scholar] [CrossRef]
- Virtanen, V.; Nippolainen, E.; Shaikh, R.; Afara, I.; Töyräs, J.; Solheim, J.; Tafintseva, V.; Zimmermann, B.; Kohler, A.; Saarakkala, S.; et al. Infrared fiber optic spectroscopy detects bovine articular cartilage degeneration. bioRxiv 2020. [Google Scholar] [CrossRef]
- Esmonde-White, K.A.; Esmonde-White, F.W.L.; Morris, M.D.; Roessler, B.J. Fiber-optic Raman spectroscopy of joint tissues. Analyst 2011, 136, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, D.A.; Roque, R.A.; Lin, P.-C.; Irrechukwu, O.; Doty, S.; Longo, D.L.; Pleshko, N.; Spencer, R.G. Mapping proteoglycan-bound water in cartilage: Improved specificity of matrix assessment using multiexponential transverse relaxation analysis. Magn. Reson. Med. 2011, 65, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasch, P. Spectral pre-processing for biomedical vibrational spectroscopy and microspectroscopic imaging. Chemometr. Intell. Lab. Syst. 2012, 117, 100–114. [Google Scholar] [CrossRef] [Green Version]
- Byrne, H.J.; Knief, P.; Keating, M.E.; Bonnier, F. Spectral pre and post processing for infrared and Raman spectroscopy of biological tissues and cells. Chem. Soc. Rev. 2016, 45, 1865–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinnan, Å.; Berg, F.v.d.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Analyt. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Saarakkala, S.; Rieppo, L.; Rieppo, J.; Jurvelin, J.S. Fourier transform infrared (FTIR) microspectroscopy of immature, mature and degenerated articular cartilage. Microscopy 2010, 1, 403–414. [Google Scholar]
- Ramakrishnan, N.; Xia, Y. Fourier-transform infrared spectroscopic imaging of articular cartilage and biomaterials: A review. Trends Appl. Spectrosc. 2013, 10, 1–23. [Google Scholar] [PubMed]
- Calce, S.E.; Kurki, H.K.; Weston, D.A.; Gould, L. Principal component analysis in the evaluation of osteoarthritis. Am. J. Phys. Anthropol. 2017, 162, 476–490. [Google Scholar] [CrossRef]
- Mao, Z.-H.; Yin, J.-H.; Zhang, X.-X.; Wang, X.; Xia, Y. Discrimination of healthy and osteoarthritic articular cartilage by Fourier transform infrared imaging and Fisher’s discriminant analysis. Biomed. Opt. Express 2016, 7, 448–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassi, R.; Harri, T.K.; Katariina, A.M.K.; Vuokko, K.; Mikko, J.L.; Juha, T.; Simo, S. Infrared microspectroscopic determination of collagen cross-links in articular cartilage. J. Biomed. Opt. 2017, 22, 1–9. [Google Scholar]
- Prakash, M.; Sarin, J.K.; Rieppo, L.; Afara, I.O.; Töyräs, J. Optimal Regression Method for Near-Infrared Spectroscopic Evaluation of Articular Cartilage. Appl. Spectrosc. 2017, 71, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, C.D.; Kaghazchi, A.; Bonassar, L.J. Measurement of local diffusion and composition in degraded articular cartilage reveals the unique role of surface structure in controlling macromolecular transport. J. Biomech. 2019, 82, 38–45. [Google Scholar] [CrossRef]
- Oinas, J.; Rieppo, L.; Finnilä, M.A.J.; Valkealahti, M.; Lehenkari, P.; Saarakkala, S. Imaging of Osteoarthritic Human Articular Cartilage using Fourier Transform Infrared Microspectroscopy Combined with Multivariate and Univariate Analysis. Sci. Rep. 2016, 6, 30008. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Saghatoleslami, N.; Mahdavi-Shahri, N.; Matin, M.M.; Gheshlaghi, R.; Moradi, A. A comparison study of different decellularization treatments on bovine articular cartilage. J. Tissue Eng. Regen. Med. 2019, 13, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Middendorf, J.M.; Dugopolski, C.; Kennedy, S.; Blahut, E.; Cohen, I.; Bonassar, L.J. Heterogeneous matrix deposition in human tissue engineered cartilage changes the local shear modulus and resistance to local construct buckling. J. Biomech. 2020, 105, 109760. [Google Scholar] [CrossRef]
- Falcon, J.M.; Chirman, D.; Veneziale, A.; Morman, J.; Bolten, K.; Kandel, S.; Querido, W.; Freeman, T.; Pleshko, N. DMOG Negatively Impacts Tissue Engineered Cartilage Development. Cartilage 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Erickson, I.E.; Choudhury, M.; Pleshko, N.; Mauck, R.L. Transient exposure to TGF-beta 3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J. Mech. Behav. Biomed. Mater. 2012, 11, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarin, J.K.; Nykänen, O.; Tiitu, V.; Mancini, I.A.D.; Brommer, H.; Visser, J.; Malda, J.; Weeren, P.R.v.; Afara, I.O.; Töyräs, J. Arthroscopic Determination of Cartilage Proteoglycan Content and Collagen Network Structure with Near-Infrared Spectroscopy. Ann. Biomed. Eng. 2019, 47, 1815–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, M.; Joukainen, A.; Torniainen, J.; Honkanen, M.K.M.; Rieppo, L.; Afara, I.O.; Kröger, H.; Töyräs, J.; Sarin, J.K. Near-infrared spectroscopy enables quantitative evaluation of human cartilage biomechanical properties during arthroscopy. Osteoarthr. Cartil. 2019, 27, 1235–1243. [Google Scholar] [CrossRef]
- Afara, I.O.; Singh, S.; Oloyede, A. Load-unloading response of intact and artificially degraded articular cartilage correlated with near infrared (NIR) absorption spectra. J. Mech. Behav. Biomed. Mater. 2013, 20, 249–258. [Google Scholar] [CrossRef]
- Afara, I.O.; Sarin, J.K.; Ojanen, S.; Finnilä, M.A.J.; Herzog, W.; Saarakkala, S.; Korhonen, R.K.; Töyräs, J. Machine Learning Classification of Articular Cartilage Integrity Using Near Infrared Spectroscopy. Cell. Mol. Bioeng. 2020, 13, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarin, J.K.; Amissah, M.; Brommer, H.; Argüelles, D.; Töyräs, J.; Afara, I.O. Near Infrared Spectroscopic Mapping of Functional Properties of Equine Articular Cartilage. Ann. Biomed. Eng. 2016, 44, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Afara, I.O.; Hauta-Kasari, M.; Jurvelin, J.S.; Oloyede, A.; Töyräs, J. Optical absorption spectra of human articular cartilage correlate with biomechanical properties, histological score and biochemical composition. Physiol. Meas. 2015, 36, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Nippolainen, E.; Shaikh, R.; Virtanen, V.; Rieppo, L.; Saarakkala, S.; Töyräs, J.; Afara, I.O. Near Infrared Spectroscopy Enables Differentiation of Mechanically and Enzymatically Induced Cartilage Injuries. Ann. Biomed. Eng. 2020, 48, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Kafian-Attari, I.; Nippolainen, E.; Semenov, D.; Hauta-Kasari, M.; Töyräs, J.; Afara, I.O. Tissue optical properties combined with machine learning enables estimation of articular cartilage composition and functional integrity. Biomed. Opt. Express 2020, 11, 6480–6494. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.A.; Posner, A.S. Measurement of non-crystalline calcium phosphate in bone mineral. Proc. Soc. Exp. Biol. Med. 1966, 122, 137–142. [Google Scholar] [CrossRef]
- Termine, J.D.; Posner, A.S. Infrared analysis of rat bone: Age dependency of amorphous and crystalline mineral fractions. Science 1966, 153, 1523–1525. [Google Scholar] [CrossRef]
- Boskey, A.L. Amorphous calcium phosphate: The contention of bone. J. Dent. Res. 1997, 76, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Bargman, R.; Posham, R.; Boskey, A.L.; DiCarlo, E.; Raggio, C.; Pleshko, N. Comparable outcomes in fracture reduction and bone properties with RANKL inhibition and alendronate treatment in a mouse model of osteogenesis imperfecta. Osteoporos. Int. 2012, 23, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Camacho, N.P.; Rinnerthaler, S.; Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L.; Fratzl, P. Complementary information on bone ultrastructure from scanning small angle X-ray scattering and Fourier-transform infrared microspectroscopy. Bone 1999, 25, 287–293. [Google Scholar] [CrossRef]
- Camacho, N.P.; Hou, L.; Toledano, T.R.; Ilg, W.A.; Brayton, C.F.; Raggio, C.L.; Root, L.; Boskey, A.L. The material basis for reduced mechanical properties in oim mice bones. J. Bone Miner. Res. 1999, 14, 264–272. [Google Scholar] [CrossRef] [PubMed]
- West, P.A.; Bostrom, M.P.G.; Torzilli, P.A.; Camacho, N.P. Fourier transform infrared spectral analysis of degenerative cartilage: An infrared fiber optic probe and imaging study. Appl. Spectrosc. 2004, 58, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A. Bone mineral crystal size. Osteoporos. Int. 2003, 14 Suppl. 5, 16–21. [Google Scholar] [CrossRef]
- Paschalis, E.P.; DiCarlo, E.; Betts, F.; Sherman, P.; Mendelsohn, R.; Boskey, A.L. FTIR microspectroscopic analysis of human osteonal bone. Calcif. Tissue Int. 1996, 59, 480–487. [Google Scholar] [CrossRef]
- Pleshko, N.; Boskey, A.; Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 1991, 60, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Querido, W.; Campos, A.P.C.; Ferreira, E.H.M.; Gil, R.; Rossi, A.M.; Farina, M. Strontium ranelate changes the composition and crystal structure of the biological bone-like apatite produced in osteoblast cell cultures. Cell Tissue Res. 2014, 357, 793–801. [Google Scholar] [CrossRef]
- Kourkoumelis, N.; Zhang, X.; Lin, Z.; Wang, J. Fourier Transform Infrared Spectroscopy of Bone Tissue: Bone Quality Assessment in Preclinical and Clinical Applications of Osteoporosis and Fragility Fracture. Clin. Rev. Bone Miner. Metab. 2019, 17, 24–39. [Google Scholar] [CrossRef]
- Hernandez, J.D.; Wesseling, K.; Pereira, R.; Gales, B.; Harrison, R.; Salusky, I.B. Technical approach to iliac crest biopsy. Clin. J. Am. Soc. Nephrol. 2008, 3 Suppl. 3, S164–S169. [Google Scholar] [CrossRef]
- Kulak, C.A.; Dempster, D.W. Bone histomorphometry: A concise review for endocrinologists and clinicians. Arq. Bras. Endocrinol. Metabol. 2010, 54, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, S.; Mayemba, C.N.; Ung, R.V.; Martel, S.; Mac-Way, F. Division of an Iliac Crest Bone Biopsy Specimen to Allow Histomorphometry, Immunohistochemical, Molecular Analysis, and Tissue Banking: Technical Aspect and Applications. JBMR Plus 2020, 4, e10424. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Subramony, S.D.; Boskey, A.L.; Pleshko, N.; Doty, S.B.; Lu, H.H. Compositional mapping of the mature anterior cruciate ligament-to-bone insertion. J. Orthop. Res. 2017, 35, 2513–2523. [Google Scholar] [CrossRef] [Green Version]
- Spalazzi, J.P.; Boskey, A.L.; Pleshko, N.; Lu, H.H. Quantitative mapping of matrix content and distribution across the ligament-to-bone insertion. PLoS ONE 2013, 8, e74349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, M.; Bora, T.; Ghaithi, A.A.; Thukral, S.; Dutta, J. Raman Spectroscopy detects changes in Bone Mineral Quality and Collagen Cross-linkage in Staphylococcus Infected Human Bone. Sci. Rep. 2018, 8, 9417. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Yang, S.; Akkus, O. Molecular spectroscopic identification of the water compartments in bone. Bone 2014, 67, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Akkus, O. Raman spectral classification of mineral- and collagen-bound water’s associations to elastic and post-yield mechanical properties of cortical bone. Bone 2015, 81, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Pasteris, J.D.; Wopenka, B.; Freeman, J.J.; Rogers, K.; Valsami-Jones, E.; van der Houwen, J.A.M.; Silva, M.J. Lack of OH in nanocrystalline apatite as a function of degree of atomic order: Implications for bone and biomaterials. Biomaterials 2004, 25, 229–238. [Google Scholar] [CrossRef]
- Unal, M.; Uppuganti, S.; Leverant, C.J.; Creecy, A.; Granke, M.; Voziyan, P.; Nyman, J.S. Assessing glycation-mediated changes in human cortical bone with Raman spectroscopy. J. Biophotonics 2018, 11, e201700352. [Google Scholar] [CrossRef] [Green Version]
- Unal, M.; Uppuganti, S.; Timur, S.; Mahadevan-Jansen, A.; Akkus, O.; Nyman, J.S. Assessing matrix quality by Raman spectroscopy helps predict fracture toughness of human cortical bone. Sci. Rep. 2019, 9, 7195. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Guerado, E.; Caso, E.; Aguilera, F.S.; Osorio, R. Biochemical assessment of nanostructures in human trabecular bone: Proposal of a Raman microspectroscopy based measurements protocol. Injury 2018, 49 Suppl. 2, S11–S21. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Robins, S.P.; Tatakis, D.N.; Klaushofer, K.; Paschalis, E.P. Identification of Pyridinoline Trivalent Collagen Cross-Links by Raman Microspectroscopy. Calcif. Tissue Int. 2017, 100, 565–574. [Google Scholar] [CrossRef]
- Goodyear, S.R.; Aspden, R.M. Raman Microscopy and Bone. Methods Mol. Biol. 2019, 1914, 651–659. [Google Scholar] [PubMed]
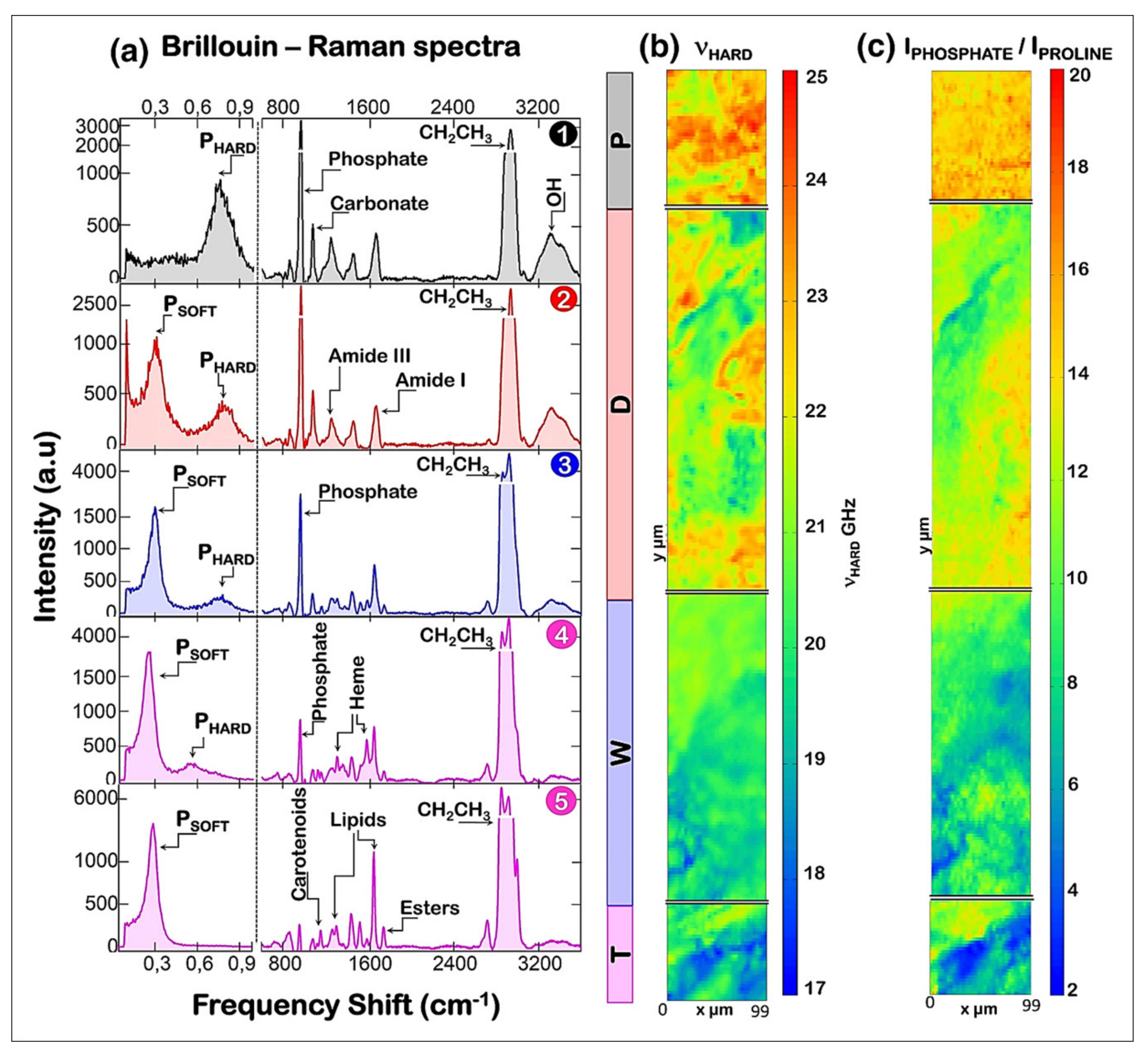
- Cardinali, M.A.; Govoni, M.; Dallari, D.; Caponi, S.; Fioretto, D.; Morresi, A. Mechano-chemistry of human femoral diaphysis revealed by correlative Brillouin-Raman microspectroscopy. Sci. Rep. 2020, 10, 17341. [Google Scholar] [CrossRef] [PubMed]
- Kochetkova, T.; Peruzzi, C.; Braun, O.; Overbeck, J.; Maurya, A.K.; Neels, A.; Calame, M.; Michler, J.; Zysset, P.; Schwiedrzik, J. Combining polarized Raman spectroscopy and micropillar compression to study microscale structure-property relationships in mineralized tissues. Acta Biomater. 2021, 119, 390–404. [Google Scholar] [CrossRef]
- Schulmerich, M.V.; Cole, J.H.; Kreider, J.M.; Esmonde-White, F.; Dooley, K.A.; Goldstein, S.A.; Morris, M.D. Transcutaneous Raman spectroscopy of murine bone in vivo. Appl. Spectrosc. 2009, 63, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Okagbare, P.I.; Esmonde-White, F.W.; Goldstein, S.A.; Morris, M.D. Development of non-invasive Raman spectroscopy for in vivo evaluation of bone graft osseointegration in a rat model. Analyst 2010, 135, 3142–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okagbare, P.I.; Begun, D.; Tecklenburg, M.; Awonusi, A.; Goldstein, S.A.; Morris, M.D. Noninvasive Raman spectroscopy of rat tibiae: Approach to in vivo assessment of bone quality. J. Biomed. Opt. 2012, 17, 090502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers, J.L.; Esmonde-White, F.W.; Esmonde-White, K.A.; Morris, M.D.; Pogue, B.W. Next-generation Raman tomography instrument for non-invasive in vivo bone imaging. Biomed. Opt. Express 2015, 6, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Chen, K.; Lynch, M.; Maher, J.R.; Awad, H.A.; Berger, A.J. Spatially offset Raman spectroscopy for in vivo bone strength prediction. Biomed. Opt. Express 2018, 9, 4781–4791. [Google Scholar] [CrossRef]




| Section | Page |
|---|---|
| 1. Overview | 1 |
| 2. Connective tissues | 2 |
| 2.1. Cartilage | 2 |
| 2.2. Bone | 3 |
| 3. Vibrational spectroscopy | 4 |
| 3.1. Vibrational spectroscopy modalities | 5 |
| 3.1.1. Mid infrared (FTIR) spectroscopy | 5 |
| 3.1.2. Near infrared spectroscopy | 7 |
| 3.1.3. Raman spectroscopy | 8 |
| 3.2. Advanced vibrational spectroscopy techniques | 9 |
| 3.2.1. Spectral imaging | 10 |
| 3.2.2. Fiber optic probes | 12 |
| 3.3. Spectral data analysis | 12 |
| 3.3.1. Pre-processing | 12 |
| 3.3.2. Post-processing | 13 |
| 4. Application of vibrational spectroscopy for connective tissue analysis | 14 |
| 4.1. Applications for cartilage assessment | 14 |
| 4.1.1. Mid infrared spectral analysis of cartilage | 14 |
| 4.1.2. Near infrared spectral analysis of cartilage | 14 |
| 4.1.3. Raman spectral analysis of cartilage | 16 |
| 4.2. Applications for bone assessment | 17 |
| 4.2.1. Mid infrared spectral analysis of bone | 17 |
| 4.2.2. Near infrared spectral analysis of bone | 19 |
| 4.2.3. Raman spectral analysis of bone | 22 |
| 5. Concluding remarks | 25 |
| Frequency (cm−1) | Tissue Component |
|---|---|
| 1740 | Lipid (ester C=O stretching) |
| 1650 | Protein (Amide I; peptide C=O stretching) |
| 1630 | Water (OH bending) |
| 1550 | Protein (Amide II; C–N stretching and N–H bending) |
| 1338 | Collagen (side chain CH2 vibration) |
| 1115 | Mineral (HPO42− stretching) |
| 1060 | Carbohydrates (sugar ring C–O stretching) |
| 1030 | Mineral (PO4 stretching) |
| 875 | Mineral (CO32− bending) |
| 856 | Sulfated proteoglycan (C–S bending) |
| Frequency (cm−1) | Tissue Component |
|---|---|
| 8500 | Water (O–H stretching and bending) |
| 7000 | Water (O–H stretching) |
| 6688 | Protein/collagen (N–H stretching) |
| 5800 | Lipid (CH2 stretching) |
| 5200 | Water (O–H stretching and bending) |
| 4890 | Protein/collagen (N–H bending) |
| 4610 | Protein/collagen (C–H stretching and deformation) |
| 4310 | Proteoglycan (sugar ring vibrations) |
| Frequency (cm−1) | Tissue Component |
|---|---|
| 1660 | Protein (Amide I; C=O stretching) |
| 1260 | Protein (Amide III; C–N stretching and N–H bending) |
| 1070 | Mineral (CO32− stretching) |
| 1060 | Sulfated proteoglycan (S=O stretching) |
| 960 | Mineral (PO4 stretching) |
| 850 | Collagen (Proline; C–C stretching) |
| Peak intensity Ratio 1 (Peak Frequencies Shown in cm−1) | Bone Compositional Property |
|---|---|
| 1030/1650 1030/1550 2 | Mineral content (or mineral-to-matrix ratio) |
| 1030/1020 960/1115 3 | Mineral crystallinity |
| 875/1030 | Mineral carbonate content (or carbonate-to-phosphate ratio) |
| 1115/1030 | Mineral HPO42− content |
| 1660/1690 | Collagen maturity (or collagen crosslink ratio) |
| Spectral Parameter (cm−1) 1 | Bone Compositional Property |
|---|---|
| 960/1660 intensity ratio | Mineral content (or mineral-to-matrix ratio) |
| Full width at half maximum (FWHM) at 960 | Mineral crystallinity |
| 1070/960 intensity ratio | Mineral carbonate content (or carbonate-to-phosphate ratio) |
| 1660/1690 intensity ratio | Collagen maturity (or collagen crosslink ratio) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Querido, W.; Kandel, S.; Pleshko, N. Applications of Vibrational Spectroscopy for Analysis of Connective Tissues. Molecules 2021, 26, 922. https://doi.org/10.3390/molecules26040922
Querido W, Kandel S, Pleshko N. Applications of Vibrational Spectroscopy for Analysis of Connective Tissues. Molecules. 2021; 26(4):922. https://doi.org/10.3390/molecules26040922
Chicago/Turabian StyleQuerido, William, Shital Kandel, and Nancy Pleshko. 2021. "Applications of Vibrational Spectroscopy for Analysis of Connective Tissues" Molecules 26, no. 4: 922. https://doi.org/10.3390/molecules26040922






