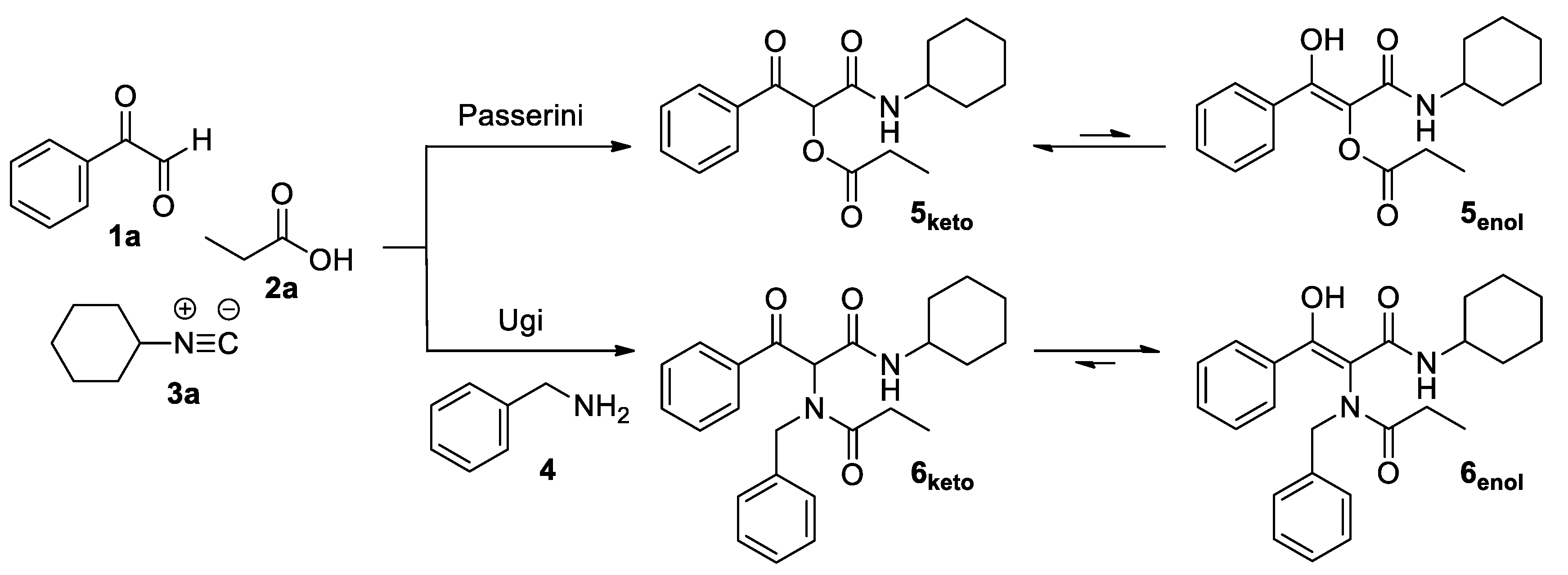
3.2. Synthesis and Characterization
3.2.1. Passerini Adducts
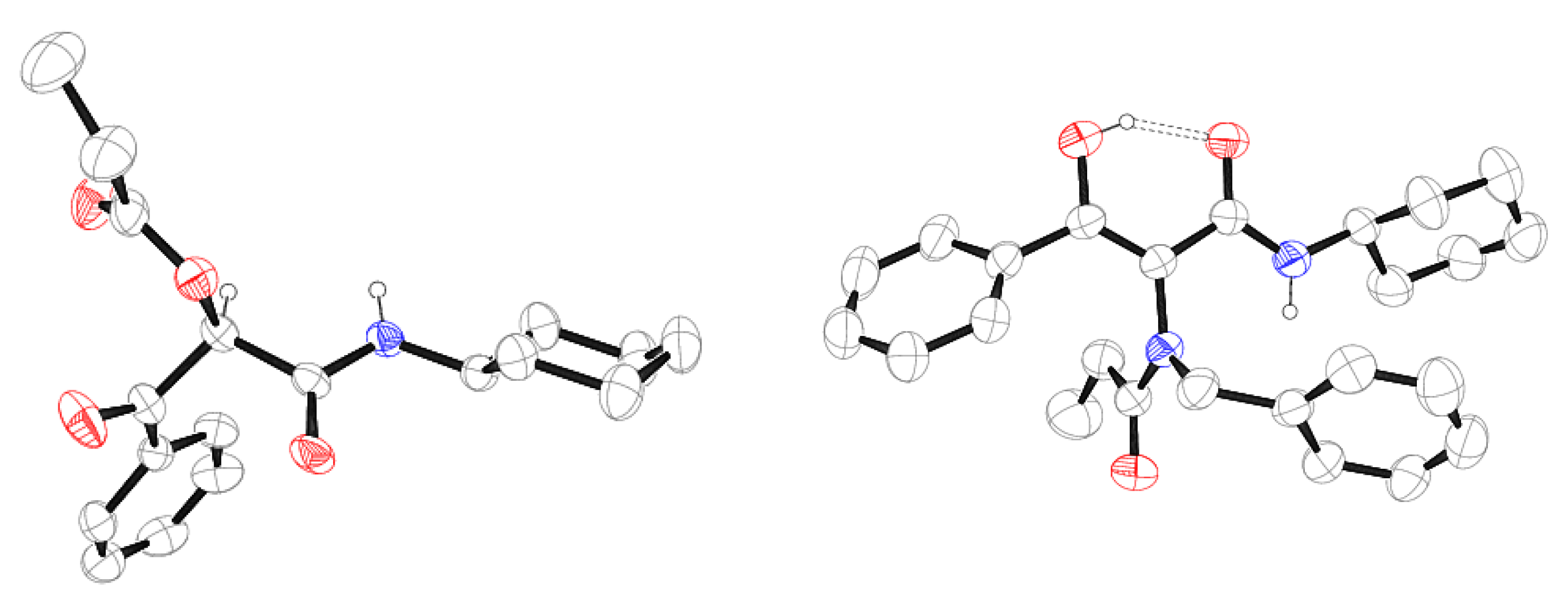
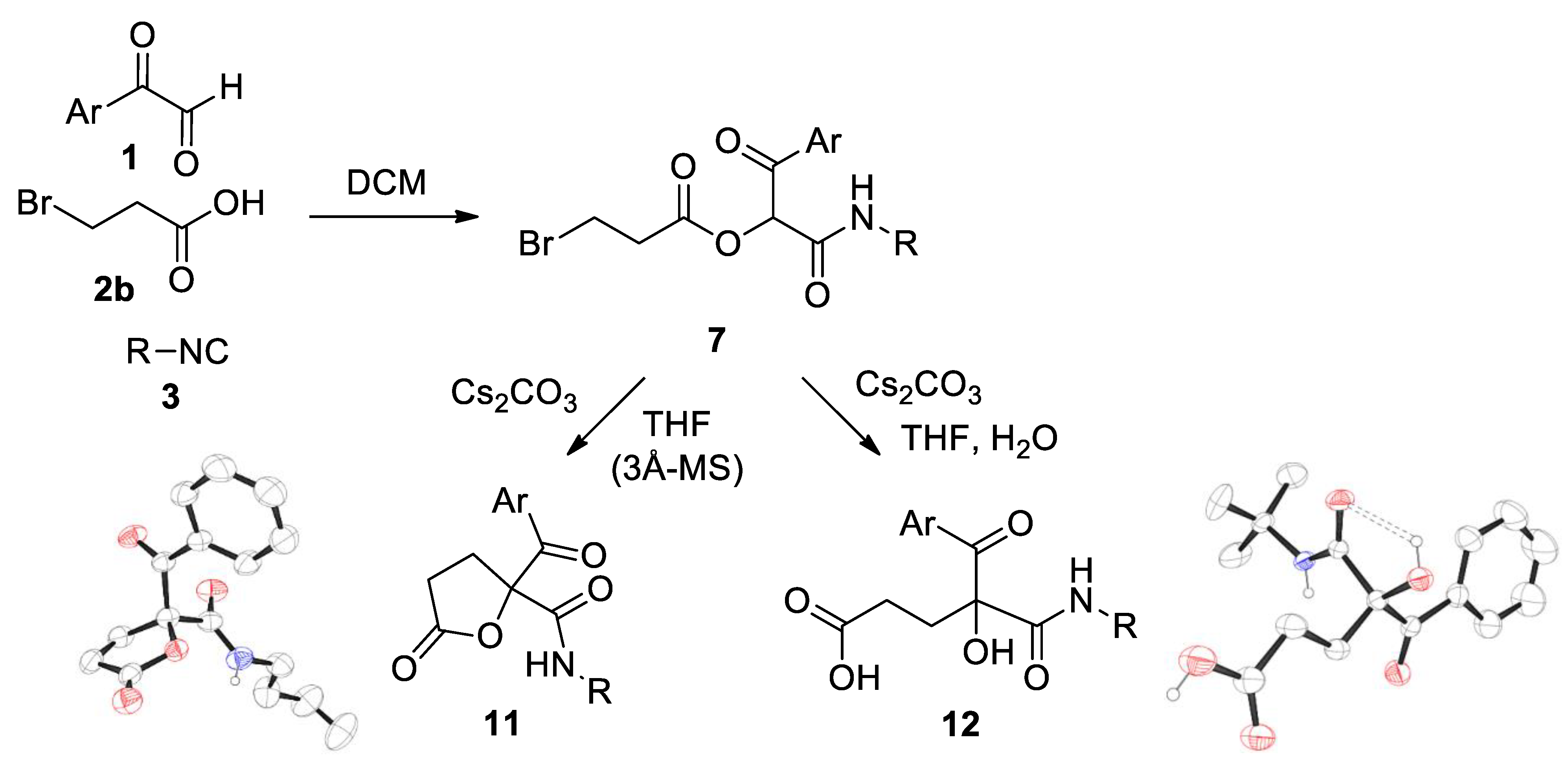
Method A: The corresponding arylglyoxal hydrate 1 (1 mmol) was dissolved in dichloromethane (10 mL), after which the corresponding acid 2a-c (1 mmol) and the corresponding isocyanide 3 (1 mmol) were added. The reaction mixture was stirred at room temperature for 24 h. A hydrochloric acid aqueous solution (1 mL) was added to neutralize the unreacted isocyanide and the mixture was washed with a sodium carbonate aqueous solution (1 × 50 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to dryness. Slow evaporation of a solution of compound 5 in a 1:1 chloroform-hexane mixture provided colorless single crystals, suitable for X-ray diffraction analysis.
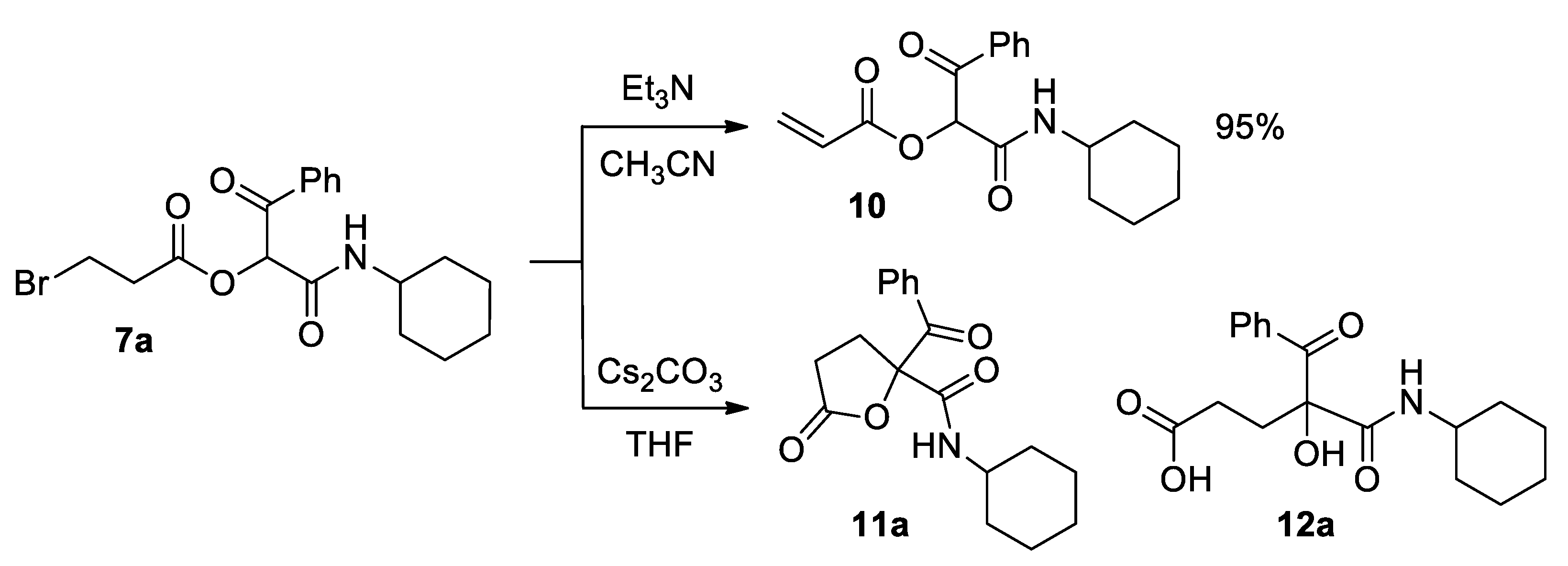
Method B: compound 10 was also prepared following a different procedure: Passerini adduct 7a (1 mmol) was dissolved in acetonitrile (10 mL) and triethylamine (1.1 mmol) was added. The reaction mixture was stirred at room temperature for 24 h and, after that, concentrated to dryness. The crude was dissolved in dichloromethane (20 mL) and this solution washed with a hydrochloric acid aqueous solution (1 × 50 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to dryness. The residue was purified by column chromatography, employing silica gel as a stationary phase and a 4:1 hexane-ethyl acetate mixture as an eluent.
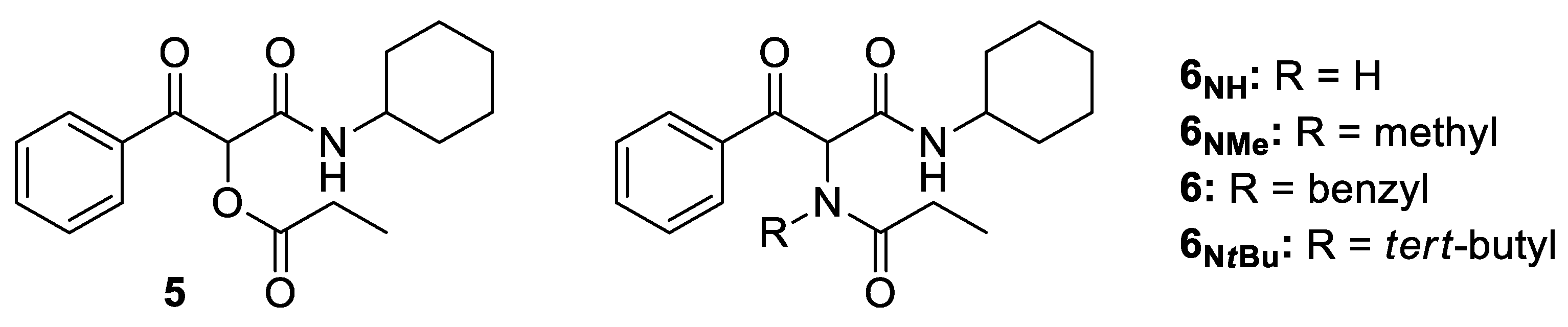
1-(Cyclohexylamino)-1,3-dioxo-3-phenylpropan-2-yl propionate (5). White solid. Yield: 82%. M. p. 110–111 °C. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.17–8.11 (m, 2H), 7.63–7.57 (m, 1H), 7.51–7.45 (m, 2H), 6.29 (s, 1H), 6.21 (d, J = 7.7 Hz, 1H, NH), 3.79–3.67 (m, 1H), 2.55 (qd, J = 15.2, 7.6 Hz, 1H), 2.54 (qd, J = 15.2, 7.6 Hz, 1H), 1.97–1.54 (m, 4H), 1.42–1.09 (m, 6H), 1.20 (t, J = 7.5 Hz, 3H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 191.9 (Cq), 172.2 (Cq), 162.7 (Cq), 134.4 (Cq), 133.8 (CHAr), 129.5 (CHAr), 128.4 (CHAr), 76.1 (CH), 48.5 (CH), 32.5 (CH2), 32.4 (CH2), 27.0 (CH2), 25.2 (CH2), 24.6 (CH2), 24.6 (CH2), 8.7 (CH3). HRMS (ESI): calculated for C18H24NO4 [MH+] 318.1700, found 318.1703.
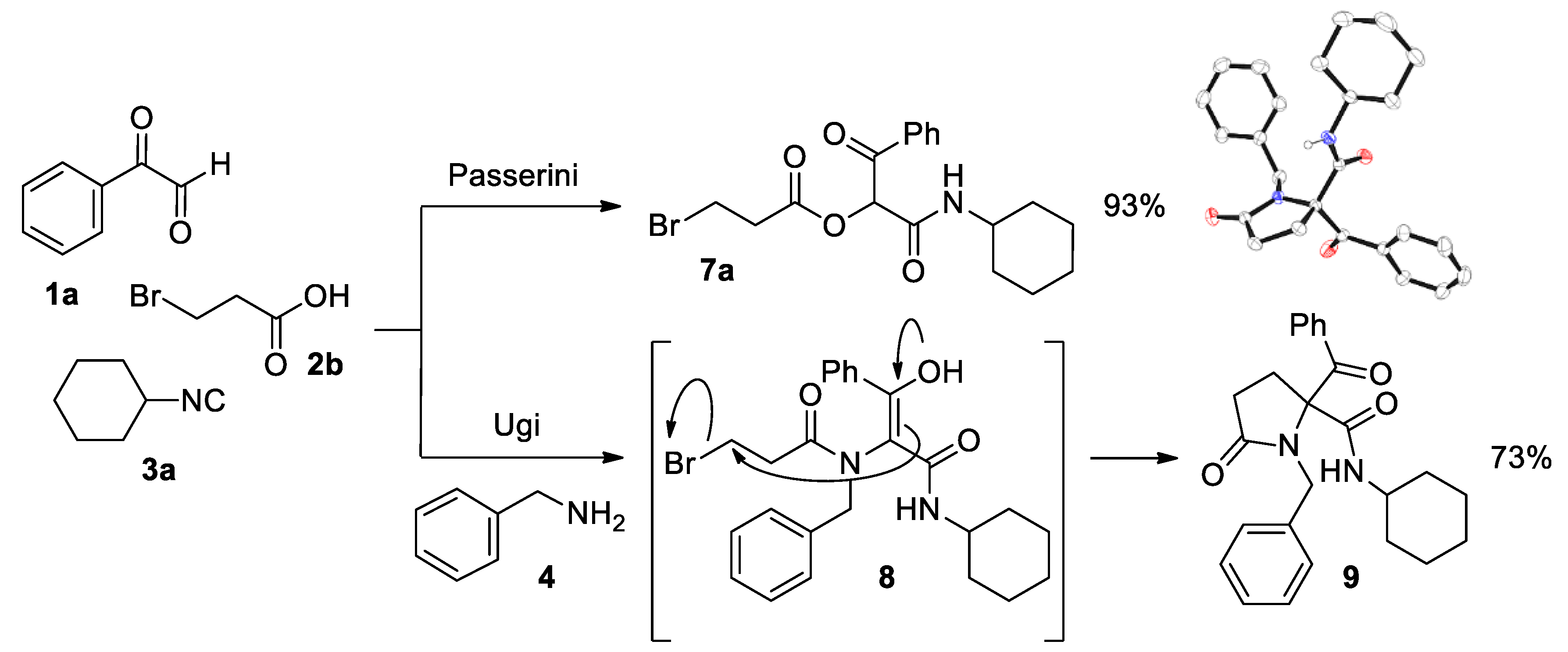
1-(Cyclohexylamino)-1,3-dioxo-3-phenylpropan-2-yl 3-bromopropanoate (7a). Pale yellow solid. Yield: 93%. M. p. 114–115 °C. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.16–8.09 (m, 2H), 7.63–7.57 (m, 1H), 7.52–7.45 (m, 2H), 6.36 (s, 1H), 6.30 (d, J = 7.7 Hz, 1H, NH), 3.78–3.66 (m, 1H), 3.61 (t, J = 6.8 Hz, 2H), 3.14 (t, J = 6.6 Hz, 2H), 1.98–1.06 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 191.5 (Cq), 168.6 (Cq), 162.4 (Cq), 134.4 (Cq), 134.3 (CHAr), 129.8 (CHAr), 128.7 (CHAr), 76.6 (CH), 48.9 (CH), 37.4 (CH2), 32.8 (CH2), 32.7 (CH2), 25.4 (CH2), 25.2 (CH2), 24.8 (CH2), 24.8 (CH2). HRMS (ESI): calculated for C18H23BrNO4 [MH+] 396.0805, found 396.0805.
1-(Butylamino)-1,3-dioxo-3-phenylpropan-2-yl 3-bromopropanoate (7b). Yellow oil. Yield: 84%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.10–8.04 (m, 2H), 7.57–7.52 (m, 1H), 7.46–7.38 (m, 2H), 6.66 (t, J = 5.5 Hz, 1H, NH), 6.33 (s, 1H), 3.56–3.50 (m, 2H), 3.27–3.09 (m, 2H), 3.08–3.02 (m, 2H), 1.45–1.36 (m, 2H), 1.29–1.17 (m, 2H), 0.82 (t, J = 7.3 Hz, 3H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 191.3 (Cq), 168.6 (Cq), 163.2 (Cq), 134.2 (Cq), 134.1 (CHAr), 129.5 (CHAr), 128.5 (CHAr), 76.3 (CH), 39.2 (CH2), 37.1 (CH2), 31.1 (CH2), 25.2 (CH2), 19.8 (CH2), 13.5 (CH3). HRMS (ESI): calculated for C16H21BrNO4 [MH+] 370.0648, found 370.0651.
1-(tert-Butylamino)-1,3-dioxo-3-phenylpropan-2-yl 3-bromopropanoate (7c). Yellow oil. Yield: 87%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.10–8.04 (m, 2H), 7.57–7.52 (m, 1H), 7.46–7.40 (m, 2H), 6.25 (sb, 1H, NH), 6.23 (s, 1H), 3.55 (t, J = 6.5 Hz, 1H), 3.54 (t, J = 6.5 Hz, 1H), 3.06 (t, J = 6.5 Hz, 2H), 1.29 (s, 9H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 191.7 (Cq), 168.5 (Cq), 162.4 (Cq), 134.4 (Cq), 134.1 (CHAr), 129.6 (CHAr), 128.6 (CHAr), 76.4 (CH), 52.0 (Cq), 37.2 (CH2), 28.4 (CH3), 25.2 (CH2). HRMS (ESI): calculated for C16H21BrNO4 [MH+] 370.0648, found 370.0650.
1-(Cyclohexylamino)-3-(4-fluorophenyl)-1,3-dioxopropan-2-yl 3-bromopropanoate (7d). Yellow oil. Yield: 86%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.22–8.15 (m, 2H), 7.20–7.12 (m, 2H), 6.35–6.28 (m, 1H, NH), 6.31 (s, 1H), 3.78–3.66 (m, 1H), 3.62 (t, J = 6.7 Hz, 1H), 3.61 (t, J = 6.7 Hz, 1H), 3.14 (t, J = 6.7 Hz, 2H), 1.97–1.08 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 189.6 (Cq), 168.5 (Cq), 166.2 (Cq, d, 1J = 257 Hz), 162.2 (Cq), 132.5 (CHAr, d, 3J = 9.6 Hz), 130.6 (Cq, d, 4J = 2.8 Hz), 115.7 (CHAr, d, 2J = 22 Hz), 76.2 (CH), 48.7 (CH), 37.1 (CH2), 32.5 (CH2), 32.4 (CH2), 25.2 (CH2), 24.6 (CH2), 24.6 (CH2). 19F NMR (300 MHz, CDCl3) δ: −102.8 (tt, J = 8.3, 5.4 Hz). HRMS (ESI): calculated for C18H22BrFNO4 [MH+] 414.0711, found 414.0714.
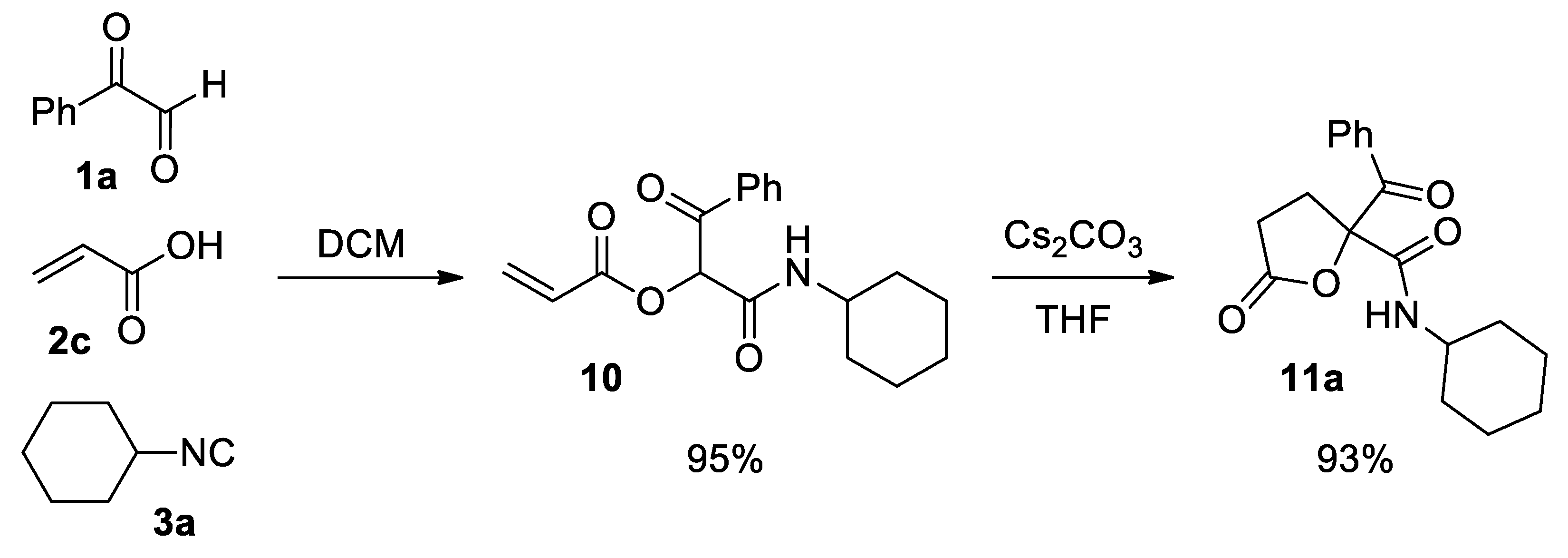
1-(Cyclohexylamino)-1,3-dioxo-3-phenylpropan-2-yl acrylate (10). Pink oil. Yield: 95% (by both method A and B). 1H NMR (300 MHz, CDCl3) δ (ppm): 8.18–8.13 (m, 2H), 7.64–7.57 (m, 1H), 7.52–7.46 (m, 2H), 6.53 (dd, J = 17.3, 1.3 Hz, 1H), 6.37 (s, 1H), 6.30 (dd, J = 17.3, 10.4 Hz, 1H), 6.23 (sb, 1H, NH), 5.99 (dd, J = 10.4, 1.3 Hz, 1H), 3.80–3.68 (m, 1H), 1.98–1.07 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 191.8 (Cq), 164.0 (Cq), 162.7 (Cq), 134.5 (Cq), 134.2 (CHAr), 133.4 (CH2), 129.8 (CHAr), 128.7 (CHAr), 126.8 (CH), 76.4 (CH), 48.8 (CH), 32.8 (CH2), 32.7 (CH2), 25.4 (CH2), 24.8 (CH2), 24.8 (CH2). HRMS (ESI): calculated for C18H22NO4 [MH+] 316.1543, found 316.1549.
3.2.2. Ugi Adducts/Lactames
Phenylglyoxal hydrate 1a (1 mmol) was dissolved in methanol (10 mL), after which benzylamine 4 (1 mmol), the corresponding acid 2a-b (1 mmol) and cyclohexyl isocyanide 3a (1 mmol) were added. The reaction mixture was stirred at room temperature for 24 h. The solvent was removed under reduced pressure and the residue dissolved in dichloromethane (20 mL). This solution was washed with a sodium hydroxide aqueous solution (2 × 50 mL) and with a hydrochloric acid aqueous solution (2 × 50 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to dryness. In the case of 6, the product was purified by column chromatography, employing silica gel as a stationary phase and a 4:1 hexane-ethyl acetate mixture as an eluent. In the case of 9, addition of ethyl acetate drove to the formation of a precipitate, which was isolated by vacuum filtration and dried in vacuo. Slow evaporation of a solution of 6 in diisopropyl ether and of a solution of 9 in acetone gave colorless single crystals, suitable for X-ray diffraction analysis.
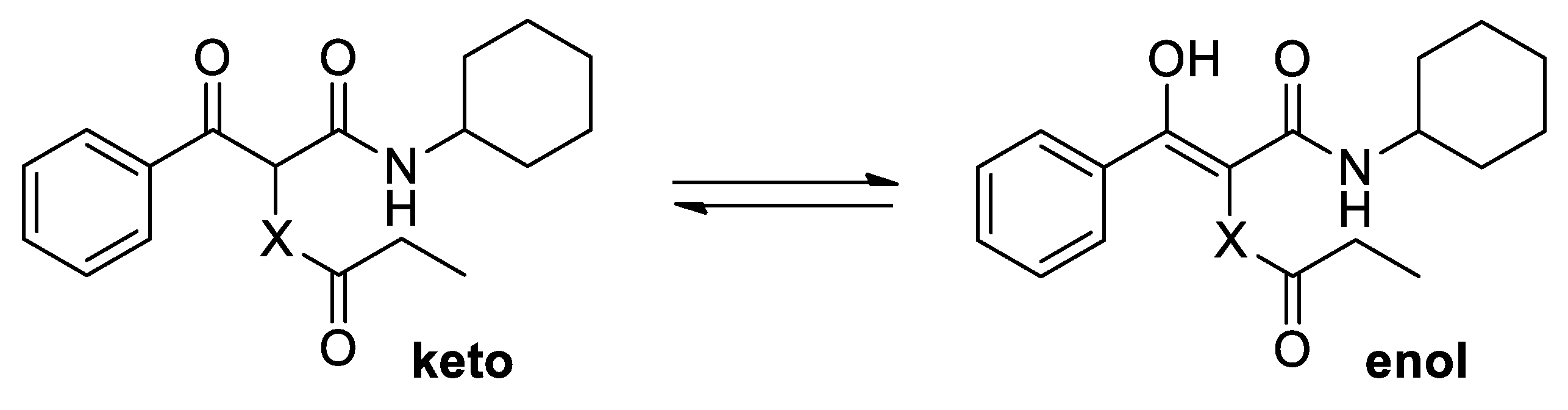
(E)-2-(N-Benzylpropionamido)-N-cyclohexyl-3-hydroxy-3-phenylacrylamide (6). Brown solid. M. p. 90–92 °C. Yield: 82%. 1H NMR (300 MHz, CDCl3) δ (ppm): 15.26 (s, 1H, OH), 7.63–7.57 (m, 2H), 7.47–7.42 (m, 3H), 7.36–7.28 (m, 5H), 5.45 (d, J = 13.7 Hz, 1H), 5.02 (d, J = 8.1 Hz, 1H, NH), 3.52–3.40 (m, 1H), 3.31 (d, J = 13.7 Hz, 1H), 2.49 (dq, J = 16.7, 7.5 Hz, 1H), 2.27 (dq, J = 16.7, 7.5 Hz, 1H), 1.72–1.07 (m, 7H), 1.14 (t, J = 7.4 Hz, 3H), 1.07–0.71 (m, 2H), 0.36–0.23 (m, 1H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 176.6 (Cq), 170.3 (Cq), 167.9 (Cq), 137.9 (Cq), 133.6 (Cq), 130.8 (CHAr), 129.7 (CHAr), 129.2 (CHAr), 128.9 (CHAr), 128.2 (CHAr), 127.3 (CHAr), 108.1 (Cq), 52.9 (CH2), 48.1 (CH), 32.8 (CH2), 31.8 (CH2), 26.9 (CH2), 25.3 (CH2), 24.7 (CH2), 24.6 (CH2), 9.5 (CH3). HRMS (ESI): calculated for C25H31N2O3 [MH+] 407.2329, found 407.2333.
2-Benzoyl-1-benzyl-N-cyclohexyl-5-oxopyrrolidine-2-carboxamide (9). White solid. Yield: 73%. M. p. 184–186 °C. 1H NMR (300 MHz, CDCl3) δ (ppm): 7.83–7.78 (m, 2H), 7.60–7.53 (m, 1H), 7.46–7.38 (m, 2H), 7.24–7.11 (m, 5H), 5.83 (d, J = 7.8 Hz, 1H, NH), 5.04 (d, J = 16.2 Hz, 1H), 4.24 (d, J = 16.2 Hz, 1H), 3.42–3.28 (m, 1H), 2.92–2.81 (m, 1H), 2.79–2.67 (m, 1H), 2.56–2.40 (m, 2H), 1.57–0.62 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 196.9 (Cq), 176.5 (Cq), 167.2 (Cq), 136.9 (Cq), 133.9 (Cq), 133.8 (CHAr), 129.3 (CHAr), 128.7 (CHAr), 128.5 (CHAr), 127.8 (CHAr), 127.3 (CHAr), 76.7 (Cq), 49.2 (CH), 46.7 (CH2), 32.1 (CH2), 31.8 (CH2), 28.9 (CH2), 25.4 (CH2), 24.6 (CH2), 24.6 (CH2). HRMS (ESI): calculated for C25H29N2O3 [MH+] 405.2173, found 405.2176.
3.2.3. Lactones
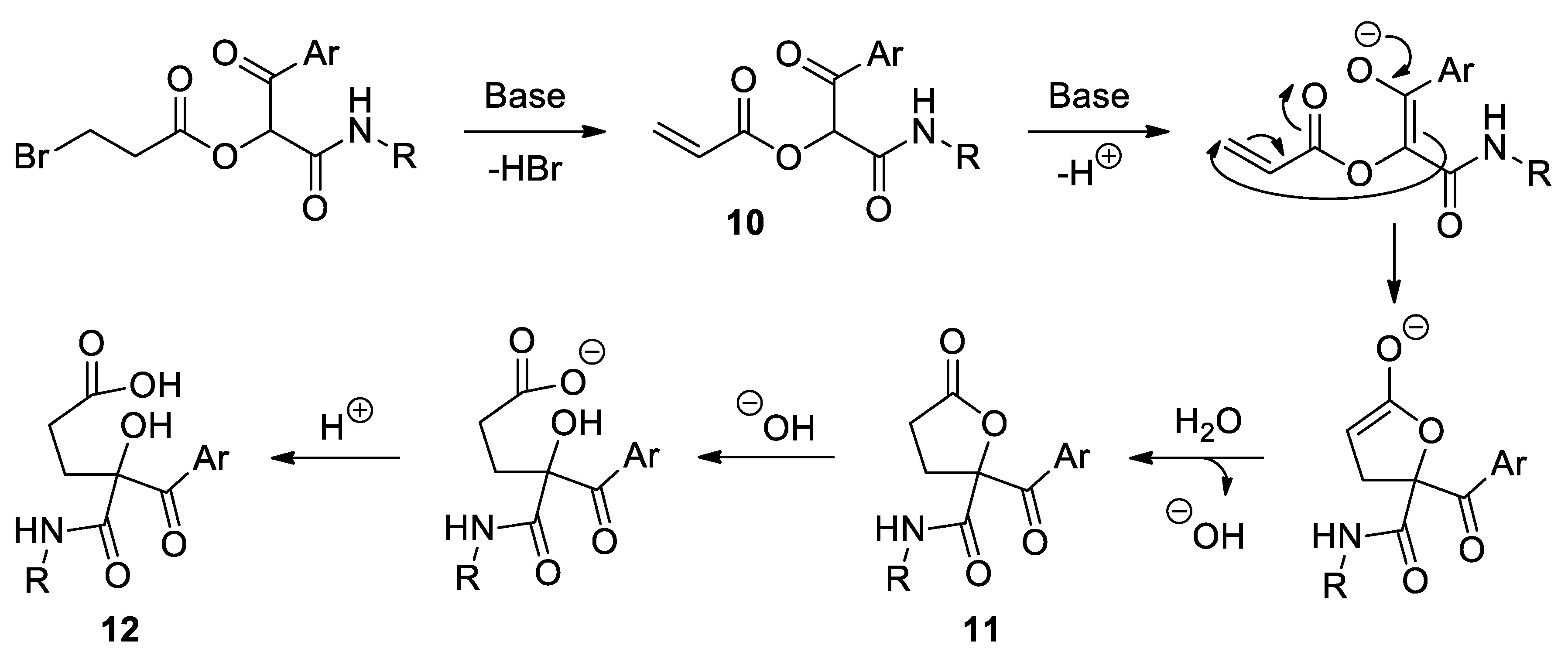
Method A: The corresponding Passerini adduct 7 (1 mmol) was dissolved in slightly wet (3 Å-MS) tetrahydrofuran (10 mL) and cesium carbonate (1.2 equiv.) was added. The reaction mixture was heated to reflux with stirring until TLC revealed the total consumption of the starting material (1–1.5 h). Subsequently, the unreacted salt was filtered off and the solvent of the filtrate removed under reduced pressure. The crude was dissolved in dichloromethane (20 mL) and the solution washed with water (2 × 20 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to dryness. The residue was purified by column chromatography, employing silica gel as a stationary phase and a 4:1 hexane-ethyl acetate mixture as an eluent. Hexane was added to the resulting oil and, in some cases, a precipitate was observed. This solid was isolated by vacuum filtration and dried in vacuo. Slow evaporation of a solution of compound 11b in a 1:1 chloroform-hexane mixture provided colorless single crystals, suitable for X-ray diffraction analysis.
Method B: compound 11a was also prepared starting from Passerini adduct 10. Reaction conditions, as well as the work-up and purification steps, are similar to those described in the previous method. In this case, TLC revealed the total consumption of the starting material after 10 min.
2-Benzoyl-N-cyclohexyl-5-oxotetrahydrofuran-2-carboxamide (11a). White solid. Yield: 71% by method A and 93% by method B. M. p. 105–106 °C. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.03–7.99 (m, 2H), 7.59–7.55 (m, 1H), 7.46–7.40 (m, 2H), 6.49 (d, J = 8.2 Hz, 1H, NH), 3.80–3.71 (m, 1H), 3.34–3.27 (m, 1H), 2.64 (dd, J = 7.0, 1.1 Hz, 1H), 2.62 (d, J = 7.0 Hz, 1H), 2.49–2.41 (m, 1H), 1.90–1.08 (m, 10H). 13C NMR {DEPT-135} (100 MHz, CDCl3) δ (ppm): 191.2 (Cq), 174.9 (Cq), 167.1 (Cq), 134.1 (CHAr), 133.5 (Cq), 129.7 (CHAr), 128.7 (CHAr), 89.1 (Cq), 48.7 (CH), 32.8 (CH2), 32.7 (CH2), 29.1 (CH2), 27.9 (CH2), 25.4 (CH2), 24.9 (CH2), 24.8 (CH2). HRMS (ESI): calculated for C18H22NO4 [MH+] 316.1543, found 316.1547.
2-Benzoyl-N-butyl-5-oxotetrahydrofuran-2-carboxamide (11b). White solid. Yield: 68%. M. p. 89–90 °C. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.09–7.99 (m, 2H), 7.61–7.55 (m, 1H), 7.48–7.40 (m, 2H), 6.65–6.50 (m, 1H, NH), 3.42–3.18 (m, 3H), 2.67–2.61 (m, 2H), 2.56–2.46 (m, 1H), 1.53–1.43 (m, 2H), 1.36–1.23 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 191.3 (Cq), 175.0 (Cq), 167.9 (Cq), 134.0 (CHAr), 133.5 (Cq), 129.7 (CHAr), 128.6 (CHAr), 89.3 (Cq), 39.4 (CH2), 31.2 (CH2), 29.1 (CH2), 27.8 (CH2), 20.0 (CH2), 13.7 (CH3). HRMS (ESI): calculated for C16H20NO4 [MH+] 290.1387, found 290.1391.
2-Benzoyl-N-(tert-butyl)-5-oxotetrahydrofuran-2-carboxamide (11c). Pale yellow oil. Yield: 70%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.03–7.99 (m, 2H), 7.60–7.54 (m, 1H), 7.47–7.41 (m, 2H), 6.33 (s, 1H, NH), 3.32–3.23 (m, 1H), 2.65–2.60 (m, 2H), 2.50–2.39 (m, 1H), 1.32 (s, 9H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 191.4 (Cq), 174.9 (Cq), 167.1 (Cq), 134.0 (CHAr), 133.5 (Cq), 129.7 (CHAr), 128.6 (CHAr), 89.1 (Cq), 52.2 (Cq), 29.0 (CH2), 28.5 (CH3), 27.9 (CH2). HRMS (ESI): calculated for C16H20NO4 [MH+] 290.1387, found 290.1393.
N-Cyclohexyl-2-(4-fluorobenzoyl)-5-oxotetrahydrofuran-2-carboxamide (11d). Pale yellow oil. Yield: 73%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.10–8.03 (m, 2H), 7.15–7.07 (m, 2H), 6.45 (d, J = 8.2 Hz, 1H, NH), 3.82–3.69 (m, 1H), 3.36–3.27 (m, 1H), 2.66–2.61 (m, 2H), 2.48–2.38 (m, 1H), 1.93–1.07 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 189.6 (Cq), 174.7 (Cq), 167.0 (Cq), 166.3 (Cq, d, 1J = 257 Hz), 132.6 (CHAr, d, 3J = 9.5 Hz), 129.9 (Cq, d, 4J = 3.0 Hz), 116.0 (CHAr, d, 2J = 22 Hz), 89.0 (Cq), 48.8 (CH), 32.8 (CH2), 32.8 (CH2), 29.1 (CH2), 27.8 (CH2), 25.4 (CH2), 24.9 (CH2), 24.8 (CH2). 19F NMR (300 MHz, CDCl3) δ: −102.9 (tt, J = 8.2, 5.3 Hz). HRMS (ESI): calculated for C18H21FNO4 [MH+] 334.1449, found 334.1458.
3.2.4. Hydrolysis Products
Carboxylic Acids
The corresponding Passerini adduct 7 (0.5 mmol) was dissolved in a tetrahydrofuran–water mixture (6 mL; 5 mL of the former and 1 mL of the latter) and cesium carbonate (1.2 equiv.) was added. The reaction mixture was heated to reflux with stirring until TLC revealed the total consumption of the starting material and the formed lactone (around 4 h). Subsequently, the unreacted salt was filtered off and the organic solvent of the filtrate removed under reduced pressure. Water (10 mL) was added to the crude and this solution washed with dichloromethane (2 × 20 mL). The organic phase was discarded and a hydrochloric acid aqueous solution (10 mL) was added. The aqueous phase was extracted with dichloromethane (2 × 20 mL) and the organic phase dried over anhydrous sodium sulfate, filtered and concentrated to dryness. The residue was purified by column chromatography, employing silica gel as a stationary phase and an increasingly polar eluent (from a 4:1 hexane-ethyl acetate mixture to pure ethyl acetate). Slow evaporation of a solution of compound 12c in acetonitrile provided colorless single crystals, suitable for X-ray diffraction analysis.
4-Benzoyl-5-(cyclohexylamino)-4-hydroxy-5-oxopentanoic acid (12a). White solid. Yield: 80%. M. p. 137–138 °C. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.26–8.21 (m, 2H), 7.60–7.53 (m, 1H), 7.47–7.40 (m, 2H), 6.71 (d, J = 8.1 Hz, 1H, NH), 5.30 (sb, 1H, OH), 3.77–3.65 (m, 1H), 2.63–2.36 (m, 4H), 1.93–1.06 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 199.6 (Cq), 178.8 (Cq), 168.7 (Cq), 134.0 (Cq), 133.9 (CHAr), 130.7 (CHAr), 128.5 (CHAr), 83.3 (Cq), 49.2 (CH), 33.4 (CH2), 32.8 (CH2), 32.8 (CH2), 28.7 (CH2), 25.5 (CH2), 24.8 (CH2). HRMS (ESI): calculated for C18H24NO5 [MH+] 334.1649, found 334.1654.
4-Benzoyl-5-(butylamino)-4-hydroxy-5-oxopentanoic acid (12b). White solid. Yield: 99%. M. p. 135–136 °C. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 12.15 (sb, 1H, OH), 8.36 (t, J = 5.8 Hz, 1H, NH), 8.05–8.00 (m, 2H), 7.61–7.54 (m, 1H), 7.48–7.40 (m, 2H), 6.75 (sb, 1H, OH), 3.18–2.99 (m, 2H), 2.40–2.02 (m, 4H), 1.45–1.33 (m, 2H), 1.29–1.17 (m, 2H), 0.83 (t, J = 7.3 Hz, 3H). 13C NMR {DEPT-135} (75 MHz, DMSO-d6) δ (ppm): 196.0 (Cq), 174.4 (Cq), 170.4 (Cq), 134.8 (Cq), 132.9 (CHAr), 129.3 (CHAr), 128.2 (CHAr), 81.3 (Cq), 38.2 (CH2), 31.3 (CH2), 31.0 (CH2), 28.2 (CH2), 19.6 (CH2), 13.7 (CH3). HRMS (ESI): calculated for C16H22NO5 [MH+] 308.1492, found 308.1480.
4-Benzoyl-5-(tert-butylamino)-4-hydroxy-5-oxopentanoic acid (12c). White solid. Yield: 99%. M. p. 125–127 °C. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.21–8.15 (m, 2H), 7.60–7.53 (m, 1H), 7.46–7.41 (m, 2H), 6.60 (s, 1H, NH), 5.24 (sb, 1H, OH), 2.55–2.35 (m, 4H), 1.32 (s, 9H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 200.3 (Cq), 168.7 (Cq), 134.4 (Cq), 133.8 (CHAr), 130.5 (CHAr), 128.5 (CHAr), 83.4 (Cq), 52.0 (Cq), 33.4 (CH2), 28.5 (CH3). HRMS (ESI): calculated for C16H22NO5 [MH+] 308.1492, found 308.1494.
5-(Cyclohexylamino)-4-(4-fluorobenzoyl)-4-hydroxy-5-oxopentanoic acid (12d). White solid. Yield: 98%. M. p. 136–137 °C. 1H NMR (300 MHz, CD3CN) δ (ppm): 8.16–8.11 (m, 2H), 7.20–7.14 (m, 3H), 3.62 (sb, 1H), 2.40–2.25 (m, 4H), 1.85–1.07 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CD3CN) δ (ppm): 196.5 (Cq), 175.5 (Cq), 170.3 (Cq), 166.5 (Cq, d, 1J = 253 Hz), 133.5 (CHAr, d, 3J = 9.4 Hz), 132.4 (Cq, d, 4J = 3.0 Hz), 116.2 (CHAr, d, 2J = 21 Hz), 83.2 (Cq), 49.6 (CH), 33.2 (CH2), 33.0 (CH2), 32.3 (CH2), 28.8 (CH2), 26.2 (CH2), 25.7 (CH2), 25.7 (CH2). 19F NMR (300 MHz, CD3CN) δ: −107.1 (tt, J = 8.9, 5.4 Hz). HRMS (ESI): calculated for C18H23FNO5 [MH+] 352.1555, found 352.1559.
Methyl Esters
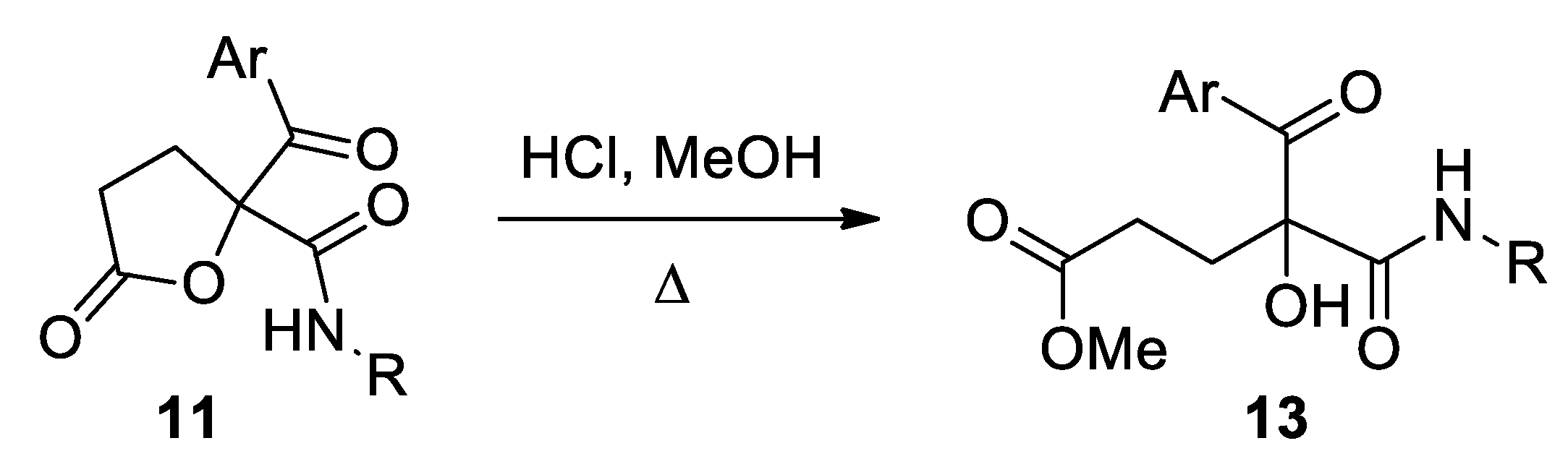
The corresponding lactone 11 (1 mmol) was dissolved in methanol (10 mL) and a solution of hydrochloric acid in methanol (0.5 M; 4 equiv.) was added. The reaction mixture was heated to reflux with stirring until TLC revealed the total consumption of the starting material (2–3 h). Subsequently, the solvent was removed under reduced pressure and the crude was dissolved in dichloromethane (20 mL). This solution was washed with a sodium carbonate aqueous solution (2 × 20 mL) and the organic phase dried over anhydrous sodium sulfate, filtered and concentrated to dryness. The residue was purified by column chromatography, employing silica gel as a stationary phase and a hexane-ethyl acetate mixture as an eluent. In the case of compound 13c, addition of hexane led to the formation of a precipitate, which was isolated by vacuum filtration and dried in vacuo.
Methyl 4-benzoyl-5-(cyclohexylamino)-4-hydroxy-5-oxopentanoate (13a). Yellow oil. Yield: 91%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.24–8.19 (m, 2H), 7.57–7.51 (m, 1H), 7.45–7.38 (m, 2H), 6.75 (d, J = 8.0 Hz, 1H, NH), 5.34 (s, 1H, OH), 3.78–3.62 (m, 1H), 3.63 (s, 3H), 2.60–2.30 (m, 4H), 1.96–1.04 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 199.5 (Cq), 173.9 (Cq), 168.8 (Cq), 134.1 (Cq), 133.7 (CHAr), 130.6 (CHAr), 128.4 (CHAr), 83.3 (Cq), 51.9 (CH3), 49.1 (CH), 33.6 (CH2), 32.8 (CH2), 32.7 (CH2), 28.7 (CH2), 25.4 (CH2), 24.8 (CH2). HRMS (ESI): calculated for C19H26NO5 [MH+] 348.1805, found 348.1816.
Methyl 4-benzoyl-5-(butylamino)-4-hydroxy-5-oxopentanoate (13b). Pale yellow oil. Yield: 93%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.26–8.20 (m, 2H), 7.58–7.52 (m, 1H), 7.46–7.38 (m, 2H), 6.88 (t, J = 5.5 Hz, 1H, NH), 5.34 (s, 1H, OH), 3.63 (s, 3H), 3.28–3.20 (m, 2H), 2.65–2.29 (m, 4H), 1.50–1.40 (m, 2H), 1.33–1.21 (m, 2H), 0.86 (t, J = 7.3 Hz, 3H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 199.5 (Cq), 173.8 (Cq), 169.7 (Cq), 134.1 (Cq), 133.8 (CHAr), 130.7 (CHAr), 128.4 (CHAr), 83.5 (Cq), 51.9 (CH3), 39.9 (CH2), 33.7 (CH2), 31.4 (CH2), 28.7 (CH2), 20.0 (CH2), 13.7 (CH3). HRMS (ESI): calculated for C17H24NO5 [MH+] 322.1649, found 322.1652.
Methyl 4-benzoyl-5-(tert-butylamino)-4-hydroxy-5-oxopentanoate (13c). White solid. Yield: 90%. M. p. 135–136 °C. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.18–8.11 (m, 2H), 7.57–7.48 (m, 1H), 7.45–7.35 (m, 2H), 6.63 (s, 1H, NH), 5.29 (s, 1H, OH), 3.61 (s, 3H), 2.52–2.30 (m, 4H), 1.29 (s, 9H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 200.1 (Cq), 173.8 (Cq), 168.7 (Cq), 134.4 (Cq), 133.6 (CHAr), 130.4 (CHAr), 128.3 (CHAr), 83.3 (Cq), 51.8 (CH3), 33.5 (CH2), 28.6 (CH2), 28.5 (CH3). HRMS (ESI): calculated for C17H24NO5 [MH+] 322.1649, found 322.1654.
Methyl 5-(cyclohexylamino)-4-(4-fluorobenzoyl)-4-hydroxy-5-oxopentanoate (13d). Yellow oil. Yield: 62%. 1H NMR (300 MHz, CDCl3) δ (ppm): 8.33–8.25 (m, 2H), 7.10–7.01 (m, 2H), 6.80 (d, J = 8.1 Hz, 1H, NH), 5.38 (s, 1H, OH), 3.73–3.59 (m, 1H), 3.60 (s, 3H), 2.54–2.28 (m, 4H), 1.91–1.03 (m, 10H). 13C NMR {DEPT-135} (75 MHz, CDCl3) δ (ppm): 197.5 (Cq), 173.8 (Cq), 168.7 (Cq), 166.0 (Cq, d, 1J = 257 Hz), 133.7 (CHAr, d, 3J = 9.4 Hz), 130.3 (Cq, d, 4J = 3.0 Hz), 115.5 (CHAr, d, 2J = 22 Hz), 83.2 (Cq), 51.9 (CH3), 49.0 (CH), 33.5 (CH2), 32.7 (CH2), 32.7 (CH2), 28.6 (CH2), 25.4 (CH2), 24.7 (CH2). 19F NMR (300 MHz, CDCl3) δ: −103.5 (tt, J = 8.3, 5.6 Hz). HRMS (ESI): calculated for C19H25FNO5 [MH+] 366.1711, found 366.1714.