Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics
Abstract
1. Introduction
2. Results and Discussion
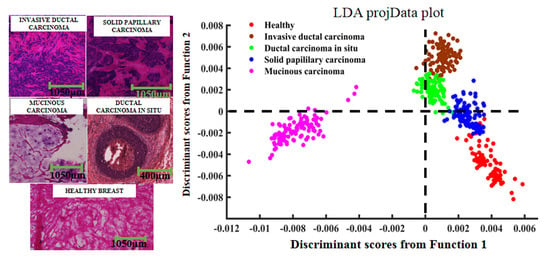
2.1. Pathological Analysis
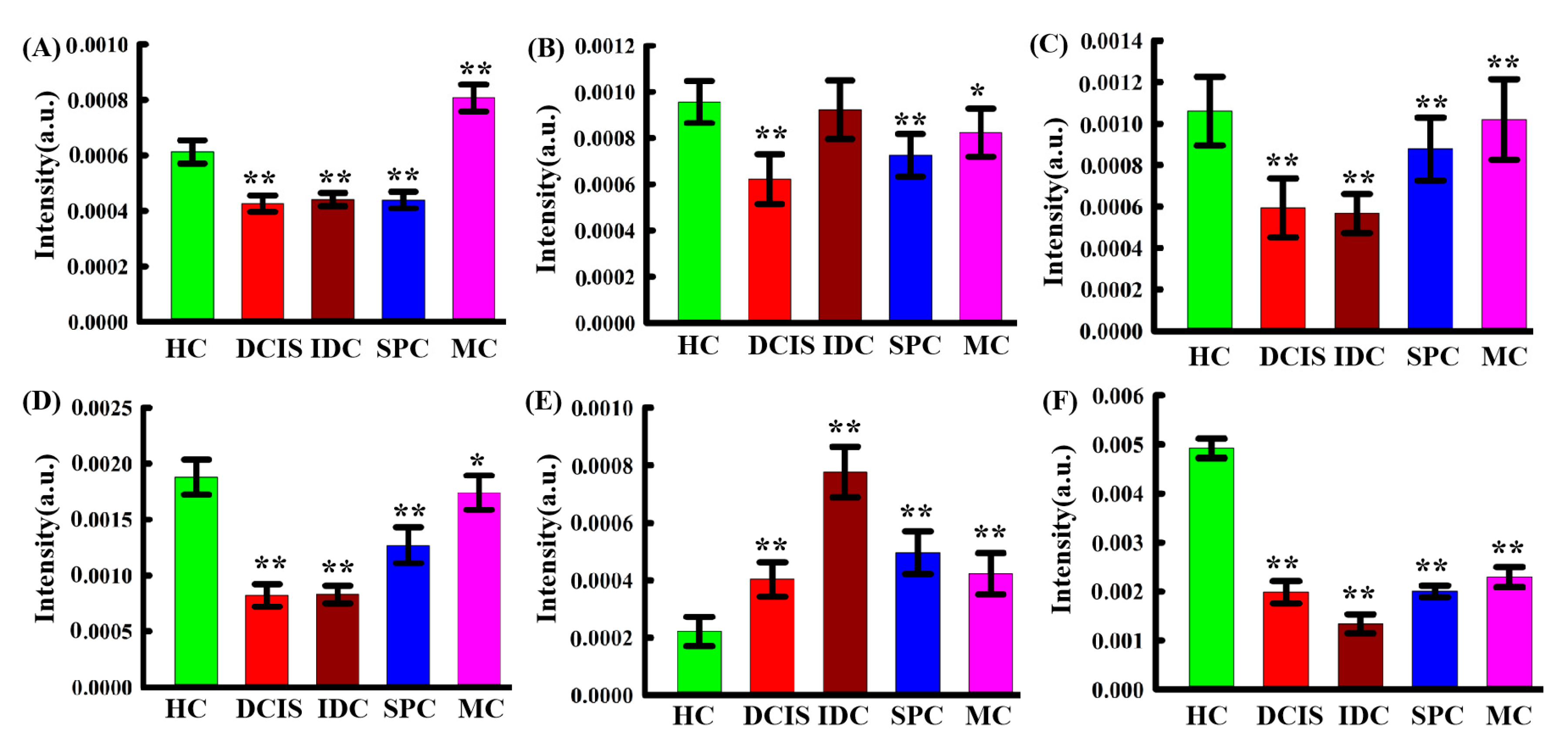
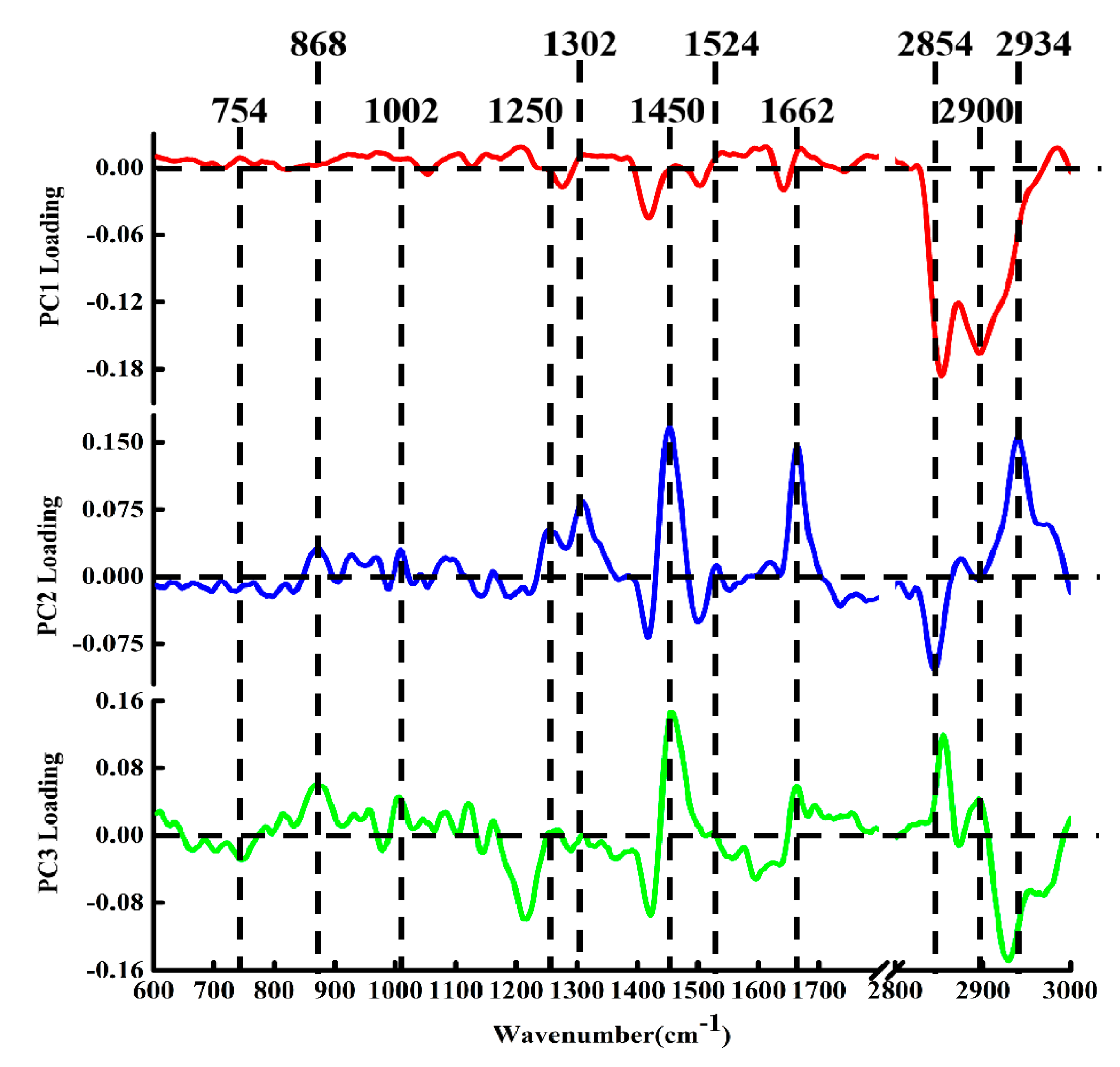
2.2. Raman Spectral Analysis
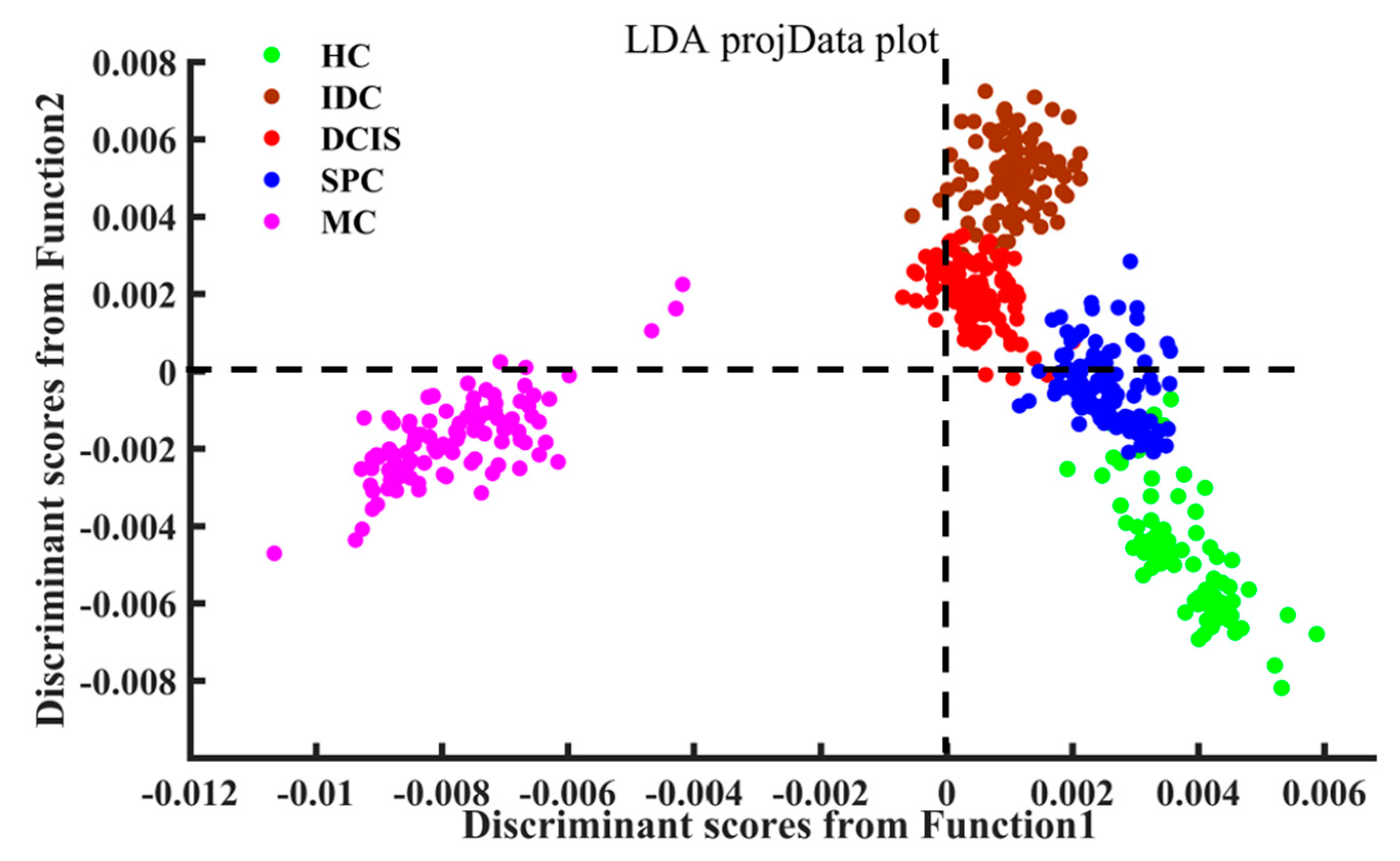
2.3. PCA–LDA Analysis
3. Experimental Section
3.1. Sample Preparation
3.2. Spectroscopic Acquisition
3.3. Data Pre-Processing and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowl, J.H.; Stein, K.D.; Alteri, R.; Jemal, D.V.M.A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Koya, K.S.K.; Salutatorian, M.; Yurgelevic, S.; Huang, C.; Werner, C.W.; Kast, R.E.; Shanley, J.; Sherman, M.; Honn, K.V.; Maddipati, K.R.; et al. Accurate identification of breast cancer margins in microenvironments of ex-vivo basal and luminal breast cancer tissues using Raman spectroscopy. Prostag. Other Lipid Mediat. 2020, 151, 106475. [Google Scholar] [CrossRef]
- Sabtu, S.N.; Sani, S.F.; Bradley, D.A.; Looi, L.M.; Osman, Z. A review of the applications of Raman spectroscopy for breast cancer tissue diagnostic and their histopathological classification of epithelial to mesenchymal transition. J. Raman Spectrosc. 2019, 51, 1–10. [Google Scholar]
- Zhao, J.; Zeng, H.; Kalia, S.; Lui, H. Wavenumber selection based analysis in Raman spectroscopy improves skin cancer diagnostic specificity. Analyst 2016, 141, 1034–1043. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Benbrahim-Tallaa, L.; Bouvard, V.; Bianchini, F.; Straif, K. Breast-Cancer Screening—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2015, 373, 2353–2358. [Google Scholar] [CrossRef]
- Evans, R.A. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer 2003, 97, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Jeevan, R.; Cromwell, D.A.; Trivella, M. Reoperation rates after breast conserving surgery for breast cancer among women in England: Retrospective study of hospital episode statistics. BMJ 2012, 345, e4505. [Google Scholar] [CrossRef] [PubMed]
- Mccahill, L.E.; Single, R.M.; Aiello Bowles, E.J.; Feigelson, H.S.; James, T.A.; Barney, T.; Engel, J.M.; Onitilo, A.A. Variability in Reexcision Following Breast Conservation Surgery. JAMA J. Am. Med. Assoc. 2012, 307, 467–475. [Google Scholar] [CrossRef]
- Keating, J.J.; Fisher, C.; Batiste, R.; Singhal, S. Advances in Intraoperative Margin Assessment for Breast Cancer. Curr. Surg. Rep. 2016, 4, 15. [Google Scholar] [CrossRef]
- Jorns, J.M.; Daignault, S.; Sabel, M.S.; Wu, A.J. Is Intraoperative Frozen Section Analysis of Reexcision Specimens of Value in Preventing Reoperation in Breast-Conserving Therapy? Am.J. Clin. Pathol. 2014, 142, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Warram, J.M.; Bland, K.I.; Zinn, K.R. The status of contemporary image-guided modalities in oncologic surgery. Ann. Surg. 2015, 261, 46–55. [Google Scholar] [CrossRef]
- Valdes, E.K.; Boolbol, S.K.; Ali, I.; Feldman, S.M.; Cohen, J.M. Intraoperative Touch Preparation Cytology for Margin Assessment in Breast-Conservation Surgery: Does It Work for Lobular Carcinoma? Ann. Surg. Oncol. 2007, 14, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- David, I.E.; David, P.C.; Lorna, A.; Steve, O.; Royston, G. Illuminating disease and enlightening biomedicine: Raman spectroscopy as a diagnostic tool. Analyst 2013, 138, 3871–3884. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman spectroscopy in cancer diagnosis. Cancer Metast. Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef]
- Song, D.; Yu, F.; Chen, S.; Chen, Y.; He, Q.; Zhang, Z.; Zhang, J.; Wang, S. Raman spectroscopy combined with multivariate analysis to study the biochemical mechanism of lung cancer microwave ablation. Biomed. Opt. Express 2020, 11, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Chen, T.; Wang, S.; Chen, S.; Li, H.; Yu, F.; Zhang, J.; Zhang, Z. Study on the biochemical mechanisms of the micro-wave ablation treatment of lung cancer by ex vivo confocal Raman microspectral imaging. Analyst 2020, 145, 626–635. [Google Scholar] [CrossRef]
- Santos, I.P.; Barroso, E.M.; Schut, T.C.B.; Caspers, P.J.; Van Lanschot, C.G.F.; Choi, D.; Der Kamp, M.F.V.; Smits, R.W.H.; Van Doorn, R.; Verdijk, R.M. Raman spectroscopy for cancer detection and cancer surgery guidance: Translation to the clinics. Analyst 2017, 142, 3025–3047. [Google Scholar] [CrossRef]
- Lieber, C.A.; Majumder, S.K.; Billheimer, D.D.; Ellis, L.M.D.D.; Mahadevanjansen, A. Raman microspectroscopy for skin cancer detection in vitro. J. Biomed. Opt. 2008, 13, 024013. [Google Scholar] [CrossRef] [PubMed]
- Vanna, R.; Morasso, C.; Piccotti, F.; Torti, E.; Altamura, D.; Albasini, S.; Agozzino, M.; Villani, L.; Sorrentino, L.; Bunk, O. Raman Spectroscopy Reveals That Biochemical Composition of Breast Microcalcifications Correlates with Histopathologic Features. Cancer Res. 2020, 80, 1762–1772. [Google Scholar] [CrossRef]
- Shipp, D.W.; Rakha, E.A.; Koloydenko, A.A.; Douglas, M.R.; Ellis, I.O.; Ioan, N. Intra-operative spectroscopic assessment of surgical margins during breast conserving surgery. Breast Cancer Res. 2018, 20, 69. [Google Scholar] [CrossRef]
- Garla, V.; Taylor, C.; Brandt, C. Semi-supervised clinical text classification with Laplacian SVMs: An application to cancer case management. J. Biomed. Inform. 2013, 46, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Kendall, C.; Shepherd, N.A.; Stone, N.J.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef]
- Huang, N.; Short, M.; Zhao, J.; Wang, H.; Lui, H.; Korbelik, M.; Zeng, H. Full range characterization of the Raman spectra of organs in a murine model. Opt. Express 2011, 19, 22892–22909. [Google Scholar] [CrossRef]
- Malini, R.; Venkatakrishna, K.; Kurien, J.; Pai, K.M.; Rao, L.; Kartha, V.B.; Krishna, C.M. Discrimination of normal, inflammatory, premalignant, and malignant oral tissue: A Raman spectroscopy study. Biopolymers 2010, 81, 179–193. [Google Scholar] [CrossRef]
- Han, B.; Du, Y.; Fu, T.; Fan, Z.; Xu, S.; Hu, C.; Bi, L.; Gao, T.; Zhang, H.; Xu, W. Differences and Relationships Between Normal and Atypical Ductal Hyperplasia, Ductal Carcinoma In Situ, and Invasive Ductal Carcinoma Tissues in the Breast Based on Raman Spectroscopy. Appl. Spectrosc. 2017, 71, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, R.J.; Kartha, V.B.; Krishna, C.M.; Solomon, J.G.R.; Ullas, G.; Devi, P.U. Tissue Raman spectroscopy for the study of radiation damage: Brain irradiation of mice. Radiat. Res. 2002, 157, 175–182. [Google Scholar] [CrossRef]
- Stone, N.J.; Kendall, C.; Smith, J.; Crow, P.; Barr, H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004, 126, 141–157. [Google Scholar] [CrossRef]
- Koljenovic, S.; Schut, T.C.B.; Vincent, A.J.P.E.; Kros, J.M.; Puppels, G.J. Detection of Meningioma in Dura Mater by Raman Spectroscopy. Anal. Chem. 2005, 77, 7958–7965. [Google Scholar] [CrossRef]
- Huang, Z.; Mcwilliams, A.; Lui, H.; Mclean, D.I.; Lam, S.; Zeng, H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davis, C.R.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef]
- Kamemoto, L.E.; Misra, A.K.; Sharma, S.K.; Goodman, M.T.; Acosta, T. Near-Infrared Micro-Raman Spectroscopy for in Vitro Detection of Cervical Cancer. Appl. Spectrosc. 2010, 64, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.; Fontana, L.C.; Raniero, L.; Ferreirastrixino, J. In vivo Raman spectroscopy of breast tumors prephotodynamic and postphotodynamic therapy. J. Raman Spectrosc. 2018, 49, 786–791. [Google Scholar] [CrossRef]
- Brozekpluska, B.; Placek, I.; Kurczewski, K.; Morawiec, Z.; Tazbir, M.; Abramczyk, H. Breast cancer diagnostics by Raman spectroscopy. J. Mol. Liq. 2008, 141, 145–148. [Google Scholar] [CrossRef]
- Bonnier, F.; Byrne, H.J. Understanding the molecular information contained in principal component analysis of vibrational spectra of biological systems. Analyst 2012, 137, 322–332. [Google Scholar] [CrossRef]
- Ruiz-Chica, A.J.; Medina, M.A.; Sánchez-Jiménez, F.; Ramírez, F.J. Characterization by Raman spectroscopy of conformational changes on guanine–cytosine and adenine–thymine oligonucleotides induced by aminooxy analogues of spermidine. J. Raman Spectrosc. 2010, 35, 93–100. [Google Scholar] [CrossRef]
- Wang, S.; Liang, Z.; Gong, Y.; Yin, Y.; Wang, K.; He, Q.; Wang, Z.; Bai, J. Confocal raman microspectral imaging of ex vivo human spinal cord tissue. J. Photochem. Photobiol. B Biol. 2016, 163, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Lui, H.; He, Q.; Zeng, H. A modular Raman microspectroscopy system for biological tissue analysis. Spectroscopy 2010, 24, 577–583. [Google Scholar] [CrossRef]
- Shim, M.G.; Wilson, B.C. The effects of ex vivo handling procedures on the near-infrared Raman spectra of normal mammalian tissues. Photochem. Photobiol. 1996, 63, 662–671. [Google Scholar]
- Li, J.; Qin, J.; Zeng, H.; Li, J.; Wang, S. Unveiling doseand time γ: Ependent osteosarcoma cell responses to the γ: Ecretase inhibitor, DAPT, by confocal Raman microscopy. J. Biophoton. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Qin, J.; Zeng, H.; Wang, S. Confocal Raman microspectroscopic analysis on the time-dependent impact of DAPT, a γ-secretase inhibitor, to osteosarcoma cells. Spectrochim. Acta A 2020, 239, 118372. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, R.; Lin, J.; Pan, J.; Chen, G.; Li, Y.; Cheng, M.; Huang, Z.; Chen, J.; Zeng, H. Nasopharyngeal cancer detection based on blood plasma surface-enhanced Raman spectroscopy and multivariate analysis. Biosens. Bioelectron. 2010, 25, 2414–2419. [Google Scholar] [CrossRef] [PubMed]



| Types of Cancer | Number of Samples | Number of Patients |
|---|---|---|
| HC | 12 | 4 |
| SPC | 3 | 3 |
| MC | 3 | 3 |
| IDC | 6 | 6 |
| DCIS | 6 | 6 |
| Actual/Predict | HC | IDC | DCIS | SPC | MC |
|---|---|---|---|---|---|
| HC | 78 | 0 | 0 | 2 | 0 |
| IDC | 0 | 77 | 3 | 0 | 0 |
| DCIS | 0 | 0 | 80 | 0 | 0 |
| SPC | 0 | 0 | 0 | 80 | 0 |
| MC | 0 | 0 | 0 | 0 | 80 |
| Actual/Predict | HC | IDC | DCIS | SPC | MC |
|---|---|---|---|---|---|
| HC | 20 | 0 | 0 | 0 | 0 |
| IDC | 0 | 20 | 0 | 0 | 0 |
| DCIS | 0 | 0 | 20 | 0 | 0 |
| SPC | 0 | 0 | 0 | 20 | 0 |
| MC | 0 | 0 | 0 | 0 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ning, T.; Yu, F.; Chen, Y.; Zhang, B.; Wang, S. Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics. Molecules 2021, 26, 921. https://doi.org/10.3390/molecules26040921
Li H, Ning T, Yu F, Chen Y, Zhang B, Wang S. Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics. Molecules. 2021; 26(4):921. https://doi.org/10.3390/molecules26040921
Chicago/Turabian StyleLi, Heping, Tian Ning, Fan Yu, Yishen Chen, Baoping Zhang, and Shuang Wang. 2021. "Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics" Molecules 26, no. 4: 921. https://doi.org/10.3390/molecules26040921
APA StyleLi, H., Ning, T., Yu, F., Chen, Y., Zhang, B., & Wang, S. (2021). Raman Microspectroscopic Investigation and Classification of Breast Cancer Pathological Characteristics. Molecules, 26(4), 921. https://doi.org/10.3390/molecules26040921