Assessing the Functional Properties of TiZr Nanotubular Structures for Biomedical Applications, through Nano-Scratch Tests and Adhesion Force Maps
Abstract
1. Introduction
2. Results and Discussion
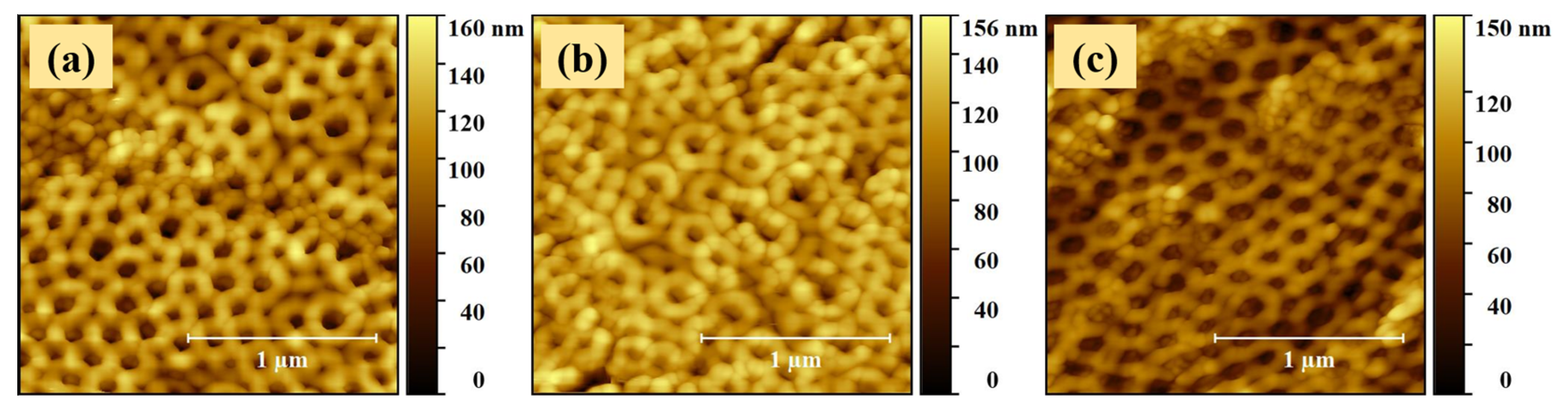
2.1. Morphological Properties
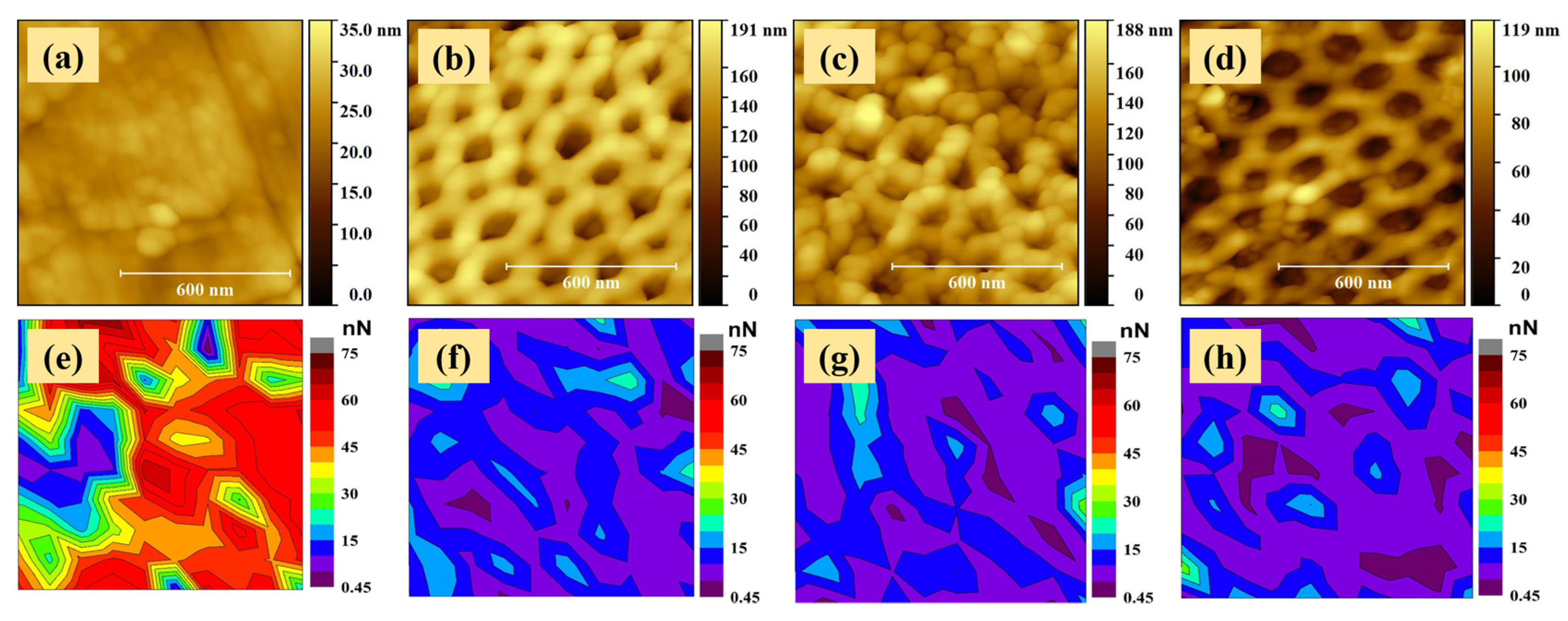
2.2. Adhesive Properties
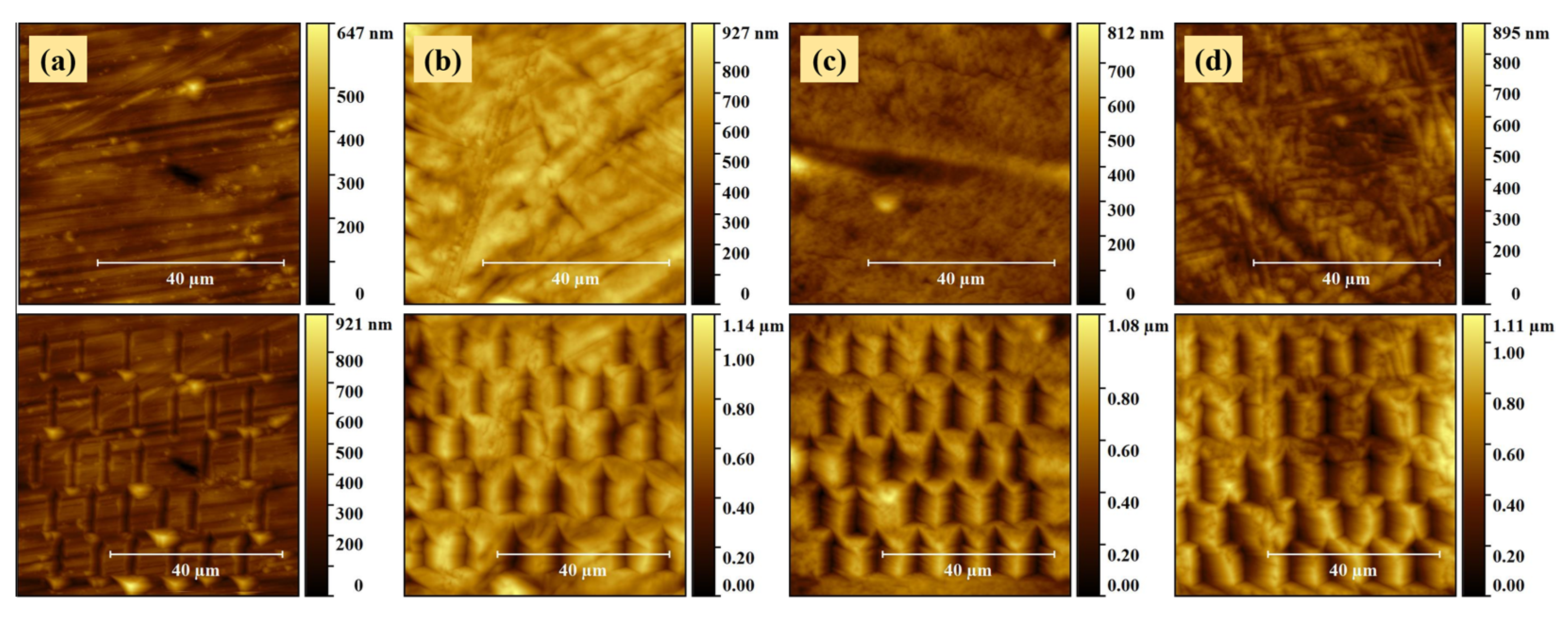
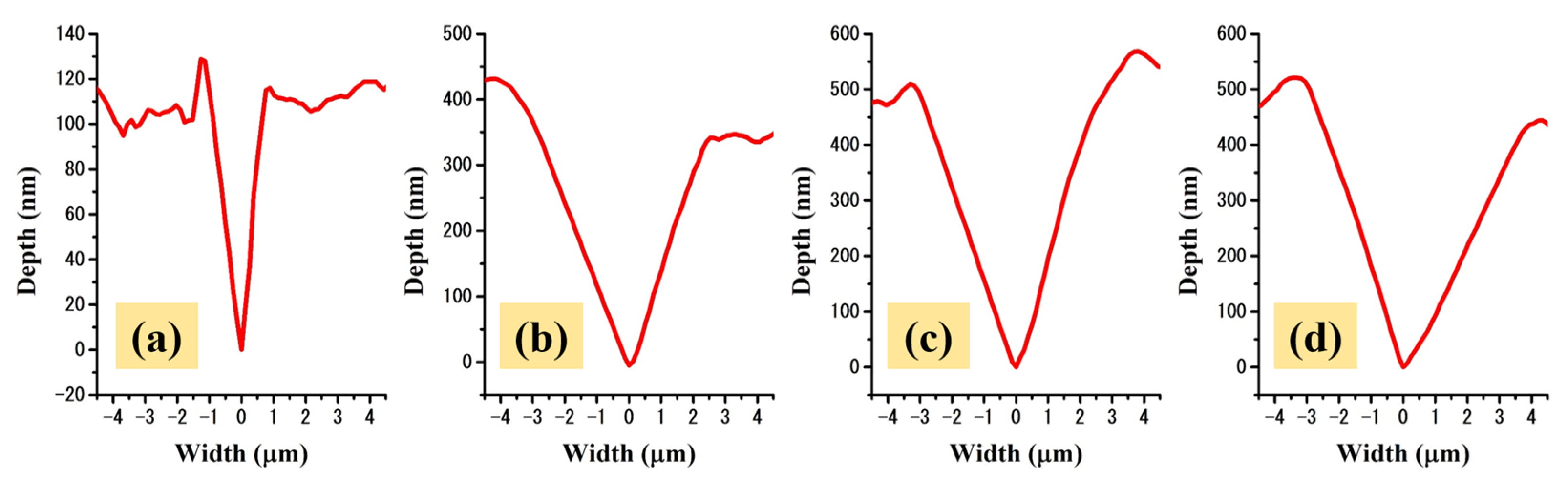
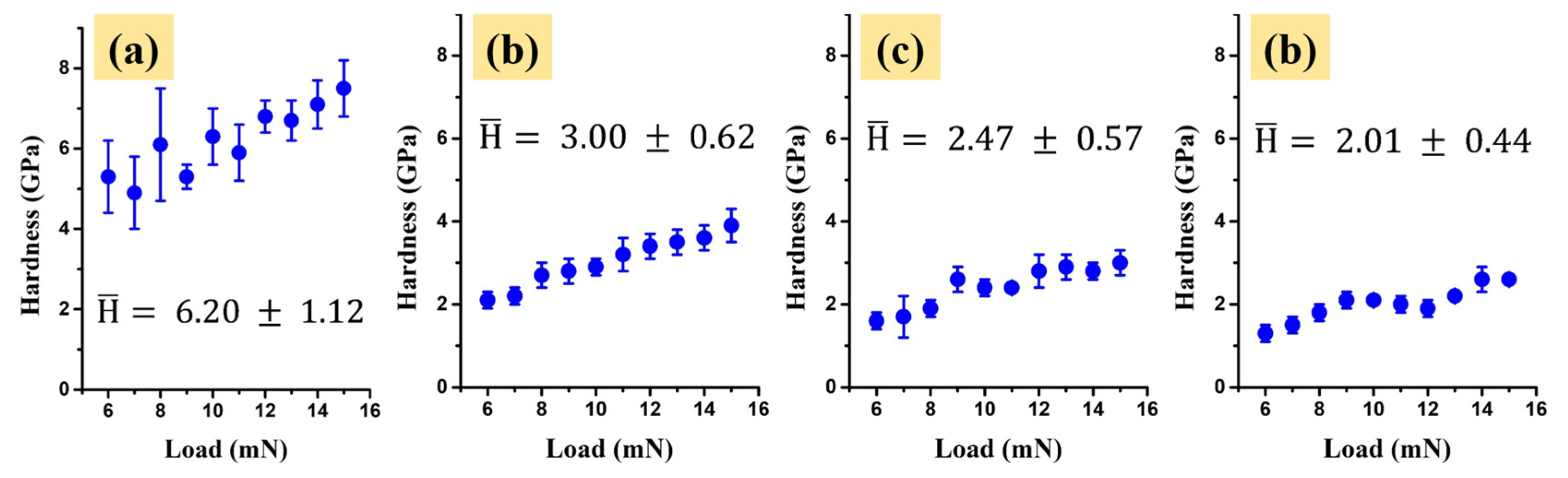
2.3. Mechanical Properties
3. Materials and Methods
3.1. Preparation of TiZr Nanostructures
3.2. Characterization Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alves, V.A.; Reis, R.Q.; Santos, I.C.B.; Souza, D.G.; de Goncalves, T.F.; Pereira-da-Silva, M.A.; Rossi, A.; da Silva, L.A. In situ impedance spectroscopy study of the electrochemical corrosion of Ti and Ti-6Al-4V in simulated body fluid at 25 degrees C and 37 degrees C. Corros. Sci. 2009, 51, 2473–2482. [Google Scholar] [CrossRef]
- Takao, H. Titanium–Tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Stoian, A.B.; Vardaki, M.; Ionita, D.; Enachescu, M.; Brancoveaun, O.; Demetrescu, I. Nanopores and nanotubes ceramic oxides elaborated on titanium alloy with zirconium by changing anodization potentials. Ceram. Int. 2018, 44, 7026–7033. [Google Scholar] [CrossRef]
- Popa, M.V.; Demetrescu, I.; Iordachescu, D.; Cimpean, A.; Vasilescu, E.; Drob, P. The relation between electrochemical tests and in vitro evaluation of titanium alloy biocompatibility. Mater. Corros. 2007, 58, 687–695. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Ionita, D.; Pirvu, C.; Stoian, A.B.; Demetrescu, I. The trends of TiZr alloy research as a viable alternative for Ti and Ti16Zr Roxolid dental implants. Coatings 2020, 10, 422. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Y. Fatigue behavior of Ti50Zr alloy for dental implant application. J. Alloys Compd. 2019, 793, 212–219. [Google Scholar] [CrossRef]
- Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar topography positively modulates osteoblast differentiation and inhibits osteoclastogenesis. Coatings 2018, 8, 294. [Google Scholar] [CrossRef]
- Trinca, L.C.; Mareci, D.; Souto, D.; Lozano-Gorrín, A.D.; Izquierdo, J.; Burtan, L.; Motrescu, I.; Vulpe, V.; Pavel, G.; Strungaru, S.; et al. Osseointegration evaluation of ZrTi alloys with hydroxyapatite-zirconia-silver layer in pig’s tibiae. Appl. Surf. Sci. 2019, 487, 127–137. [Google Scholar] [CrossRef]
- Mareci, D.; Bolat, G.; Chelariu, R.; Sutiman, D.; Munteanu, C. The estimation of corrosion behaviour of ZrTi binary alloys for dental applications using electrochemical techniques. Mater. Chem. Phys. 2013, 141, 362–369. [Google Scholar] [CrossRef]
- Akimoto, T.; Ueno, T.; Tsutsumi, Y.; Doi, H.; Hanawa, T.; Wakabayashi, N. Evaluation of corrosion resistance of implant-use Ti-Zr binary alloys with a range of compositions. J. Biomed. Mater. Res. B 2018, 106, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.C.; Popa, M.; Ivanescu, S.; Vasilescu, C.; Drob, S.I.; Neacsu, E.I.; Popa, M.V. Microstructure, mechanical properties, and corrosion resistance of Ti-20Zr alloy in undoped and NaF doped artificial saliva. Met. Mater. Int. 2014, 20, 177–187. [Google Scholar] [CrossRef]
- Chelariu, R.; Mareci, D.; Munteanu, C. Preliminary electrochemical testing of some Zr–Ti alloys in 0.9% NaCl solution. Mater. Corros. 2012, 64, 585–591. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Shugurov, V.V.; Koval, N.N.; Shulepov, I.A.; Surmenev, R.A. Physico-mechanical properties of Ti-Zr coatings fabricated via ion-assisted arc-plasma deposition. Vacuum 2018, 149, 129–133. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.E.; Yamada, Y.; Hodgson, P.D. Fabrication of novel TiZr alloy foams for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1439–1444. [Google Scholar] [CrossRef]
- Vardaki, M.; Mohajernia, S.; Pantazi, A.; Nica, I.C.; Enachescu, M.; Mazare, A.; Demetrescu, I.; Schmuki, P. Post treatments effect on TiZr nanostructures fabricated via anodizing. J. Mater. Res. Technol. 2019, 8, 5802–5812. [Google Scholar] [CrossRef]
- Pantazi, A.; Vardaki, M.; Mihai, G.; Ionita, D.; Stoian, A.B.; Enachescu, M.; Demetrescu, I. Understanding surface and interface properties of modified Ti50Zr with nanotubes. Appl. Surf. Sci. 2020, 506, 144661. [Google Scholar] [CrossRef]
- Zalnezhad, E.; Baradaran, S.; Bushroa, A.R.; Sarhan, A.A.D. Mechanical property enhancement of Ti-6Al-4V by multilayer thin solid film Ti/TiO2 nanotubular array coating for biomedical application. Metall. Mater. Trans. A 2014, 45, 785–797. [Google Scholar] [CrossRef]
- Arkusz, K.; Paradowska, E.; Nycz, M.; Krasicka-Cydzik, E. Influence of thermal modification and morphology of TiO2 nanotubes on their electrochemical properties for biosensors applications. J. Nanosci. Nanotechnol. 2018, 18, 3713–3721. [Google Scholar] [CrossRef]
- Fontes, A.C.A.; Sopchenski, L.; Laurindo, C.A.; Torres, R.D.; Popat, K.C.; Soares, P. Annealing temperature effect on tribocorrosion and biocompatibility properties of TiO2 nanotubes. J. Bio Tribo Corros. 2020, 64. [Google Scholar] [CrossRef]
- Nycz, M.; Paradowska, E.; Arkusz, K.; Pijanowska, D.G. Influence of geometry and annealing temperature in argon atmosphere of TiO2 nanotubes on their electrochemical properties. Acta Bioeng. Biomech. 2020, 22, 165–177. [Google Scholar] [CrossRef]
- Mazare, A.; Ionita, D.; Totea, G.; Demetrescu, I. Calcination condition effect on microstructure, electrochemical and hemolytic behavior of amorphous nanotubes on Ti6Al7Nb alloy. Surf. Coat. Technol. 2014, 252, 87–92. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Wang, Z.; Han, A.; Shan, Z.; Yang, X.; Zhu, X. Essential distinction between one-step anodizing and two-step anodizing of Ti. Mater. Res. Bull. 2017, 95, 444–450. [Google Scholar] [CrossRef]
- Ying Chin, L.; Zainal, Z.; Khusaimi, Z.; Ismail, S.S. Electrochemical synthesis of ordered titania nanotubes in mixture of ethylene glycol and glycerol electrolyte. Malays. J. Anal. Sci. 2016, 20, 373–381. [Google Scholar] [CrossRef]
- Gerber, C.; Lang, H.P. How the doors to the nanoworld were opened. Nat. Nanotechnol. 2006, 1, 3–5. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Valdez-Salas, B.; Escamilla, A.; Curiel, M.; Valdez-Salas, E.; Nedev, N.; Bastidas, J.M. Disinfection of titanium dioxide nanotubes using super-oxidized water decrease bacterial viability without disrupting osteoblast behavior. Mater. Sci. Eng. C 2016, 60, 239–245. [Google Scholar] [CrossRef]
- Paterlini, T.T.; Nogueira, L.F.B.; Tovani, C.B.; Cruz, M.A.E.; Derradi, R.; Ramos, A.P. The role played by modified bioinspired surfaces in interfacial properties of biomaterials. Biophys. Rev. 2017, 9, 683–698. [Google Scholar] [CrossRef]
- Gaillard, Y.; Tromas, C.; Woirgard, J. Study of the dislocation structure involved in a nanoindentation test by atomic force microscopy and controlled chemical etching. Acta Mater. 2006, 51, 1059–1065. [Google Scholar] [CrossRef]

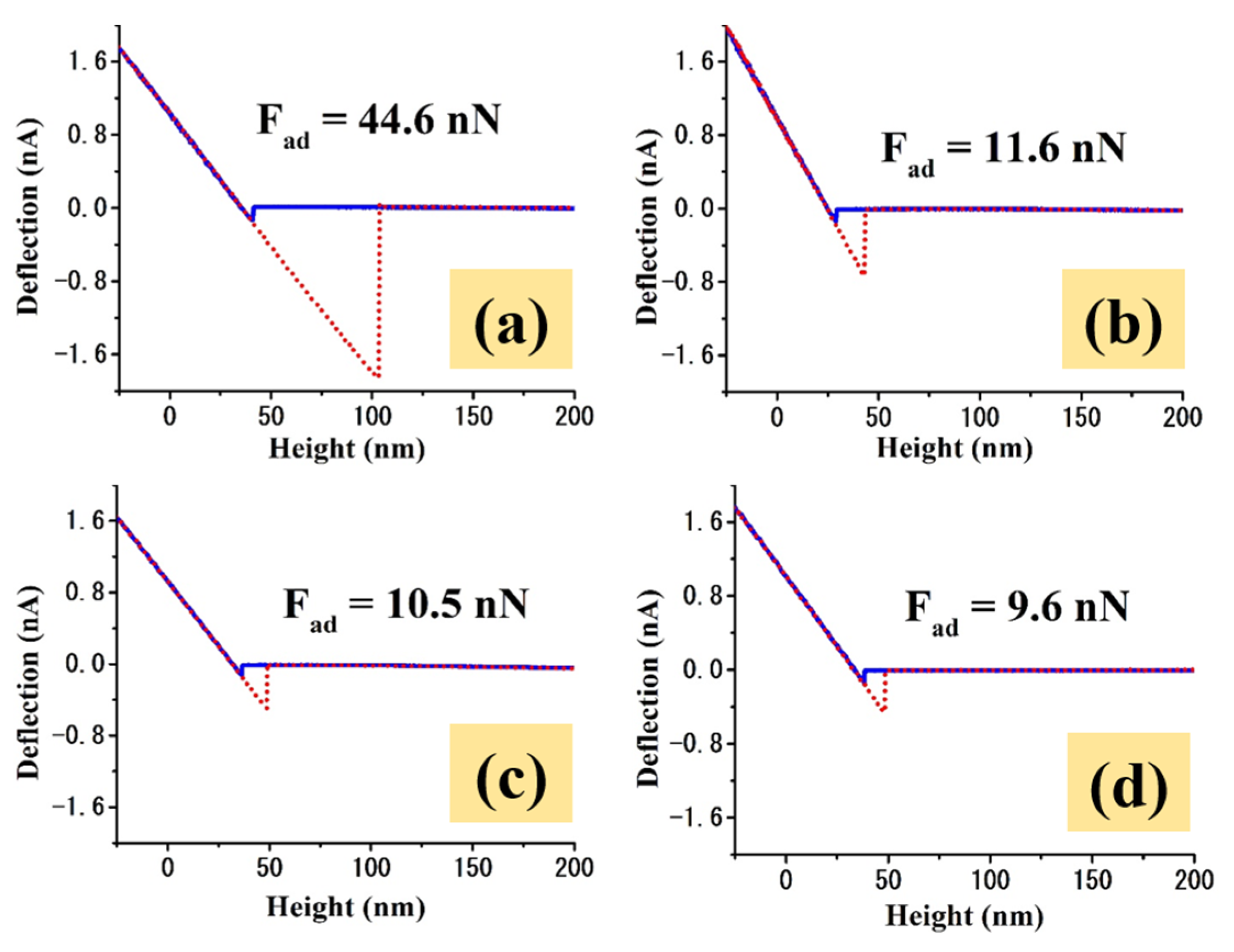
| Sample | Scan Size | Dominant Fad Range [nN] | [nN] | |||
|---|---|---|---|---|---|---|
| 2 × 2 µm2 | 1 × 1 µm2 | |||||
| RMS (nm) | Ra (nm) | RMS (nm) | Ra (nm) | |||
| TiZr un-coated | - | - | 8.7 ± 3 | 6.6 ± 2 | 40–60 | 51.7 ± 5.2 |
| S1 | 30.3 ± 1.4 | 24.1 ± 0.8 | 28.2 ± 0.7 | 22.1 ± 0.8 | 5–20 | 11.3 ± 3 |
| S2 | 27.6 ± 1.4 | 21.8 ± 1.6 | 20.8 ± 3.5 | 16.5 ± 3 | 5–15 | 9.9 ± 2.2 |
| S3 | 27.8 ± 2.3 | 20.52 ± 1.9 | 22.1 ± 3.7 | 16.6 ± 2.9 | 5–15 | 9.3 ± 2 |
| Sample Code | Elaboration Protocol | Post-Treatment |
|---|---|---|
| X | un-coated | – |
| S1 | 2-step anodizing | – |
| S2 | 2-step anodizing | air annealing |
| S3 | 2-step anodizing | air annealing and reduction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardaki, M.; Pantazi, A.; Demetrescu, I.; Enachescu, M. Assessing the Functional Properties of TiZr Nanotubular Structures for Biomedical Applications, through Nano-Scratch Tests and Adhesion Force Maps. Molecules 2021, 26, 900. https://doi.org/10.3390/molecules26040900
Vardaki M, Pantazi A, Demetrescu I, Enachescu M. Assessing the Functional Properties of TiZr Nanotubular Structures for Biomedical Applications, through Nano-Scratch Tests and Adhesion Force Maps. Molecules. 2021; 26(4):900. https://doi.org/10.3390/molecules26040900
Chicago/Turabian StyleVardaki, Maria, Aida Pantazi, Ioana Demetrescu, and Marius Enachescu. 2021. "Assessing the Functional Properties of TiZr Nanotubular Structures for Biomedical Applications, through Nano-Scratch Tests and Adhesion Force Maps" Molecules 26, no. 4: 900. https://doi.org/10.3390/molecules26040900
APA StyleVardaki, M., Pantazi, A., Demetrescu, I., & Enachescu, M. (2021). Assessing the Functional Properties of TiZr Nanotubular Structures for Biomedical Applications, through Nano-Scratch Tests and Adhesion Force Maps. Molecules, 26(4), 900. https://doi.org/10.3390/molecules26040900








