Selected Thoughts on Hydrophobicity in Drug Design
Abstract
1. Introduction
2. Hydrophobicity Matters
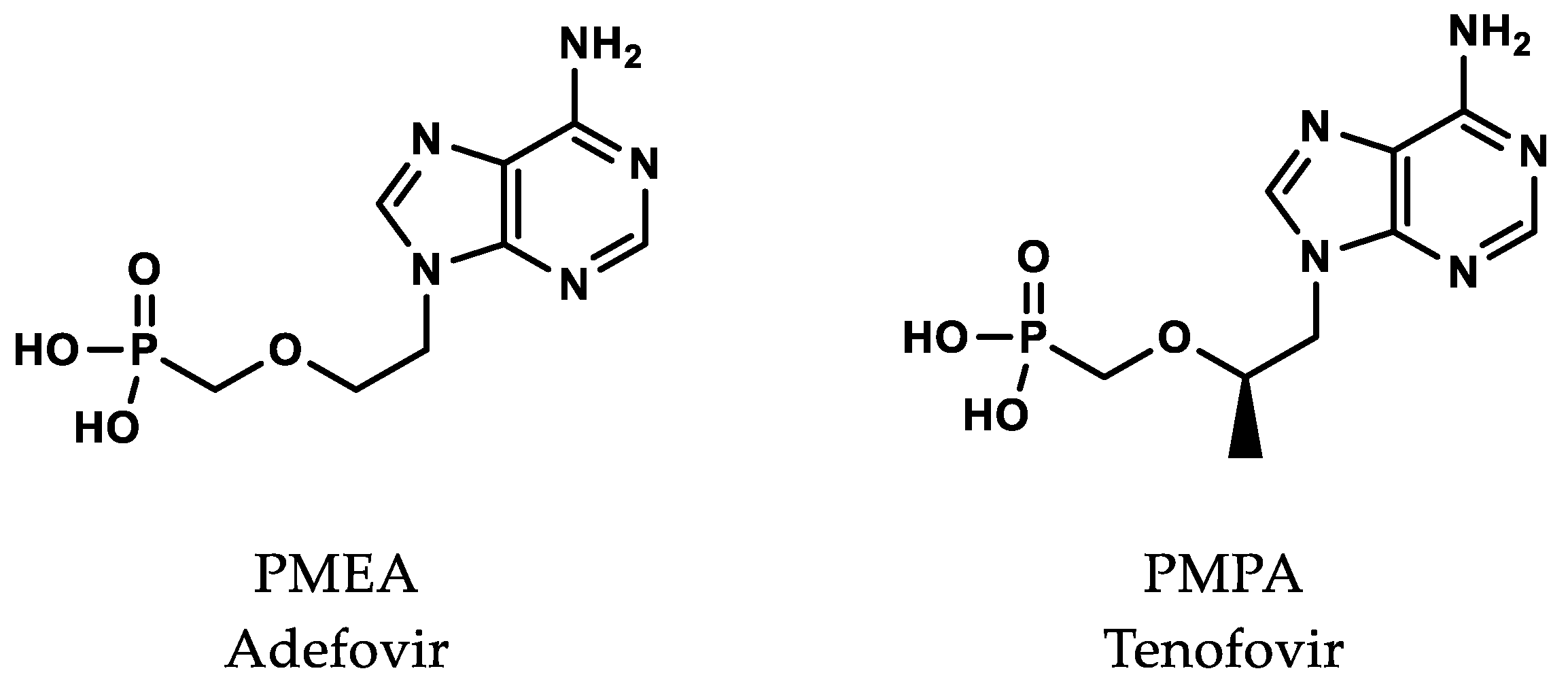
3. Antiviral Nucleotide Analogues
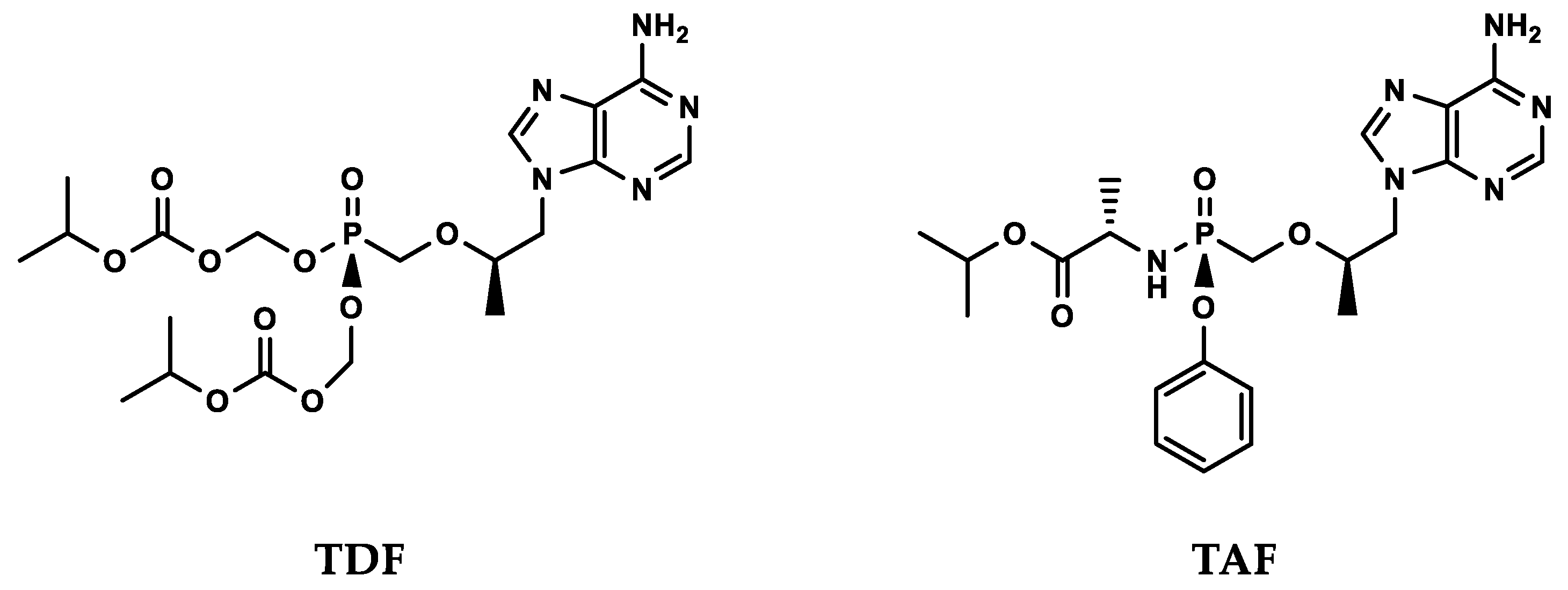
4. Prodrugs
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.C.; Dvorak, G.A.; Smee, D.F.; Matthews, T.R.; Verheyden, J.P. 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine: A new potent and selective antiherpes agent. J. Med. Chem. 1983, 26, 759–761. [Google Scholar] [CrossRef]
- Smee, D.F.; Martin, J.C.; Verheyden, J.P.; Matthews, T.R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob. Agents Chemother. 1983, 23, 676–682. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral activity of 5-substituted 2′-deoxyuridines. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 46. [Google Scholar]
- Martin, J.C.; Jeffrey, G.A.; McGee, D.P.C.; Tippie, M.A.; Smee, D.F.; Matthews, T.R.; Verheyden, J.P.H. Synthesis and antiviral activity of acyclic nucleosides related to DHPG. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 43. [Google Scholar]
- Robins, R.K.; Revankar, G.R.; Srivastava, P.C.; Kirsi, J.; North, J.A.; Murray, B.; McKernan, P.A. New nucleosides with broad spectrum antiviral activity. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 45. [Google Scholar]
- Fox, J.J. 2′-Flouro-arabinosyl pyrimidine nucleosides: A new source of anti-herpes virus agents. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 44. [Google Scholar]
- De Clercq, E.; Holý, A.; Rosenberg, I.; Sakuma, T.; Balzarini, J.; Maudgal, P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, 464–467. [Google Scholar] [CrossRef]
- Prisbe, E.J.; Martin, J.C.; McGee, D.P.; Barker, M.F.; Smee, D.F.; Duke, A.E.; Matthews, T.R.; Verheyden, J.P. Synthesis and antiherpes virus activity of phosphate and phosphonate derivatives of 9-[(1,3-dihydroxy-2-propoxy)methyl]guanine. J. Med. Chem. 1986, 29, 671–675. [Google Scholar] [CrossRef]
- Holý, A.; De Clercq, E.; Votruba, I. Phosphonylmethyl esters of nucleosides and their acyclic analogues. In Nucleotide Analogues as Antiviral Agents; Martin, J.C., Ed.; American Chemical Society: Washington, DC, USA, 1989; pp. 51–71. [Google Scholar]
- Martin, J.C.; Hitchcock, M.J.; De Clercq, E.; Prusoff, W.H. Early nucleoside reverse transcriptase inhibitors for the treatment of HIV: A brief history of stavudine (D4T) and its comparison with other dideoxynucleosides. Antivir. Res. 2010, 85, 34–38. [Google Scholar] [CrossRef]
- Barreiro, E.J.; Kummerle, A.E.; Fraga, C.A. The methylation effect in medicinal chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef]
- Leung, C.S.; Leung, S.S.; Tirado-Rives, J.; Jorgensen, W.L. Methyl effects on protein-ligand binding. J. Med. Chem. 2012, 55, 4489–4500. [Google Scholar] [CrossRef] [PubMed]
- Schonherr, H.; Cernak, T. Profound methyl effects in drug discovery and a call for new C-H methylation reactions. Angew. Chem. Int. Ed. Engl. 2013, 52, 12256–12267. [Google Scholar] [CrossRef]
- Wagner, R.W.; Matteucci, M.D.; Lewis, J.G.; Gutierrez, A.J.; Moulds, C.; Froehler, B.C. Antisense gene inhibition by oligonucleotides containing C-5 propyne pyrimidines. Science 1993, 260, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Froehler, B.C.W.S.; Terhorst, T.J.; Gerrard, S.R. Oligonucleotides containing C-5 propyne analogs of 2′-deoxyuridine and 2′-deoxycytidine. Tetrahedron Lett. 1992, 33, 5307–5310. [Google Scholar] [CrossRef]
- Kim, C.U.; Chen, X.; Mendel, D.B. Neuraminidase inhibitors as anti-influenza virus agents. Antivir. Chem. Chemother. 1999, 10, 141–154. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: Design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Jones, G.H.; Moffatt, J.G. The synthesis of 6′-deoxyhomonucleoside-6′-phosphonic acids. J. Am. Chem. Soc. 1968, 90, 5336–5338. [Google Scholar] [CrossRef] [PubMed]
- Starrett, J.E., Jr.; Tortolani, D.R.; Hitchcock, M.J.; Martin, J.C.; Mansuri, M.M. Synthesis and in vitro evaluation of a phosphonate prodrug: Bis(pivaloyloxymethyl) 9-(2-phosphonylmethoxyethyl)adenine. Antivir. Res. 1992, 19, 267–273. [Google Scholar] [CrossRef]
- Yu, K.L.; Bronson, J.J.; Yang, H.; Patick, A.; Alam, M.; Brankovan, V.; Datema, R.; Hitchcock, M.J.; Martin, J.C. Synthesis and antiviral activity of methyl derivatives of 9-[2-(phosphonomethoxy)ethyl]guanine. J. Med. Chem. 1992, 35, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Holy, A.; Jindrich, J.; Naesens, L.; Snoeck, R.; Schols, D.; De Clercq, E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: Potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993, 37, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Chilar, T.; Chen, M.S. Incorporation of selected nucleoside phosphonates and anti-human immunodeficiency virus nucleotide analogues into DNA by human DNA polymerases α, β and γ. Antivir. Chem. Chemother. 1997, 8, 187–195. [Google Scholar]
- Cherrington, J.M.; Allen, S.J.W.; Bischofberger, N.; Chen, M.S. Kinetic interaction of the diphosphates of 9-(2-phosphonylmethoxyethyl)adenine and other anti-HIV active purine congeners with HIV reverse transcriptase and human DNA polymerase α, β and γ. Antivir. Chem. Chemother. 1995, 6, 217–221. [Google Scholar] [CrossRef][Green Version]
- Heijtink, R.A.; Kruining, J.; de Wilde, G.A.; Balzarini, J.; De Clercq, E.; Schalm, S.W. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 1994, 38, 2180–2182. [Google Scholar] [CrossRef] [PubMed]
- Delaney, W.E., IV; Ray, A.S.; Yang, H.; Qi, X.; Xiong, S.; Zhu, Y.; Miller, M.D. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob. Agents Chemother. 2006, 50, 2471–2477. [Google Scholar] [CrossRef]
- Lee, W.A.; He, G.X.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K.C. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005, 49, 1898–1906. [Google Scholar] [CrossRef]
- Callebaut, C.; Stepan, G.; Tian, Y.; Miller, M.D. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob. Agents Chemother. 2015, 59, 5909–5916. [Google Scholar] [CrossRef]
- Murakami, E.; Wang, T.; Park, Y.; Hao, J.; Lepist, E.I.; Babusis, D.; Ray, A.S. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob. Agents Chemother. 2015, 59, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Ruane, P.J.; DeJesus, E.; Berger, D.; Markowitz, M.; Bredeek, U.F.; Callebaut, C.; Zhong, L.; Ramanathan, S.; Rhee, M.S.; Fordyce, M.W.; et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J. Acquir. Immune Defic. Syndr. 2013, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Fung, S.K.; Nguyen, T.T.; Cheng, W.; Sicard, E.; Ryder, S.D.; Flaherty, J.F.; Lawson, E.; Zhao, S.; Subramanian, G.M.; et al. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J. Hepatol. 2015, 62, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.L.; Lee, B.T.; Tien, A.; Chang, M.; Lim, C.; Ahn, A.; Bae, H.S. Improvement of bone mineral density and markers of proximal renal tubular function in chronic hepatitis B patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. J. Viral Hepat. 2019, 26, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Lou, L. Advances in Nucleotide Antiviral Development from Scientific Discovery to Clinical Applications: Tenofovir Disoproxil Fumarate for Hepatitis B. J. Clin. Transl. Hepatol. 2013, 1, 33–38. [Google Scholar] [PubMed]
| Disease | Drug (Common Name) | Launch |
|---|---|---|
| HIV/AIDS | Viread (TDF) | 2001 |
| Truvada® (TDF/emtricitabine) | 2004 | |
| Atripla® (TDF/emtricitabine/efavirenz) 1 | 2006 | |
| Complera® (TDF/emtricitabine/rilpivirine) 1 | 2011 | |
| Stribild® (TDF/emtricitabine/elvitegravir/cobicistat) 1 | 2012 | |
| Genvoya® (TAF/emtricitabine/elvitegravir/cobicistat) 1 | 2015 | |
| Odefsey® (TAF/emtricitabine/rilpivirine) 1 | 2016 | |
| Descovy® (TAF/emtricitabine) | 2016 | |
| Bitarvy® (TAF/emtricitabine/bictegravir) 1 | 2018 | |
| HIV PrEP 2 | Truvada (TDF/emtricitabine) | 2012 |
| Descovy (TAF/emtricitabine) | 2019 | |
| Hepatitis B | Hepsera (adefovir dipivoxil) | 2002 |
| Viread (TDF) | 2008 | |
| Vemlidy (TAF) | 2016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, L.L.; Martin, J.C. Selected Thoughts on Hydrophobicity in Drug Design. Molecules 2021, 26, 875. https://doi.org/10.3390/molecules26040875
Lou LL, Martin JC. Selected Thoughts on Hydrophobicity in Drug Design. Molecules. 2021; 26(4):875. https://doi.org/10.3390/molecules26040875
Chicago/Turabian StyleLou, Lillian L., and John C. Martin. 2021. "Selected Thoughts on Hydrophobicity in Drug Design" Molecules 26, no. 4: 875. https://doi.org/10.3390/molecules26040875
APA StyleLou, L. L., & Martin, J. C. (2021). Selected Thoughts on Hydrophobicity in Drug Design. Molecules, 26(4), 875. https://doi.org/10.3390/molecules26040875





