Abstract
The fundamental aim of drug design in research and development is to invent molecules with selective affinity towards desired disease-associated targets. At the atomic loci of binding surfaces, systematic structural variations can define affinities between drug candidates and biomolecules, and thereby guide the optimization of safety, efficacy and pharmacologic properties. Hydrophobic interaction between biomolecules and drugs is integral to binding affinity and specificity. Examples of antiviral drug discovery are discussed.
1. Introduction
We are pleased to contribute to a collection of manuscripts dedicated to Professor Erik De Clercq on the occasion of his 80th birthday. One of us (J.C.M.) has a four-decade collaboration with Professor De Clercq dating back to our early and independent work on potential antiviral drugs since I synthesized ganciclovir [1,2]. My career received a substantial boost as one of four plenary speakers at an American Chemical Society Symposium at the ACS National Meeting in 1983 in Seattle. Erik and I were the younger up-and-coming scientists, discussing BVDU (bromovinyldeoxyuridine) [3] and ganciclovir (Cytovene®) [4], respectively. The more established scientists were Roland Robins (ribavirin, Virazole®) [5] and Jack Fox (2′-fluoro-arabinosyl pyrimidine nucleosides) [6]. Erik and I were already friends; however, that symposium began a long series of frequent meetings at conferences around the world to explore our common scientific interests, especially the chemistry of antivirals. The decade of the 1980s was a great start to our collaboration, subsequent to our independent publication of the first examples of antiviral nucleotide analogues [7,8]. The formal collaboration was initiated in 1986 along with Professor Antonín (Tony) Holý in Prague to explore the potential of phosphonomethyl nucleotide analogues as antiviral drug candidates. Just two years into the collaboration, I presided over a full day the ACS Symposium (Los Angeles, CA, USA, 1988), covering the then newly discovered antiviral potential of nucleotide analogues. New results and concepts of this emerging field were captured in a book, Nucleotide Analogues as Antiviral Agents, edited by me, where 12 chapters were contributed by a number of pioneering labs including Erik and Tony (Chapter 4) [9]. Along the way we collaborated on other endeavors including the anti-HIV drug stavudine (Zerit®) [10], and shared many a stage as experts in the field, most recently as guests of Jean-Marie Lehn at the University of Strasbourg, 23 September 2019.
The following covers some brief observations of drug discovery concerning the power of lipophilic interactions in drug design and for nucleotide analogues.
2. Hydrophobicity Matters
The value of hydrophobic interactions to improve inhibitor affinity and selectivity in drug design has been well recognized and plays out over and over in a variety of research efforts. Even the addition of a methyl group can be profound [11,12,13]. A nucleobase modification discovered at Gilead Sciences was the higher affinity of 5-propynyl substituted pyrimidine antisense oligonucleotide analogues. It was previously known that a 5-methyl-C substitution led to higher affinity binding of oligonucleotides to RNA. The rational assumption by Brian Froehler that creating more hydrophobic stacking by occupying more available space with the linear propyne group proved accurate [14,15].
Around the same time at Gilead, a much more impactful observation was made by serendipity. Choung Kim’s practice of not making assumptions about possible biological activity routinely assayed his chemical intermediates. By this means, oseltamivir (Tamiflu®) for influenza virus infection was discovered when a hydrophobic pocket at the virus neuraminidase active site was uncovered then exploited, greatly improving affinity through hydrophobic interactions thus achieving potent inhibition of the influenza virus [16,17].
These lessons have been applied with various success but to no greater degree than subsequent research on nucleotides, discussed below.
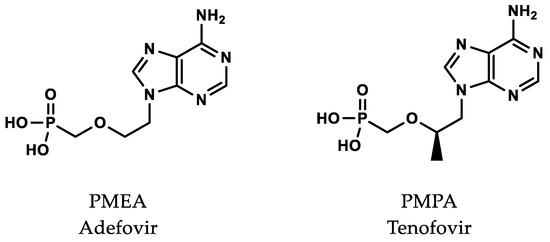
4. Prodrugs
To make the nucleotide molecules drug-like, prodrugs were employed to allow oral dosing and improved pharmacokinetics. After considerable additional effort, two prodrugs of PMPA (tenofovir) were successfully developed. Tenofovir disoproxil fumarate (TDF 300 mg, Viread®) was approved in 2001 for HIV/AIDS and in 2008 for chronic hepatitis B, and tenofovir alafenamide (TAF 40 mg, Vemlidy®) was approved in 2016 (Figure 2).
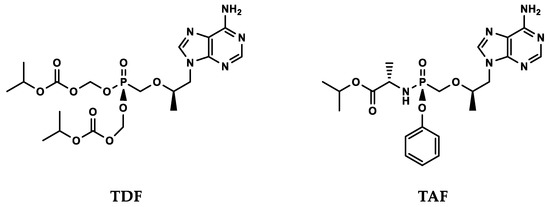
Figure 2.
Two prodrugs of tenofovir—once daily oral dosing. TDF: tenofovir disoproxil fumarate; TAF: tenofovir alafenamide.
Modification of tenofovir by prodrug moieties markedly increased uptake into cells, as TDF is 50-fold and TAF several hundred- to one thousand-fold more active in vitro than tenofovir [25,26,27]. As the first prodrug in development, TDF’s clinical potency is limited by the rapid hydrolysis to tenofovir in blood, resulting in suboptimal levels of TDF in circulation. The second prodrug TAF has much improved stability in circulation, efficiency in cellular uptake and selective proteolytic conversion to tenofovir inside specific target cells [26,28]. Consequently, TAF can achieve equivalent clinical antiviral efficacy as TDF when dose is lowered by 8- to 10-fold [29,30]. The resulting decrease in off-target effects renders an improved safety profile [31].
Both TDF and TAF were subsequently combined with other agents to produce single tablet regimens, which transformed the care of HIV patients. The various formations are shown in Table 1. The later developed formulations containing TAF have supplanted the earlier TDF combination products. This is because TAF has a superior safety profile which is important for patients with HIV and HBV [32] who are being individually treated for decades.

Table 1.
Tenofovir-containing therapeutics for HIV and HBV.
5. Concluding Remarks
The collaboration with Erik in Belgium and Tony in the Czech Republic has spanned 35 years and involved hundreds of scientists working together to achieve remarkable benefits for patients, allowing many to live normal productive lives instead of succumbing to fatal disease. Starting about 15 years ago, global efforts brought tenofovir-containing regimens to low-income countries, greatly expanding the benefits of tenofovir around the world. This collaboration, which has paralleled the long careers of many of our colleagues, demonstrates Erik’s contribution to have created many scientific progeny who carry on important research in virology to this day, including current efforts to control COVID-19, the disease caused by SARS-CoV-2.
Author Contributions
Both authors contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The manuscript writing received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable
Data Availability Statement
All data described are from published reports as referenced and are publicly accessible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martin, J.C.; Dvorak, G.A.; Smee, D.F.; Matthews, T.R.; Verheyden, J.P. 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine: A new potent and selective antiherpes agent. J. Med. Chem. 1983, 26, 759–761. [Google Scholar] [CrossRef]
- Smee, D.F.; Martin, J.C.; Verheyden, J.P.; Matthews, T.R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob. Agents Chemother. 1983, 23, 676–682. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral activity of 5-substituted 2′-deoxyuridines. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 46. [Google Scholar]
- Martin, J.C.; Jeffrey, G.A.; McGee, D.P.C.; Tippie, M.A.; Smee, D.F.; Matthews, T.R.; Verheyden, J.P.H. Synthesis and antiviral activity of acyclic nucleosides related to DHPG. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 43. [Google Scholar]
- Robins, R.K.; Revankar, G.R.; Srivastava, P.C.; Kirsi, J.; North, J.A.; Murray, B.; McKernan, P.A. New nucleosides with broad spectrum antiviral activity. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 45. [Google Scholar]
- Fox, J.J. 2′-Flouro-arabinosyl pyrimidine nucleosides: A new source of anti-herpes virus agents. In Proceedings of the 185th American Chemical Society National Meeting, Seattle, WA, USA, 20–25 March 1983. Abstract 44. [Google Scholar]
- De Clercq, E.; Holý, A.; Rosenberg, I.; Sakuma, T.; Balzarini, J.; Maudgal, P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, 464–467. [Google Scholar] [CrossRef]
- Prisbe, E.J.; Martin, J.C.; McGee, D.P.; Barker, M.F.; Smee, D.F.; Duke, A.E.; Matthews, T.R.; Verheyden, J.P. Synthesis and antiherpes virus activity of phosphate and phosphonate derivatives of 9-[(1,3-dihydroxy-2-propoxy)methyl]guanine. J. Med. Chem. 1986, 29, 671–675. [Google Scholar] [CrossRef]
- Holý, A.; De Clercq, E.; Votruba, I. Phosphonylmethyl esters of nucleosides and their acyclic analogues. In Nucleotide Analogues as Antiviral Agents; Martin, J.C., Ed.; American Chemical Society: Washington, DC, USA, 1989; pp. 51–71. [Google Scholar]
- Martin, J.C.; Hitchcock, M.J.; De Clercq, E.; Prusoff, W.H. Early nucleoside reverse transcriptase inhibitors for the treatment of HIV: A brief history of stavudine (D4T) and its comparison with other dideoxynucleosides. Antivir. Res. 2010, 85, 34–38. [Google Scholar] [CrossRef]
- Barreiro, E.J.; Kummerle, A.E.; Fraga, C.A. The methylation effect in medicinal chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef]
- Leung, C.S.; Leung, S.S.; Tirado-Rives, J.; Jorgensen, W.L. Methyl effects on protein-ligand binding. J. Med. Chem. 2012, 55, 4489–4500. [Google Scholar] [CrossRef] [PubMed]
- Schonherr, H.; Cernak, T. Profound methyl effects in drug discovery and a call for new C-H methylation reactions. Angew. Chem. Int. Ed. Engl. 2013, 52, 12256–12267. [Google Scholar] [CrossRef]
- Wagner, R.W.; Matteucci, M.D.; Lewis, J.G.; Gutierrez, A.J.; Moulds, C.; Froehler, B.C. Antisense gene inhibition by oligonucleotides containing C-5 propyne pyrimidines. Science 1993, 260, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Froehler, B.C.W.S.; Terhorst, T.J.; Gerrard, S.R. Oligonucleotides containing C-5 propyne analogs of 2′-deoxyuridine and 2′-deoxycytidine. Tetrahedron Lett. 1992, 33, 5307–5310. [Google Scholar] [CrossRef]
- Kim, C.U.; Chen, X.; Mendel, D.B. Neuraminidase inhibitors as anti-influenza virus agents. Antivir. Chem. Chemother. 1999, 10, 141–154. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: Design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Jones, G.H.; Moffatt, J.G. The synthesis of 6′-deoxyhomonucleoside-6′-phosphonic acids. J. Am. Chem. Soc. 1968, 90, 5336–5338. [Google Scholar] [CrossRef] [PubMed]
- Starrett, J.E., Jr.; Tortolani, D.R.; Hitchcock, M.J.; Martin, J.C.; Mansuri, M.M. Synthesis and in vitro evaluation of a phosphonate prodrug: Bis(pivaloyloxymethyl) 9-(2-phosphonylmethoxyethyl)adenine. Antivir. Res. 1992, 19, 267–273. [Google Scholar] [CrossRef]
- Yu, K.L.; Bronson, J.J.; Yang, H.; Patick, A.; Alam, M.; Brankovan, V.; Datema, R.; Hitchcock, M.J.; Martin, J.C. Synthesis and antiviral activity of methyl derivatives of 9-[2-(phosphonomethoxy)ethyl]guanine. J. Med. Chem. 1992, 35, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Holy, A.; Jindrich, J.; Naesens, L.; Snoeck, R.; Schols, D.; De Clercq, E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: Potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993, 37, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Chilar, T.; Chen, M.S. Incorporation of selected nucleoside phosphonates and anti-human immunodeficiency virus nucleotide analogues into DNA by human DNA polymerases α, β and γ. Antivir. Chem. Chemother. 1997, 8, 187–195. [Google Scholar]
- Cherrington, J.M.; Allen, S.J.W.; Bischofberger, N.; Chen, M.S. Kinetic interaction of the diphosphates of 9-(2-phosphonylmethoxyethyl)adenine and other anti-HIV active purine congeners with HIV reverse transcriptase and human DNA polymerase α, β and γ. Antivir. Chem. Chemother. 1995, 6, 217–221. [Google Scholar] [CrossRef][Green Version]
- Heijtink, R.A.; Kruining, J.; de Wilde, G.A.; Balzarini, J.; De Clercq, E.; Schalm, S.W. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 1994, 38, 2180–2182. [Google Scholar] [CrossRef] [PubMed]
- Delaney, W.E., IV; Ray, A.S.; Yang, H.; Qi, X.; Xiong, S.; Zhu, Y.; Miller, M.D. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob. Agents Chemother. 2006, 50, 2471–2477. [Google Scholar] [CrossRef]
- Lee, W.A.; He, G.X.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K.C. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005, 49, 1898–1906. [Google Scholar] [CrossRef]
- Callebaut, C.; Stepan, G.; Tian, Y.; Miller, M.D. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob. Agents Chemother. 2015, 59, 5909–5916. [Google Scholar] [CrossRef]
- Murakami, E.; Wang, T.; Park, Y.; Hao, J.; Lepist, E.I.; Babusis, D.; Ray, A.S. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob. Agents Chemother. 2015, 59, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Ruane, P.J.; DeJesus, E.; Berger, D.; Markowitz, M.; Bredeek, U.F.; Callebaut, C.; Zhong, L.; Ramanathan, S.; Rhee, M.S.; Fordyce, M.W.; et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J. Acquir. Immune Defic. Syndr. 2013, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Fung, S.K.; Nguyen, T.T.; Cheng, W.; Sicard, E.; Ryder, S.D.; Flaherty, J.F.; Lawson, E.; Zhao, S.; Subramanian, G.M.; et al. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J. Hepatol. 2015, 62, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.L.; Lee, B.T.; Tien, A.; Chang, M.; Lim, C.; Ahn, A.; Bae, H.S. Improvement of bone mineral density and markers of proximal renal tubular function in chronic hepatitis B patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. J. Viral Hepat. 2019, 26, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Lou, L. Advances in Nucleotide Antiviral Development from Scientific Discovery to Clinical Applications: Tenofovir Disoproxil Fumarate for Hepatitis B. J. Clin. Transl. Hepatol. 2013, 1, 33–38. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).