Synthesis of 2-Oxazolines from Ring Opening Isomerization of 3-Amido-2-Phenyl Azetidines
Abstract
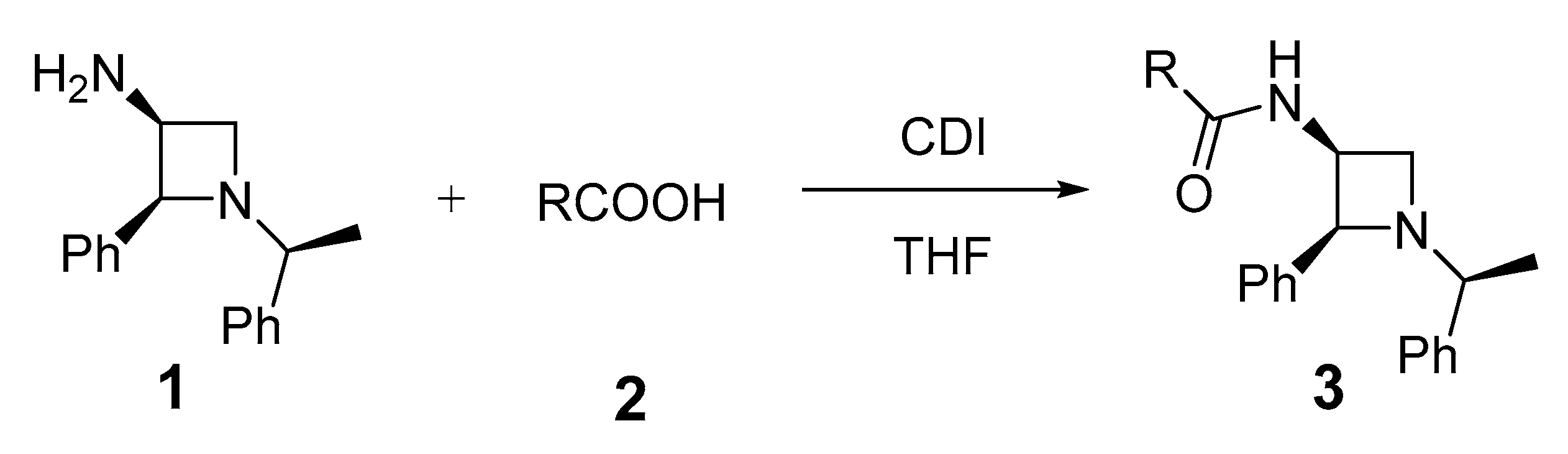
1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Isomeization of Amide 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wong, G.S.K.; Wu, W. 2-oxazolines. Chem. Heterocycl. Compd. 2004, 60, 331–528. [Google Scholar]
- Wipf, P.; Miller, C.P. Total synthesis of westiellamide. J. Am. Chem. Soc. 1992, 114, 10975–10977. [Google Scholar] [CrossRef]
- Xiao, S.-J.; Guo, D.-L.; Zhang, M.-S.; Chen, F.; Ding, L.-S.; Zhou, Y. Three new oxazoline alkaloids from Gymnotheca chinensis. J. Asian Nat. Prod. Res. 2016, 18, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Campiani, G.; De Angelis, M.; Armaroli, S.; Fattorusso, C.; Catalanotti, B.; Ramunno, A.; Nacci, V.; Novellino, E.; Grewer, C.; Ionescu, D.; et al. A rational approach to the design of selective substrates and potent nontransportable inhibitors of the excitatory amino acid transporter EAAC1 (EAAT3). New glutamate and aspartate analogues as potential neuroprotective agents. J. Med. Chem. 2001, 44, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Alanis, F.G.; Hernandez-Fernandez, E.; Carranza-Rosales, P.; Lopez-Cortina, S.; Hernandez-Fernandez, J.; Ordonez, M.; Guzman-Delgado, N.E.; Morales-Vargas, A.; Velazquez-Moreno, V.M.; Santiago-Mauricio, M.G. Synthesis, antimycobacterial and cytotoxic activity of α,β-unsaturated amides and 2,4-disubstituted oxazoline derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Woods, K.W.; Claiborne, A.; Gwaltney, S.L., II; Barr, K.J.; Liu, G.; Gehrke, L.; Credo, R.B.; Hui, Y.H.; Lee, J.; et al. Synthesis and Biological Evaluation of 2-Indolyloxazolines as a New Class of Tubulin Polymerization Inhibitors. Discovery of A-289099 as an Orally Active Antitumor Agent. Bioorg. Med. Chem. Lett. 2002, 12, 465–469. [Google Scholar] [CrossRef]
- Ye, X.-Y.; Liang, Z.-Q.; Jin, C.; Lang, Q.-W.; Chen, G.-Q.; Zhang, X. Design of oxa-spirocyclic PHOX ligands for the asymmetric synthesis of lorcaserin via iridium-catalyzed asymmetric hydrogenation. Chem. Commun. 2021, 57, 195–198. [Google Scholar] [CrossRef]
- Xi, C.-C.; Zhao, X.-J.; Tian, J.-M.; Chen, Z.-M.; Zhang, K.; Zhang, F.-M.; Tu, Y.-Q.; Dong, J.-W. Atroposelective Synthesis of Axially Chiral 3-Arylindoles by Copper-Catalyzed Asymmetric Cross-Coupling of Indoles with Quinones and Naphthoquinones. Org. Lett. 2020, 22, 4995–5000. [Google Scholar] [CrossRef]
- Park, J.-U.; Ahn, H.-I.; Cho, H.-J.; Xuan, Z.; Kim, J.H. Asymmetric Synthesis of N-Fused 1,3-Oxazolidines via Pd-Catalyzed Decarboxylative (3+2) Cycloaddition. Adv. Synth. Catal. 2020, 362, 1836–1840. [Google Scholar] [CrossRef]
- Qiu, Z.; Sun, R.; Yang, K.; Teng, D. Spiro indan-based phosphine-oxazolines as highly efficient P,N ligands for enantioselective Pd-catalyzed allylic alkylation of indoles and allylic etherification. Molecules 2019, 24, 1575. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Sibi, M.P. Dibenzofuran-4,6-bis(oxazoline) (DBFOX). A novel trans-chelating bis(oxazoline) ligand for asymmetric reactions. Org. Biomol. Chem. 2018, 16, 5551–5565. [Google Scholar] [CrossRef]
- Frump, J.A. Oxazolines. Their preparation, reactions, and applications. Chem. Rev. 1971, 71, 483–506. [Google Scholar] [CrossRef]
- Goud, D.R.; Pathak, U. A mild and efficient synthesis of 2-oxazolines via transamidation-cyclodehydrosulfurization of thioamides with 2-aminoethanol. Synthesis 2012, 44, 3678–3682. [Google Scholar] [CrossRef]
- Brandstatter, M.; Roth, F.; Luedtke, N.W. Synthesis of 2-Oxazolines by in Situ Desilylation and Cyclodehydration of β-Hydroxyamides. J. Org. Chem. 2015, 80, 40–51. [Google Scholar] [CrossRef]
- Huang, H.; Yang, W.; Chen, Z.; Lai, Z.; Sun, J. A mild catalytic synthesis of 2-oxazolines via oxetane ring-opening: Rapid access to a diverse family of natural products. Chem. Sci. 2019, 10, 9586–9590. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.J.; Jenkins, I.D.; Loughlin, W.A. The use of phosphonium anhydrides for the synthesis of 2-oxazolines, 2-thiazolines and 2-dihydrooxazine under mild conditions. Org. Biomol. Chem. 2009, 7, 739–746. [Google Scholar] [CrossRef]
- Sharma, R.; Vadivel, S.K.; Duclos, R.I.; Makriyannis, A. Open vessel mode microwave-assisted synthesis of 2-oxazolines from carboxylic acids. Tetrahedron Lett. 2009, 50, 5780–5782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajaram, S.; Sigman, M.S. Modular Synthesis of Amine-Functionalized Oxazolines. Org. Lett. 2002, 4, 3399–3401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, X.; Li, Y.; Zhang, Z.; Zheng, Z.-b. Synthesis of chiral 4,5-dihydrothiazol-2-amines and 4,5-dihydrooxazol-2-amines through an unexpected ring opening reaction of azetidines. Tetrahedron Lett. 2016, 57, 1236–1238. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, X.; Liu, R.; Zi, G. Synthesis of new chiral cis-3-aminoazetidines and their use in catalytic asymmetric reactions. Inorg. Chim. Acta 2009, 362, 1687–1691. [Google Scholar] [CrossRef]
- Higgins, R.H.; Faircloth, W.J.; Baughman, R.G.; Eaton, Q.L. Ring Opening of Azetidinols by Phenols: Regiochemistry and Stereochemistry. J. Org. Chem. 1994, 59, 2172–2178. [Google Scholar] [CrossRef]
- Purkarthofer, T.; Gruber, K.; Gruber-Khadjawi, M.; Waich, K.; Skranc, W.; Mink, D.; Griengl, H. A biocatalytic Henry reaction—The hydroxynitrile lyase from Hevea brasiliensis also catalyzes nitroaldol reactions. Angew. Chem. Int. Ed. 2006, 45, 3454–3456. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Minakata, S.; Takahashi, T.; Oderaotoshi, Y.; Komatsu, M. Asymmetric N1 Unit Transfer to Olefins with a Chiral Nitridomanganese Complex: Novel Stereoselective Pathways to Aziridines or Oxazolines. J. Org. Chem. 2002, 67, 2101–2110. [Google Scholar] [CrossRef] [PubMed]

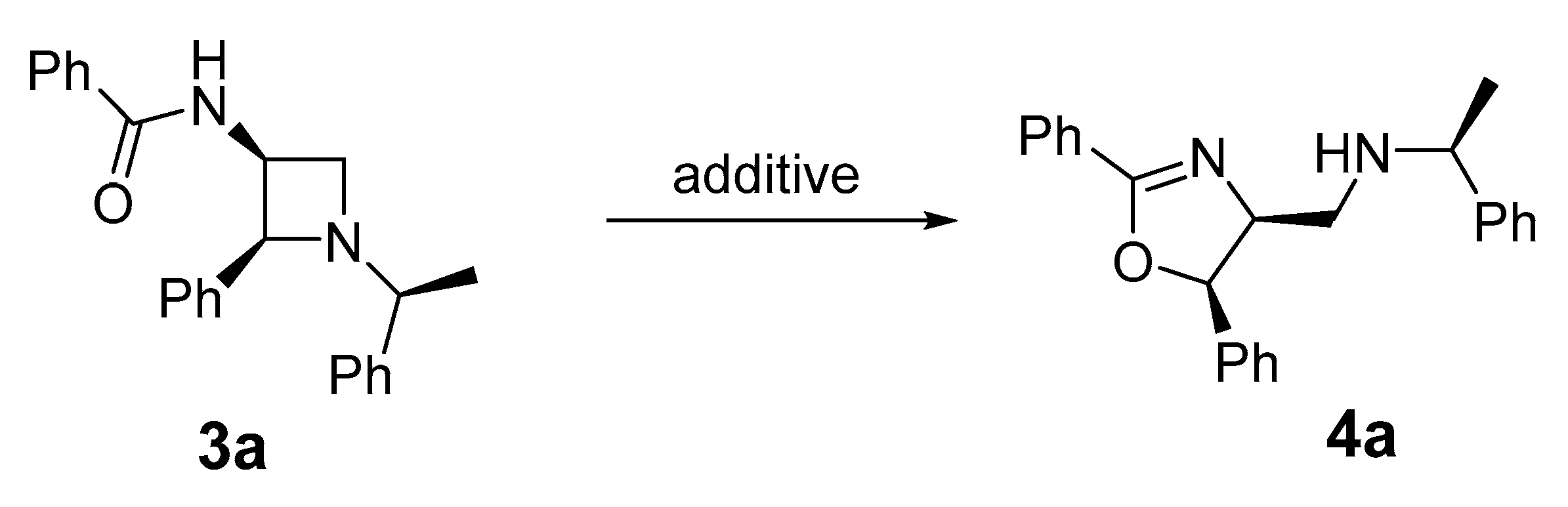

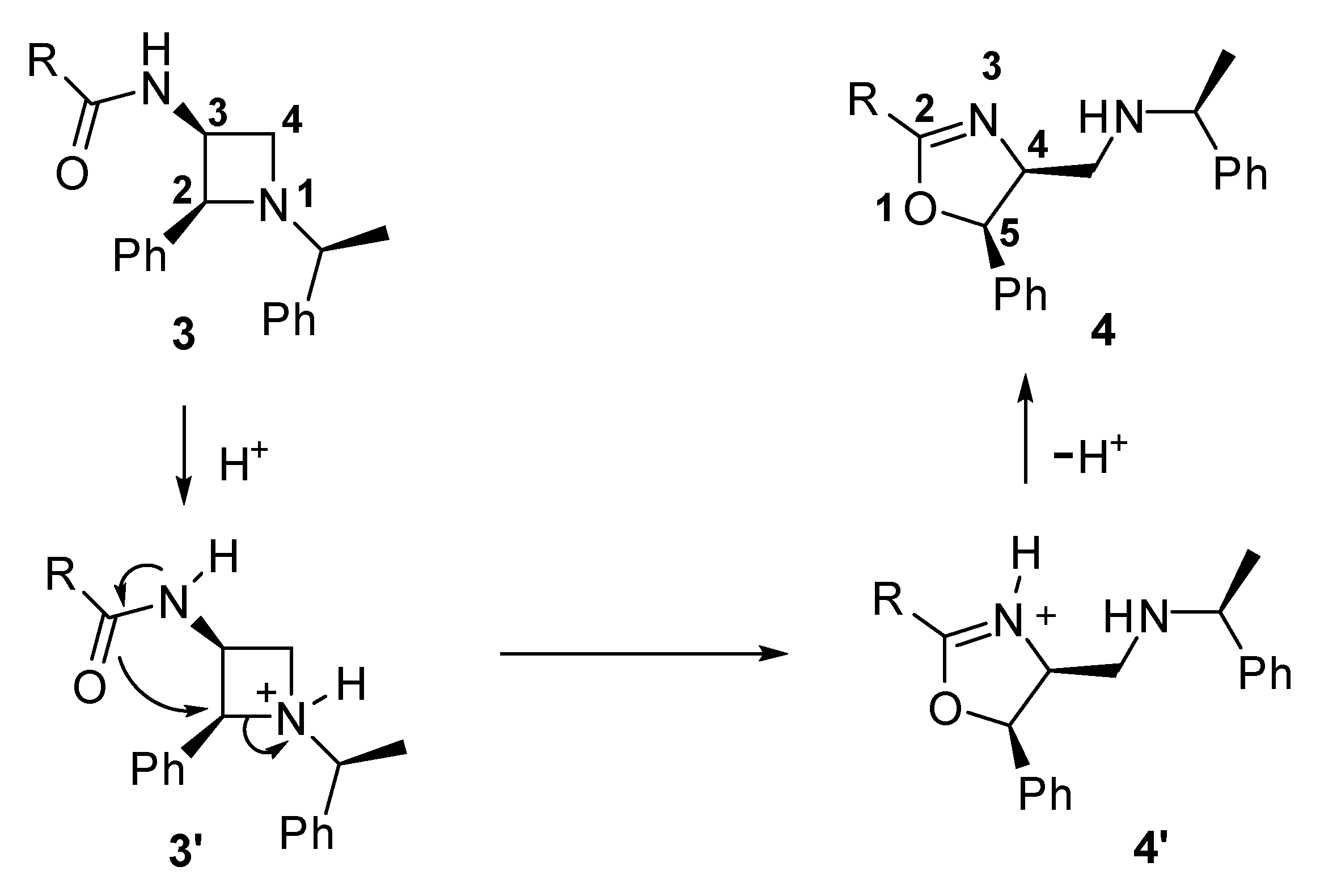
| Entry | Additive (Equiv.) | Solvent | Time (h) | Yield (%) 2 |
| 1 | - | ClCH2CH2Cl | 6 | n.r. |
| 2 | - | Toluene | 6 | n.r. |
| 3 | DABCO (1) | ClCH2CH2Cl | 6 | n.r. |
| 4 | t-BuOK (1) | THF | 6 | n.r. |
| 5 | NaH (1) | THF | 6 | n.r. |
| 6 | HClO4 (1) | ClCH2CH2Cl | 0.5 | 87 |
| 7 | CF3SO3H (1) | ClCH2CH2Cl | 0.5 | 65 |
| 8 | CH3COOH (1) | CH2Cl2 | 12 | n.r. |
| 9 | CF3COOH (1) | CH2Cl2 | 12 | 37 |
| 10 | CF3COOH (1) | ClCH2CH2Cl | 0.5 | 91 |
| 11 | CF3COOH (1) | ClCH2CH2Cl | 2 | 86 |
| 12 | CF3COOH (0.75) | ClCH2CH2Cl | 0.5 | 71 |
| 13 | TMSOTf (1) | ClCH2CH2Cl | 4 | 72 |
| 14 | Cu(OTf)2 (1) | ClCH2CH2Cl | 4 | 93 |
| 15 | Cu(OTf)2 (0.5) | ClCH2CH2Cl | 4 | 90 |
| 16 | BF3·Et2O (1) | ClCH2CH2Cl | 6 | 87 |
| Entry | R | Product | Yield (%) 2 |
| 1 | 3a: C6H5- | 4a | 96 |
| 2 | 3b: 4-MeOC6H4- | 4b | 94 |
| 3 | 3c: 2-MeOC6H4- | 4c | 93 |
| 4 | 3d: 4-O2NC6H4- | 4d | 94 |
| 5 | 3e: 2-O2NC6H4- | 4e | 91 |
| 6 | 3f: 4-ClC6H4- | 4f | 79 |
| 7 | 3g: 2-ClC6H4- | 4g | 85 |
| 8 | 3h: 4-pyridyl | 4h | 82 |
| 9 | 3i: 2-pyridyl | 4i | 87 |
| 10 3 | 3j: 2-HOC6H4- | 4j | 87 |
| 11 3 | 3k: 2-H2NC6H4- | 4k | 82 |
| 12 3 | 3l: 1-hydroxynaphthalen-2-yl | 4l | 88 |
| 13 | 3m: thiophen-2-yl | 4m | 90 |
| 14 | 3n: furan-2-yl | 4n | 84 |
| 15 | 3o: Me- | 4o | 85 |
| 16 | 3p: C6H5CH2- | 4p | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Mao, B.; Zhang, Z. Synthesis of 2-Oxazolines from Ring Opening Isomerization of 3-Amido-2-Phenyl Azetidines. Molecules 2021, 26, 857. https://doi.org/10.3390/molecules26040857
Zhou X, Mao B, Zhang Z. Synthesis of 2-Oxazolines from Ring Opening Isomerization of 3-Amido-2-Phenyl Azetidines. Molecules. 2021; 26(4):857. https://doi.org/10.3390/molecules26040857
Chicago/Turabian StyleZhou, Xin, Baiyi Mao, and Zhanbin Zhang. 2021. "Synthesis of 2-Oxazolines from Ring Opening Isomerization of 3-Amido-2-Phenyl Azetidines" Molecules 26, no. 4: 857. https://doi.org/10.3390/molecules26040857
APA StyleZhou, X., Mao, B., & Zhang, Z. (2021). Synthesis of 2-Oxazolines from Ring Opening Isomerization of 3-Amido-2-Phenyl Azetidines. Molecules, 26(4), 857. https://doi.org/10.3390/molecules26040857






