Lycium Barbarum Polysaccharides and Wolfberry Juice Prevent DEHP-Induced Hepatotoxicity via PXR-Regulated Detoxification Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Design
2.2.1. LBP Intervention before DEHP Exposure
2.2.2. LBP and WJ Intervention after DEHP Exposure
2.3. Reagents
2.4. ELISA Procedures
2.5. Statistical Analysis
3. Results
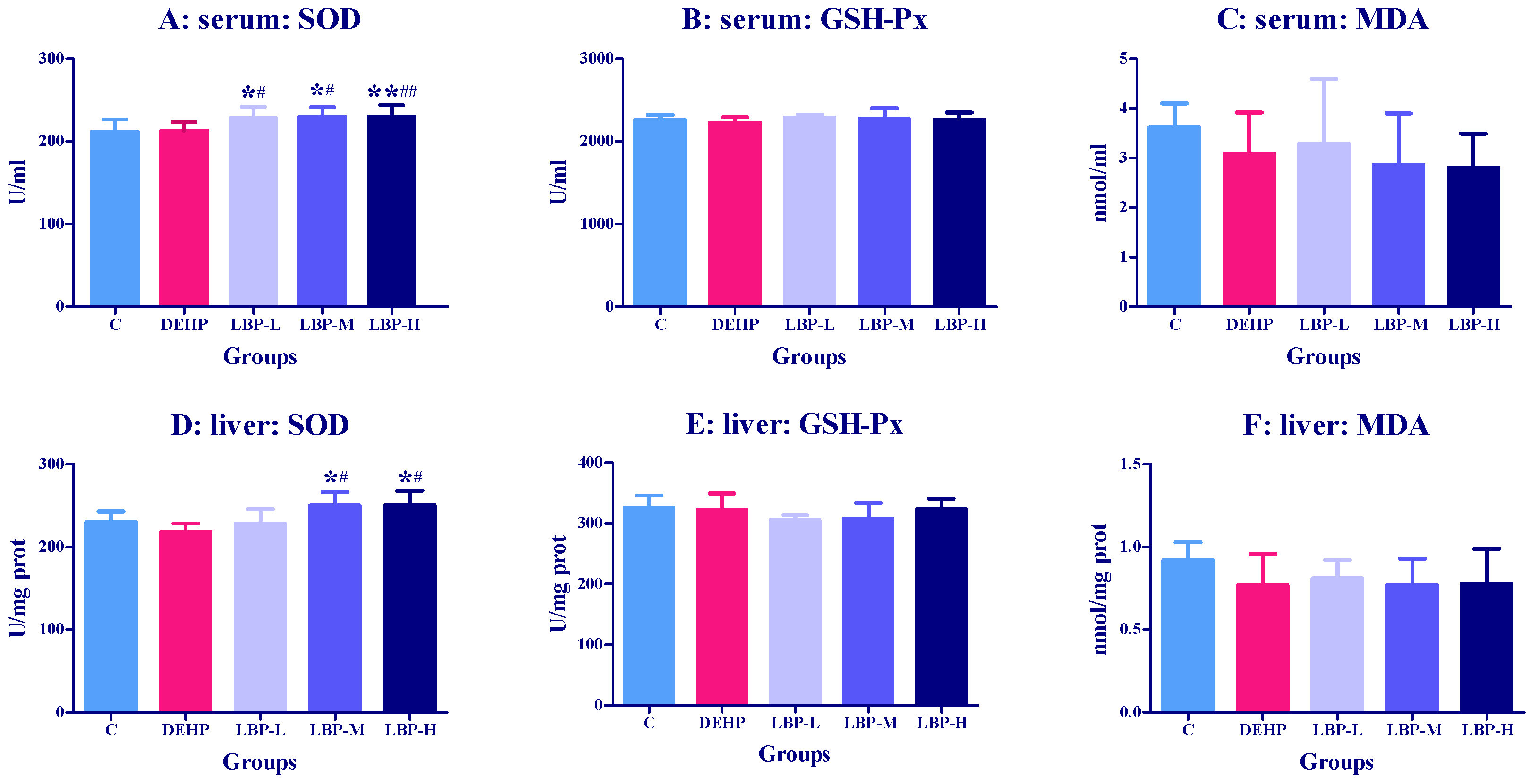
3.1. Effects of LBP Intervention before DEHP Exposure on Serum and Liver Oxidative Stress Index in Rats
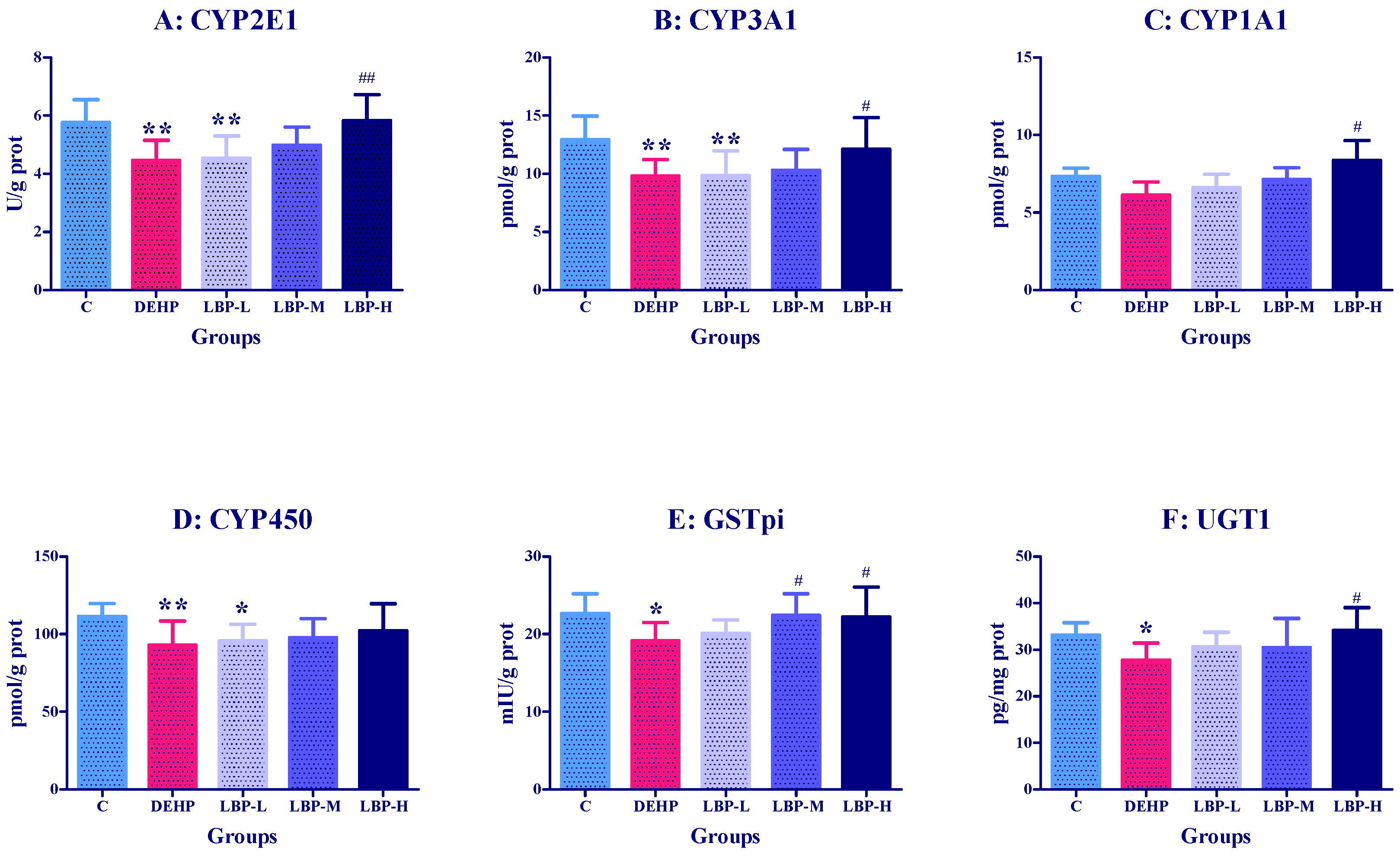
3.2. Effects of LBP Intervention before DEHP Exposure on Liver Metabolic Enzyme Activities in Rats
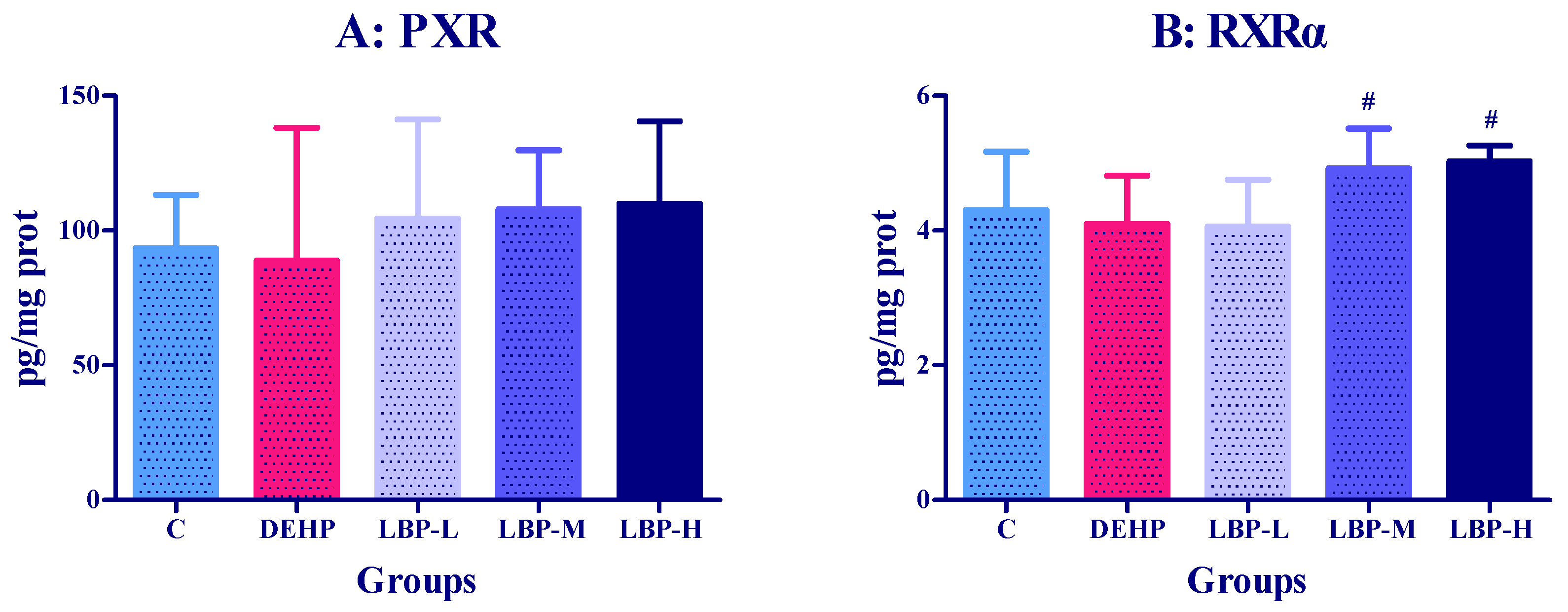
3.3. Effects of LBP Intervention before DEHP Exposure on PXR Concentration in Rats
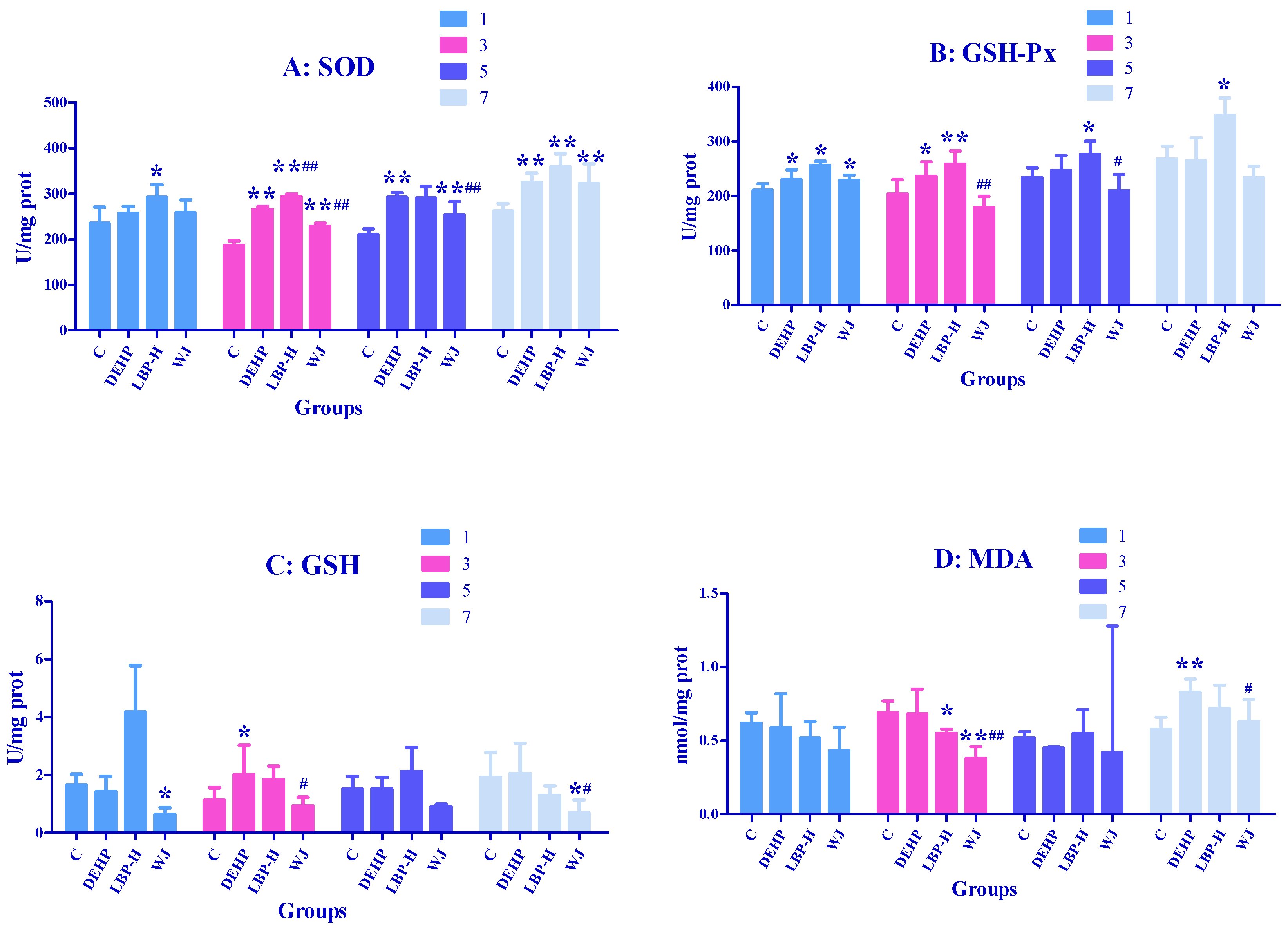
3.4. Effects of LBP and WJ Intervention after DEHP Exposure on Serum Oxidative Stress Index in Rats Exposed to DEHP
3.5. Effects of LBP and WJ Intervention after DEHP Exposure on Liver Oxidative Stress Index in Rats Exposed to DEHP
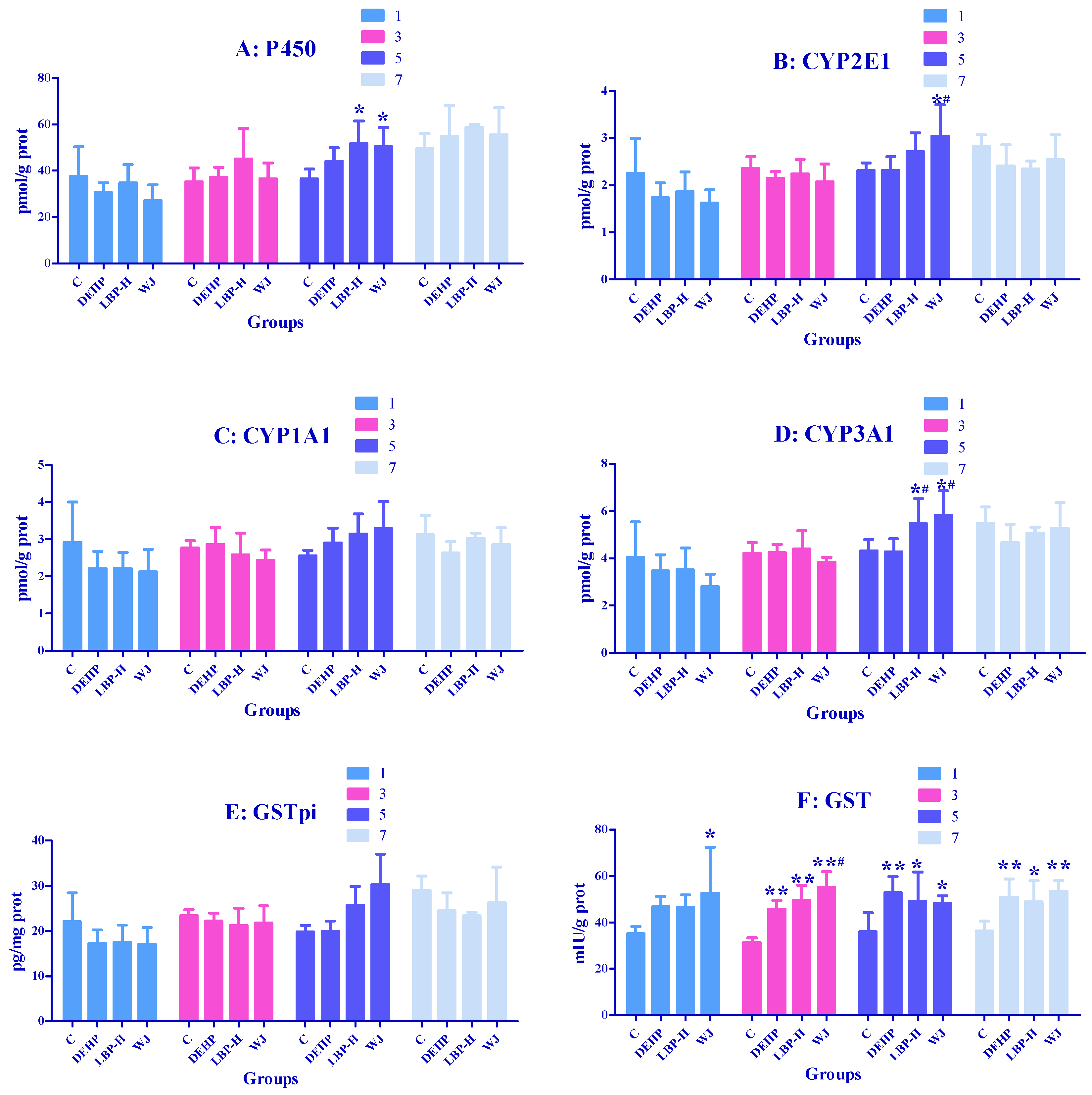
3.6. Effects of LBP and WJ Intervention after DEHP Exposure on Liver Metabolic Enzyme Activities in Rats Exposed to DEHP
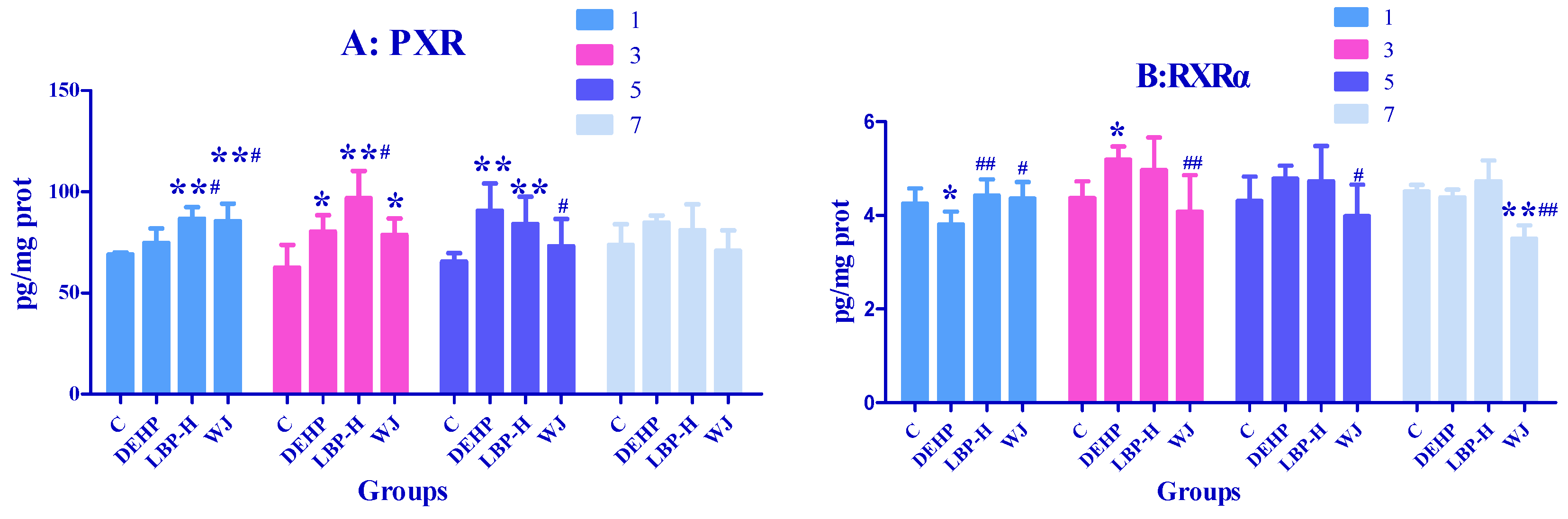
3.7. Effects of LBP and WJ Intervention after DEHP Exposure on PXR Activity in Rats Exposed to DEHP
4. Discussion
4.1. LBP Intervention before DEHP Exposure
4.2. LBP and WJ Intervention after DEHP Exposure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halden, R.U. Plastics and Health Risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef]
- Luo, Y.; Li, X.-N.; Zhao, Y.; Du, Z.-H.; Li, J.-L. DEHP triggers cerebral mitochondrial dysfunction and oxidative stress in quail (Coturnix japonica) via modulating mitochondrial dynamics and biogenesis and activating Nrf2-mediated defense response. Chemosphere 2019, 224, 626–633. [Google Scholar] [CrossRef]
- Junaid, M.; Jia, P.; Tang, Y.-M.; Xiong, W.-X.; Huang, H.-Y.; Strauss, P.R.; Li, W.-G.; Pei, D.-S. Mechanistic toxicity of DEHP at environmentally relevant concentrations (ERCs) and ecological risk assessment in the Three Gorges Reservoir Area, China. Environ. Pollut. 2018, 242, 1939–1949. [Google Scholar] [CrossRef]
- Heinemeyer, G.; Sommerfeld, C.; Springer, A.; Heiland, A.; Lindtner, O.; Greiner, M.; Heuer, T.; Krems, C.; Conrad, A. Estimation of dietary intake of bis(2-ethylhexyl)phthalate (DEHP) by consumption of food in the German population. Int. J. Hyg. Environ. Health 2013, 216, 472–480. [Google Scholar] [CrossRef]
- Autian, J. Toxicity and health threats of phthalate esters: Review of the literature. Environ. Health Perspect. 1973, 4, 3–26. [Google Scholar] [CrossRef]
- Kaul, A.F.; Souney, P.F.; Osathanondh, R. A Review of Possible Toxicity of DI-2-Ethylhexylphthalate (DEHP) in Plastic Intravenous Containers: Effects on Reproduction. Drug Intell. Clin. Pharm. 1982, 16, 689–692. [Google Scholar] [CrossRef]
- Tickner, J.; Schettler, T.; Guidotti, T.; McCally, M.; Rossi, M. Health risks posed by use of Di2-ethylhexyl phthalate (DEHP) in PVC medical devices: A critical review. Am. J. Ind. Med. 2001, 39, 100–111. [Google Scholar] [CrossRef]
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Eckstein, R.; Weisbach, V.; Angerer, J. Intravenous exposure to di(2-ethylhexyl)phthalate (DEHP): Metabolites of DEHP in urine after a voluntary platelet donation. Arch. Toxicol. 2005, 79, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Habeebu, S.S.; Klaassen, C.D. Testicular toxicity of di-(2-ethylhexyl)phthalate in young Sprague–Dawley rats. Toxicology 2002, 171, 105–115. [Google Scholar] [CrossRef]
- Gao, H.-T.; Xu, R.; Cao, W.-X.; Qian, L.-L.; Wang, M.; Lu, L.; Xu, Q.; Yu, S.-Q. Effects of six priority controlled phthalate esters with long-term low-dose integrated exposure on male reproductive toxicity in rats. Food Chem. Toxicol. 2017, 101, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Drexler, H.; Angerer, J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health 2003, 206, 77–83. [Google Scholar] [CrossRef]
- Ito, Y.; Kamijima, M.; Hasegawa, C.; Tagawa, M.; Kawai, T.; Miyake, M.; Hayashi, Y.; Naito, H.; Nakajima, T. Species and inter-individual differences in metabolic capacity of di(2-ethylhexyl)phthalate (DEHP) between human and mouse livers. Environ. Health Prev. Med. 2013, 19, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.; Orton, T.C.; Pratt, I.S.; Batten, P.L.; Bratt, H.; Jackson, S.J.; Elcombe, C.R. Comparative pharmacokinetics and subacute toxicity of di(2-ethylhexyl) phthalate (DEHP) in rats and marmosets: Extrapolation of effects in rodents to man. Environ. Health Perspect. 1986, 65, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.-M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Joo, H.; Campbell, J.L.; Andersen, M.E.; Clewell, H.J. In vitro intestinal and hepatic metabolism of Di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicol. Vitr. 2013, 27, 1451–1457. [Google Scholar] [CrossRef]
- Eveillard, A.; Lasserre, F.; De Tayrac, M.; Polizzi, A.; Claus, S.; Canlet, C.; Mselli-Lakhal, L.; Gotardi, G.; Paris, A.; Guillou, H. Identification of potential mechanisms of toxicity after di-(2-ethylhexyl)-phthalate (DEHP) adult exposure in the liver using a systems biology approach. Toxicol. Appl. Pharmacol. 2009, 236, 282–292. [Google Scholar] [CrossRef]
- Matic, M.; Mahns, A.; Tsoli, M.; Corradin, A.; Polly, P.; Robertson, G.R. Pregnane X Receptor: Promiscuous regulator of detoxification pathways. Int. J. Biochem. Cell Biol. 2007, 39, 478–483. [Google Scholar] [CrossRef]
- Wang, L.-Q. Inhibition of Sulfotransferases by Xenobiotics. Curr. Drug Metab. 2006, 7, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Makkonen, H.; Dunlop, T.W.; Matilainen, M.; Väisänen, S.; Carlberg, C. Identification of Pregnane X Receptor Binding Sites in the Regulatory Regions of Genes Involved in Bile Acid Homeostasis. J. Mol. Biol. 2005, 346, 505–519. [Google Scholar] [CrossRef]
- Chung, R.-S.; Chen, C.-C.; Ng, L.-T. Nitrogen fertilization affects the growth performance, betaine and polysaccharide concentrations of Lycium barbarum. Ind. Crop. Prod. 2010, 32, 650–655. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Yu, M.-S.; Yang, X.-F.; So, K.-F.; Yuen, W.-H.; Chang, R.C.-C. Neuroprotective Effects of Polysaccharides from Wolfberry, the Fruits of Lycium barbarum, Against Homocysteine-induced Toxicity in Rat Cortical Neurons. J. Alzheimer’s Dis. 2010, 19, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Q.; Xiao, J.; Fan, H.-X.; Yu, Y.; He, R.-R.; Feng, X.-L.; Kurihara, H.; So, K.-F.; Yao, X.-S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Chung, W.Y.; Szeto, Y.T.; Benzie, I.F. Fasting plasma zeaxanthin response to Fructus barbarum L. (wolfberry; Kei Tze) in a food-based human supplementation trial. Br. J. Nutr. 2005, 93, 123–130. [Google Scholar] [CrossRef]
- Li, X.; Zhou, A. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497. [Google Scholar] [CrossRef]
- Wu, H.; Guo, H.; Zhao, R. Effect of Lycium barbarum Polysaccharide on the Improvement of Antioxidant Ability and DNA Damage in NIDDM Rats. Yakugaku Zasshi 2006, 126, 365–371. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Meng, J.; Lu, Z.; Sun, C.; Chang, C. Advances in the pharmacologic of Lycium barbarum polysaccharide. Lishizhen Med. Mater. Med. Res. 2018, 29, 2489–2493. [Google Scholar]
- Wu, G.; Jia, Y.; Yuan, S. Progress in immunopharmacology of lycium barbarum anticancer. China Pharm. 2002, 13, 54–55. [Google Scholar]
- Miao, T.; Yan, Q.; Wang, J.; Zhang, L. Research progress of lycium barbarum polysaccharide against aging. Asia Pac. Trad. Med. 2019, 15, 178–180. [Google Scholar]
- Huang, T.; Zheng, X.; Qiu, X.; Wang, Y. The protective effect of lycium barbarum polysaccharide on model of autoimmune premature ovarian failure in mice. J. Pharmaceut. Res. 2014, 33, 437–440. [Google Scholar] [CrossRef]
- Xia, G.; Xin, N.; Liu, W.; Yao, H.; Hou, Y.; Qi, J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol. Med. Rep. 2014, 9, 1237–1241. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, H.; Shi, S.; Li, M. Lycium ruthenicum polysaccharide attenuates inflammation through inhibiting TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2014, 67, 330–335. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Xiang, Z.; Xu, D.; So, K.-F.; Vardi, N.; Xu, Y. Lycium Barbarum Polysaccharides Protect Retina in rd1 Mice During Photoreceptor Degeneration. Investig. Opthalmology Vis. Sci. 2018, 59, 597–611. [Google Scholar] [CrossRef]
- Amagase, H.; Nance, D.M. A Randomized, Double-Blind, Placebo-Controlled, Clinical Study of the General Effects of a Standardized Lycium barbarum (Goji) Juice, GoChi™. J. Altern. Complement. Med. 2008, 14, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Liu, L.; Huang, L.; Bai, L.; Jin, S. Effects of DBP on the Structure and Function of Testes in the Male Rats. J. Beihua Univ. 2010, 11, 9. [Google Scholar]
- Chen, X.; Wang, J.S.; Jiang, Y. Effects of Co-exposure of Di-(2-ethylhexyl) Phthalate and Benzo(a)pyrene on CYP1A1 and CYP3A4 Enzyme Activities in HepG2 Cells. Int. J. Occup. Med. Environ. Health 2011, 28, 525–530. [Google Scholar]
- Court, M.H.; Zhang, X.; Ding, X.; Yee, K.K.; Hesse, L.M.; Finel, M. Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica 2011, 42, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Zeybek, N.D.; Giray, B.; Rachidi, W.; Kızılgün, M.; Hininger-Favier, I.; Favier, A.; Asan, E.; Hincal, F. The effects of di(2-ethylhexyl)phthalate on rat liver in relation to selenium status. Int. J. Exp. Pathol. 2014, 95, 64–77. [Google Scholar] [CrossRef]
- Doerge, D.R.; Twaddle, N.C.; Churchwell, M.I.; Chang, H.C.; Newbold, R.R.; Delclos, K. Mass spectrometric determination of p-nonylphenol metabolism and disposition following oral administration to Sprague-Dawley rats. Reprod. Toxicol. 2002, 16, 45–56. [Google Scholar] [CrossRef]
- Burchell, B.; Nebert, D.W.; Nelson, D.R.; Bock, K.W.; Iyanagi, T.; Jansen, P.L.; Lancet, R.; Mulder, G.J.; Chowdhury, J.R.; Siest, G.; et al. The UDP Glucuronosyltransferase Gene Super family: Suggested Nomenclature Based on Evolutionary Divergence. DNA Cell Biol. 1991, 10, 487–494. [Google Scholar] [CrossRef]
- Castro-Caldas, M.; Carvalho, A.N.; Peixeiro, I.; Rodrigues, E.; Lechner, M.C.; Gama, M.J. GSTpi Expression in MPTP-Induced Dopaminergic Neurodegeneration of C57BL/6 Mouse Midbrain and Striatum. J. Mol. Neurosci. 2008, 38, 114–127. [Google Scholar] [CrossRef][Green Version]
- He, Y.J.; Huang, S.W.; Liu, R.J.; Liu, C.H. Intervention effects of Lycium barbarum juice on the metabolism of DEHP based on the UGT1 detoxification pathway. Food. Sci. 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Rataj, F.; Mller, F.J.; Jhne, M.; Zierau, O.; Diel, P.; Vollmer, G.; Kretzschmar, G. Regulation of uterine AHR battery gene ex-pression by 17β-Estradiol is predominantly mediated by estrogen receptor α. Arch. Toxicol. 2012, 86, 1603–1612. [Google Scholar] [CrossRef]
- Cao, S.; Du, J.; Hei, Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017, 14, 4919–4927. [Google Scholar] [CrossRef]
- Hurst, C.H.; Waxman, D.J. Environmental phthalate monoesters activate pregnane X receptor-mediated transcription. Toxicol. Appl. Pharmacol. 2004, 199, 266–274. [Google Scholar] [CrossRef]
- Du, Z.-H.; Xia, J.; Sun, X.-C.; Li, X.-N.; Zhang, C.; Zhao, H.-S.; Zhu, S.-Y.; Li, J.-L. A novel nuclear xenobiotic receptors (AhR/PXR/CAR)-mediated mechanism of DEHP-induced cerebellar toxicity in quails (Coturnix japonica) via disrupting CYP enzyme system homeostasis. Environ. Pollut. 2017, 226, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, W.; Qin, Q.; Wang, J.; Zheng, H.; Xiong, W.; Yuan, J. Mono(2-ethylhexyl) phthalate induces apoptosis in p53-silenced L02 cells via activation of both mitochondrial and death receptor pathways. Environ. Toxicol. 2015, 30, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-F.; Fang, H.-Y.; Qu, H. Optimization of micellar electrokinetic capillary chromatography method using central composite design for the analysis of components in Yangwei granule. J. Zhejiang Univ. Sci. B 2011, 12, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Ping, L.I.; Zhang, X.L. Effect of angelica Sinensis polysaccharide on the contents of ant-oxidative enzymes and nitric oxide in mice with chemical liver injured. Modern. Prev. Med. 2011, 38, 4725–4727. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhou, X.; Huang, S.; Yang, J.; Liu, R.; Liu, C. Lycium Barbarum Polysaccharides and Wolfberry Juice Prevent DEHP-Induced Hepatotoxicity via PXR-Regulated Detoxification Pathway. Molecules 2021, 26, 859. https://doi.org/10.3390/molecules26040859
Liu H, Zhou X, Huang S, Yang J, Liu R, Liu C. Lycium Barbarum Polysaccharides and Wolfberry Juice Prevent DEHP-Induced Hepatotoxicity via PXR-Regulated Detoxification Pathway. Molecules. 2021; 26(4):859. https://doi.org/10.3390/molecules26040859
Chicago/Turabian StyleLiu, Huan, Xiong Zhou, Shaowen Huang, Jie Yang, Ruijing Liu, and Chunhong Liu. 2021. "Lycium Barbarum Polysaccharides and Wolfberry Juice Prevent DEHP-Induced Hepatotoxicity via PXR-Regulated Detoxification Pathway" Molecules 26, no. 4: 859. https://doi.org/10.3390/molecules26040859
APA StyleLiu, H., Zhou, X., Huang, S., Yang, J., Liu, R., & Liu, C. (2021). Lycium Barbarum Polysaccharides and Wolfberry Juice Prevent DEHP-Induced Hepatotoxicity via PXR-Regulated Detoxification Pathway. Molecules, 26(4), 859. https://doi.org/10.3390/molecules26040859





