Structural Insights into Ligand—Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors
Abstract
1. Introduction
2. β-Adrenergic Receptors
3. Dopaminergic Receptors
4. Muscarinic Receptors
5. Serotonin Receptors
6. Opioid Receptors
7. Angiotensin Receptor
8. Conclusions
Funding
Conflicts of Interest
References
- Lefkowitz, R.J. A brief history of G-protein coupled receptors (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2013, 52, 6366–6378. [Google Scholar] [CrossRef]
- Kobilka, B. The structural basis of G-protein-coupled receptor signaling (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2013, 52, 6380–6388. [Google Scholar] [CrossRef]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2017, 117, 139–155. [Google Scholar] [CrossRef]
- Manglik, A.; Kruse, A.C. Structural Basis for G Protein-Coupled Receptor Activation. Biochemistry 2017, 56, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Biased Receptor Signaling in Drug Discovery. Pharmacol. Rev. 2019, 71, 267–315. [Google Scholar] [CrossRef]
- Wingler, L.M.; Lefkowitz, R.J. Conformational Basis of G Protein-Coupled Receptor Signaling Versatility. Trends Cell Biol. 2020, 30, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front. Pharmacol. 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.E.; Meicher, K.; Xu, H.E. Understanding the GPCR biased signaling through G protein and arrestin complex structures. Curr. Opin. Struct. Biol. 2017, 45, 150–159. [Google Scholar] [CrossRef]
- Rankovic, Z.; Brust, T.F.; Bohn, L.M. Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg. Med. Chem. Lett. 2016, 26, 241–250. [Google Scholar] [CrossRef]
- Kenakin, T.; Miller, L.J. Seven transmembrane receptors as shapeshifting proteins: The impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010, 62, 265–304. [Google Scholar] [CrossRef]
- Shonberg, J.; Lopez, L.; Scammells, P.J.; Christopoulos, A.; Capuano, B.; Lane, J.R. Biased agonism at G protein-coupled receptors: The promise and the challenges--a medicinal chemistry perspective. Med. Res. Rev. 2014, 34, 1286–1330. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Booe, J.M.; Pioszak, A.A. Structural insights into ligand recognition and selectivity for classes A, B, and C GPCRs. Eur. J. Pharmacol. 2015, 763 Pt B, 196–205. [Google Scholar] [CrossRef]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Shonberg, J.; Kling, R.C.; Gmeiner, P.; Löber, S. GPCR crystal structures: Medicinal chemistry in the pocket. Bioorg. Med. Chem. 2015, 23, 3880–3906. [Google Scholar] [CrossRef]
- Kolinski, M.; Plazinska, A.; Jozwiak, K. Recent progress in understanding of structure, ligand interactions and the mechanism of activation of the β₂-adrenergic receptor. Curr. Med. Chem. 2012, 19, 1155–1163. [Google Scholar] [CrossRef]
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Marchionni, N.; Fornasari, D. beta-blockers: Their new life from hypertension to cancer and migraine. Pharmacol. Res. 2020, 151, 104587. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A. To block it, or not to block it? J. Cancer Res. Clin. Oncol. 2017, 143, 2631–2633. [Google Scholar] [CrossRef]
- Sinagra, G.; Corrà, U.; Contini, M.; Magrì, D.; Paolillo, S.; Perrone Filardi, P.; Sciomer, S.; Badagliacca, R.; Agostoni, P. Choosing among β-blockers in heart failure patients according to β-receptors’ location and functions in the cardiopulmonary system. Pharmacol. Res. 2020, 156, 104785. [Google Scholar] [CrossRef]
- Warne, T.; Edwards, P.C.; Leslie, A.G.; Tate, C.G. Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol. Structure 2012, 20, 841–849. [Google Scholar] [CrossRef]
- Wisler, J.W.; DeWire, S.M.; Whalen, E.J.; Violin, J.D.; Drake, M.T.; Ahn, S.; Shenoy, S.K.; Lefkowitz, R.J. A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 16657–16662. [Google Scholar] [CrossRef] [PubMed]
- Warne, T.; Edwards, P.C.; Doré, A.S.; Leslie, A.G.W.; Tate, C.G. Molecular basis for high-affinity agonist binding in GPCRs. Science 2019, 364, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. In Methods in Neurosciences; Sealfon, S.C., Ed.; Academic Press: Cambridge, MA, USA, 1995; Volume 25, pp. 366–428. [Google Scholar]
- Xiao, R.P.; Ji, X.; Lakatta, E.G. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol. Pharmacol. 1995, 47, 322–329. [Google Scholar] [PubMed]
- Xiao, R.P.; Avdonin, P.; Zhou, Y.Y.; Cheng, H.; Akhter, S.A.; Eschenhagen, T.; Lefkowitz, R.J.; Koch, W.J.; Lakatta, E.G. Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999, 84, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.Y.; Jozwiak, K.; Toll, L.; Tanga, M.J.; Kozocas, J.A.; Jimenez, L.; Huang, Y.; Song, Y.; Plazinska, A.; Pajak, K.; et al. Tyrosine 308 is necessary for ligand-directed Gs protein-biased signaling of β2-adrenoceptor. J. Biol. Chem. 2014, 289, 19351–19363. [Google Scholar] [CrossRef]
- Jozwiak, K.; Khalid, C.; Tanga, M.J.; Berzetei-Gurske, I.; Jimenez, L.; Kozocas, J.A.; Woo, A.; Zhu, W.; Xiao, R.P.; Abernethy, D.R.; et al. Comparative molecular field analysis of the binding of the stereoisomers of fenoterol and fenoterol derivatives to the beta2 adrenergic receptor. J. Med. Chem. 2007, 50, 2903–2915. [Google Scholar] [CrossRef]
- Jozwiak, K.; Woo, A.Y.; Tanga, M.J.; Toll, L.; Jimenez, L.; Kozocas, J.A.; Plazinska, A.; Xiao, R.P.; Wainer, I.W. Comparative molecular field analysis of fenoterol derivatives: A platform towards highly selective and effective beta(2)-adrenergic receptor agonists. Bioorg. Med. Chem. 2010, 18, 728–736. [Google Scholar] [CrossRef]
- Plazinska, A.; Pajak, K.; Rutkowska, E.; Jimenez, L.; Kozocas, J.; Koolpe, G.; Tanga, M.; Toll, L.; Wainer, I.W.; Jozwiak, K. Comparative molecular field analysis of fenoterol derivatives interacting with an agonist-stabilized form of the β₂-adrenergic receptor. Bioorg. Med. Chem. 2014, 22, 234–246. [Google Scholar] [CrossRef][Green Version]
- Jozwiak, K.; Toll, L.; Jimenez, L.; Woo, A.Y.; Xiao, R.P.; Wainer, I.W. The effect of stereochemistry on the thermodynamic characteristics of the binding of fenoterol stereoisomers to the beta(2)-adrenoceptor. Biochem. Pharmacol. 2010, 79, 1610–1615. [Google Scholar] [CrossRef]
- Toll, L.; Jimenez, L.; Waleh, N.; Jozwiak, K.; Woo, A.Y.; Xiao, R.P.; Bernier, M.; Wainer, I.W. β2-adrenergic receptor agonists inhibit the proliferation of 1321N1 astrocytoma cells. J. Pharmacol. Exp. Ther. 2011, 336, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.; Sadowska, M.; Paul, R.K.; Singh, N.S.; Boguszewska-Czubara, A.; Jimenez, L.; Abdelmohsen, K.; Toll, L.; Jozwiak, K.; Bernier, M.; et al. Activation of β2-adrenergic receptor by (R,R’)-4’-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells. Cell Signal. 2015, 27, 997–1007. [Google Scholar] [CrossRef][Green Version]
- Wnorowski, A.; Such, J.; Paul, R.K.; Wersto, R.P.; Indig, F.E.; Jozwiak, K.; Bernier, M.; Wainer, I.W. Concurrent activation of β2-adrenergic receptor and blockage of GPR55 disrupts pro-oncogenic signaling in glioma cells. Cell Signal. 2017, 36, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Tschammer, N.; Bollinger, S.; Kenakin, T.; Gmeiner, P. Histidine 6.55 is a major determinant of ligand-biased signaling in dopamine D2L receptor. Mol. Pharmacol. 2011, 79, 575–585. [Google Scholar] [CrossRef]
- Fowler, J.C.; Bhattacharya, S.; Urban, J.D.; Vaidehi, N.; Mailman, R.B. Receptor conformations involved in dopamine D(2L) receptor functional selectivity induced by selected transmembrane-5 serine mutations. Mol. Pharmacol. 2012, 81, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Michino, M.; Free, R.B.; Doyle, T.B.; Sibley, D.R.; Shi, L. Structural basis for Na(+)-sensitivity in dopamine D2 and D3 receptors. Chem. Commun. (Camb) 2015, 1, 8618–8621. [Google Scholar] [CrossRef]
- Free, R.B.; Chun, L.S.; Moritz, A.E.; Miller, B.N.; Doyle, T.B.; Conroy, J.L.; Padron, A.; Meade, J.A.; Xiao, J.; Hu, X.; et al. Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor. Mol. Pharmacol. 2014, 86, 96–105. [Google Scholar] [CrossRef]
- Sanchez-Soto, M.; Verma, R.K.; Willette, B.K.A.; Gonye, E.C.; Moore, A.M.; Moritz, A.E.; Boateng, C.A.; Yano, H.; Free, R.B.; Shi, L.; et al. A structural basis for how ligand binding site changes can allosterically regulate GPCR signaling and engender functional selectivity. Sci. Signal. 2020, 13, eaaw5885. [Google Scholar] [CrossRef]
- Ring, A.M.; Manglik, A.; Kruse, A.C.; Enos, M.D.; Weis, W.I.; Garcia, K.C.; Kobilka, B.K. Adrenaline-activated structure of beta2-adrenoceptor stabilized by an engineered nanobody. Nature 2013, 502, 575–579. [Google Scholar] [CrossRef]
- Wacker, D.; Wang, C.; Katritch, V.; Han, G.W.; Huang, X.P.; Vardy, E.; McCorvy, J.D.; Jiang, Y.; Chu, M.; Siu, F.Y.; et al. Structural features for functional selectivity at serotonin receptors. Science 2013, 340, 615–619. [Google Scholar] [CrossRef]
- Christopoulos, A.; Kenakin, T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002, 54, 323–374. [Google Scholar] [CrossRef]
- Bock, A.; Merten, N.; Schrage, R.; Dallanoce, C.; Bätz, J.; Klöckner, J.; Schmitz, J.; Matera, C.; Simon, K.; Kebig, A.; et al. The allosteric vestibule of a seven transmembrane helical receptor controls G-protein coupling. Nat. Commun. 2012, 3, 1044. [Google Scholar] [CrossRef]
- Kruse, A.C.; Ring, A.M.; Manglik, A.; Hu, J.; Hu, K.; Eitel, K.; Hübner, H.; Pardon, E.; Valant, C.; Sexton, P.M.; et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 2013, 504, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.; Wang, S.; McCorvy, J.D.; Betz, R.M.; Venkatakrishnan, A.J.; Levit, A.; Lansu, K.; Schools, Z.L.; Che, T.; Nichols, D.E.; et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 2017, 168, 377–389.e12. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Frescas, S.; Marona-Lewicka, D.; Kurrasch-Orbaugh, D.M. Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD). J. Med. Chem. 2002, 45, 4344–4349. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Wacker, D.; Wang, S.; Agegnehu, B.; Liu, J.; Lansu, K.; Tribo, A.R.; Olsen, R.H.J.; Che, T.; Jin, J.; et al. Structural determinants of 5-HT2B receptor activation and biased agonism. Nat. Struct. Mol. Biol. 2018, 25, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Raehal, K.M.; Walker, K.L.; Bohn, L.M. Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 2005, 314, 1195–1201. [Google Scholar] [CrossRef]
- Conibear, A.E.; Kelly, E. A Biased View of μ-Opioid Receptors? Mol. Pharmacol. 2019, 96, 542–549. [Google Scholar] [CrossRef]
- Hothersall, J.D.; Torella, R.; Humphreys, S.; Hooley, M.; Brown, A.; McMurray, G.; Nickolls, S.A. Residues W320 and Y328 within the binding site of the mu-opioid receptor influence opiate ligand bias. Neuropharmacology 2017, 118, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.X.; Cheng, T.; Li, W.H.; Liu, G.X.; Zhu, W.L.; Tang, Y. Computational insights into the G-protein-biased activation and inactivation mechanisms of the μ opioid receptor. Acta Pharmacol. Sin. 2018, 39, 154–164. [Google Scholar] [CrossRef]
- de Waal, P.W.; Shi, J.; You, E.; Wang, X.; Melcher, K.; Jiang, Y.; Xu, H.E.; Dickson, B.M. Molecular mechanisms of fentanyl mediated β-arrestin biased signaling. PLoS Comput. Biol. 2020, 16, e1007394. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Majumdar, S.; Zaidi, S.A.; Ondachi, P.; McCorvy, J.D.; Wang, S.; Mosier, P.D.; Uprety, R.; Vardy, E.; Krumm, B.E.; et al. Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 2018, 172, 55–67.e15. [Google Scholar] [CrossRef] [PubMed]
- Fenalti, G.; Giguere, P.M.; Katritch, V.; Huang, X.P.; Thompson, A.A.; Cherezov, V.; Roth, B.L.; Stevens, R.C. Molecular control of delta-opioid receptor signalling. Nature 2014, 506, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Laroche, G.; Wang, X.; Ågren, H.; Bowman, G.R.; Giguère, P.M.; Tu, Y. Propagation of the Allosteric Modulation Induced by Sodium in the δ-Opioid Receptor. Chemistry 2017, 23, 4615–4624. [Google Scholar] [CrossRef]
- Holloway, A.C.; Qian, H.; Pipolo, L.; Ziogas, J.; Miura, S.; Karnik, S.; Southwell, B.R.; Lew, M.J.; Thomas, W.G. Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol. Pharmacol. 2002, 61, 768–777. [Google Scholar] [CrossRef]
- Ryba, D.M.; Li, J.; Cowan, C.L.; Russell, B.; Wolska, B.M.; Solaro, R.J. Long-Term Biased β-Arrestin Signaling Improves Cardiac Structure and Function in Dilated Cardiomyopathy. Circulation 2017, 135, 1056–1070. [Google Scholar] [CrossRef]
- Wingler, L.M.; Skiba, M.A.; McMahon, C.; Staus, D.P.; Kleinhenz, A.L.W.; Suomivuori, C.M.; Latorraca, N.R.; Dror, R.O.; Lefkowitz, R.J.; Kruse, A.C. Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science 2020, 367, 888–892. [Google Scholar] [CrossRef]
- Suomivuori, C.M.; Latorraca, N.R.; Wingler, L.M.; Eismann, S.; King, M.C.; Kleinhenz, A.L.W.; Skiba, M.A.; Staus, D.P.; Kruse, A.C.; Lefkowitz, R.J.; et al. Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science 2020, 367, 881–887. [Google Scholar] [CrossRef]
- Manglik, A.; Kobilka, B. The role of protein dynamics in GPCR function: Insights from the β2AR and rhodopsin. Curr. Opin. Cell Biol. 2014, 27, 136–143. [Google Scholar] [CrossRef]
- Sutkeviciute, I.; Vilardaga, J.P. Structural insights into emergent signaling modes of G protein-coupled receptors. J. Biol. Chem. 2020, 295, 11626–11642. [Google Scholar] [CrossRef]
- Huang, W.; Masureel, M.; Qu, Q.; Janetzko, J.; Inoue, A.; Kato, H.E.; Robertson, M.J.; Nguyen, K.C.; Glenn, J.S.; Skiniotis, G.; et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 2020, 579, 303–308. [Google Scholar] [CrossRef]
- Lee, Y.; Warne, T.; Nehmé, R.; Pandey, S.; Dwivedi-Agnihotri, H.; Chaturvedi, M.; Edwards, P.C.; García-Nafría, J.; Leslie, A.G.W.; Shukla, A.K.; et al. Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor. Nature 2020, 583, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.G.; DeVree, B.T.; Zou, Y.; Kruse, A.C.; Chung, K.Y.; Kobilka, T.S.; Thian, F.S.; Chae, P.S.; Pardon, E.; Calinski, D.; et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 2011, 477, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B. Novel Therapies for Heart Failure—Where Do They Stand? Circ. J. 2016, 80, 1882–1891. [Google Scholar] [CrossRef]
- Viscusi, E.R.; Webster, L.; Kuss, M.; Daniels, S.; Bolognese, J.A.; Zuckerman, S.; Soergel, D.G.; Subach, R.A.; Cook, E.; Skobieranda, F. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain 2016, 157, 264–272. [Google Scholar] [CrossRef] [PubMed]
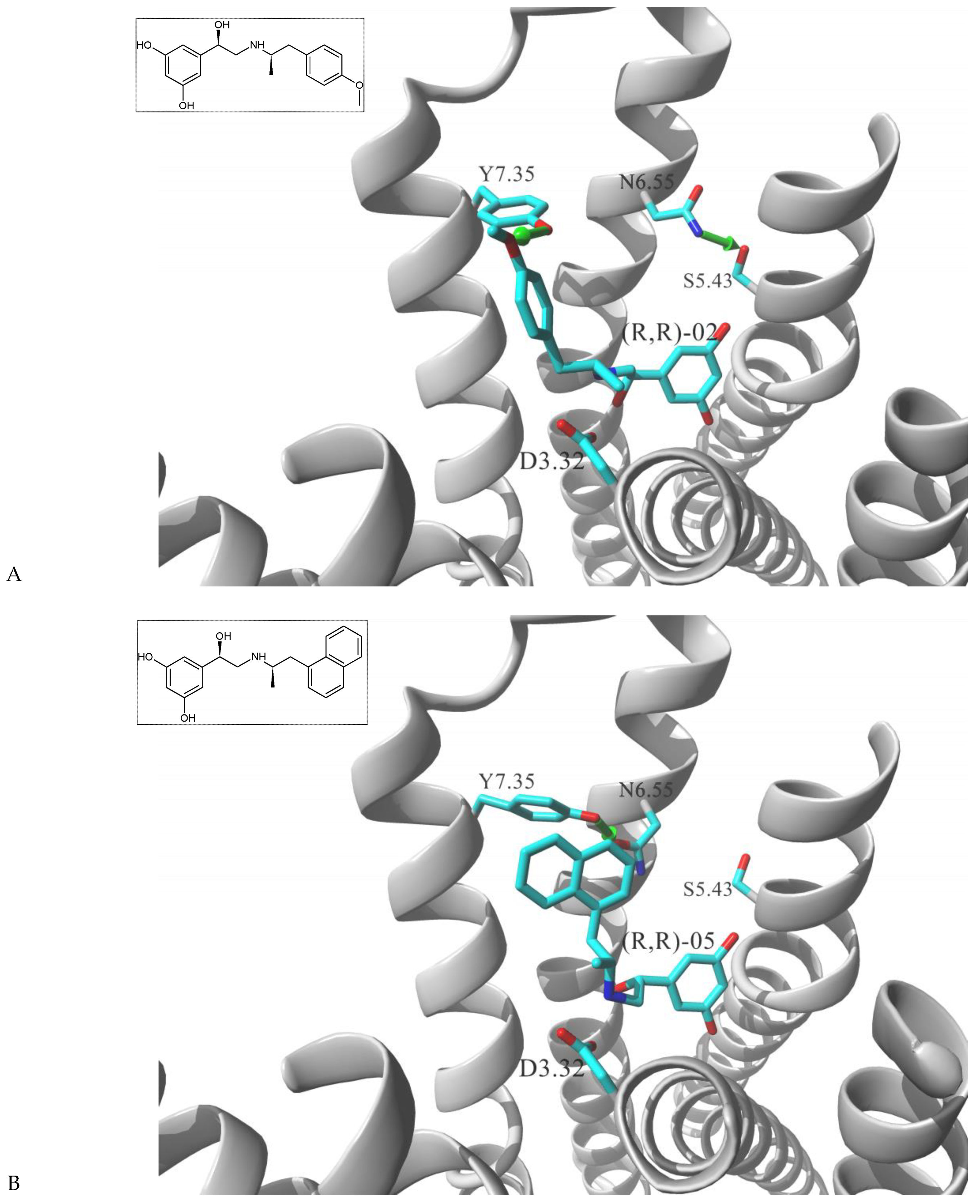
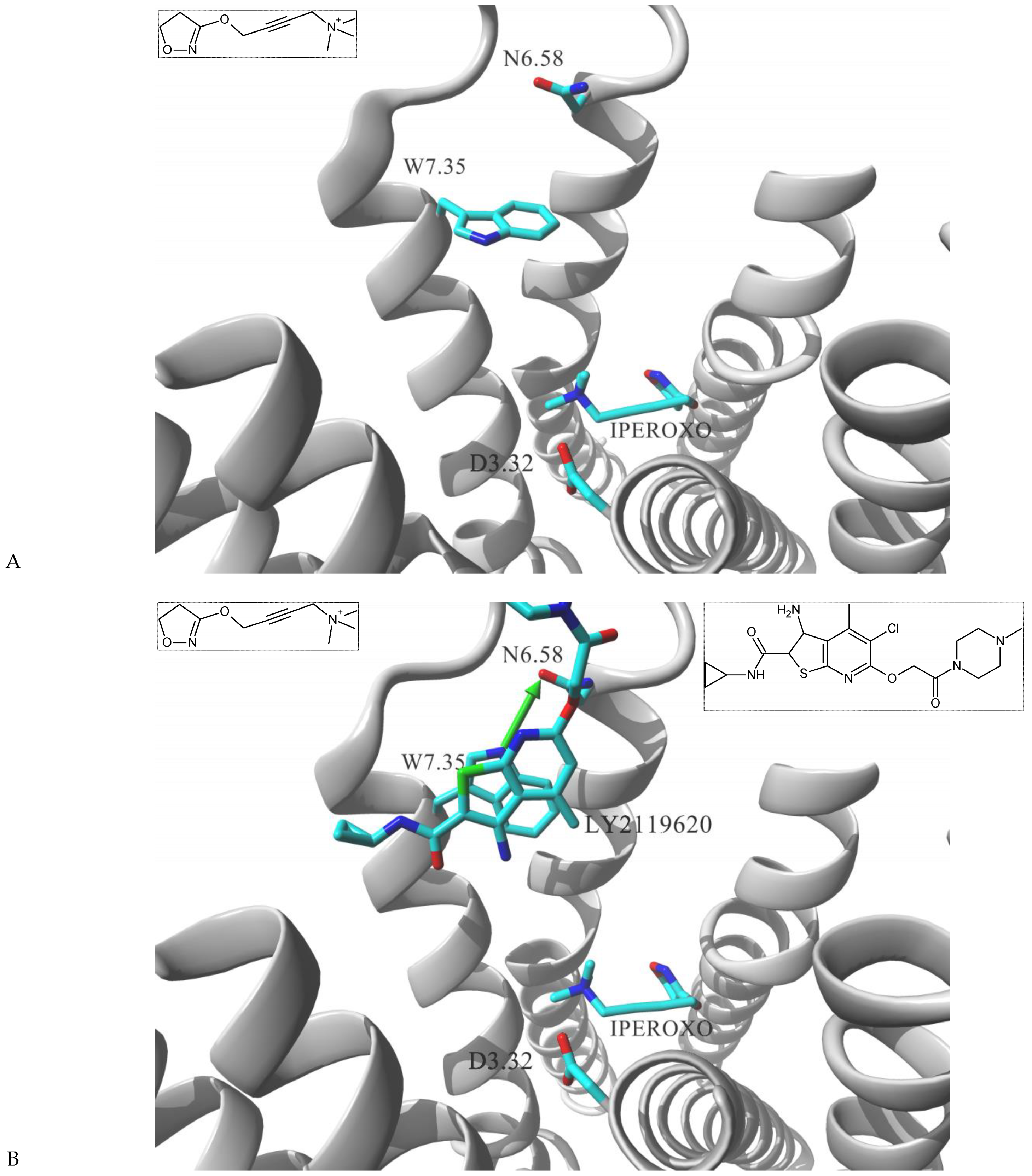
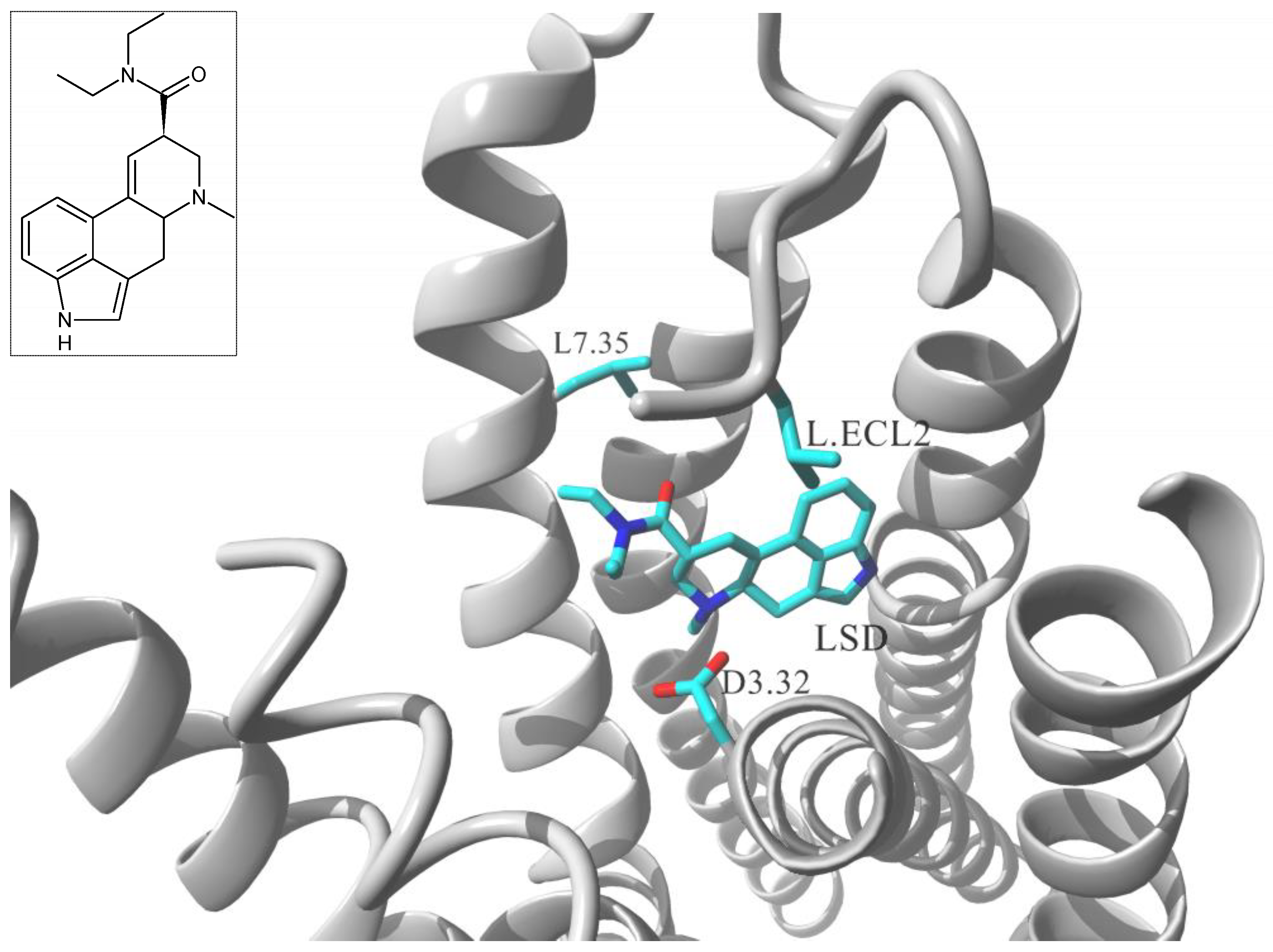
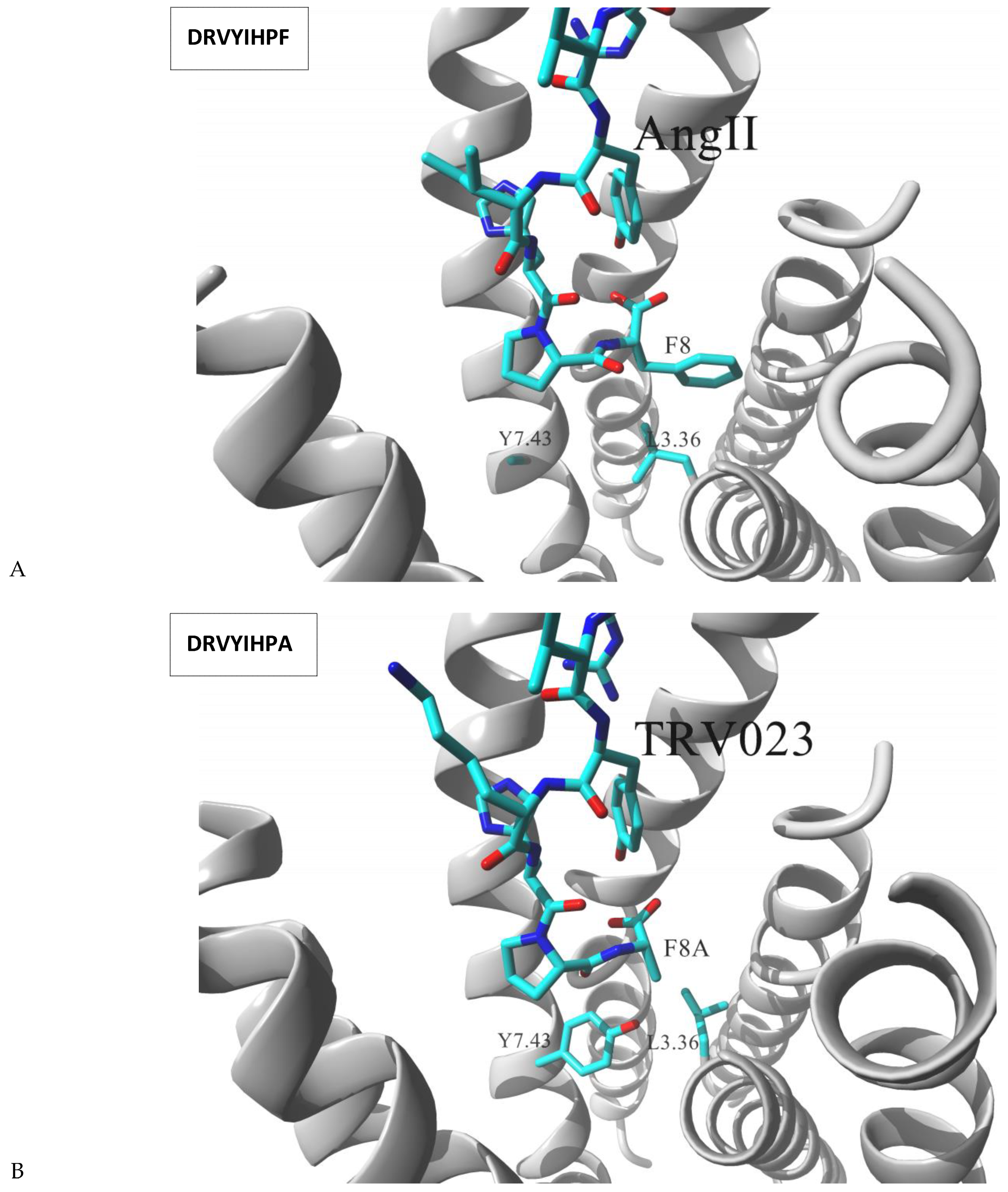
| Gs Signaling Ligands (PTx Insensitive) | [Gs+Gi] Signaling Ligands (PTx Sensitive) |
|---|---|
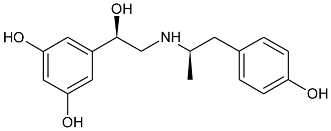 (R,R)-01 (R,R)-01 | 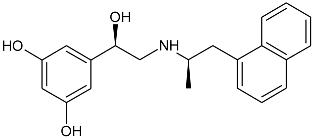 (R,R)-05 (R,R)-05 |
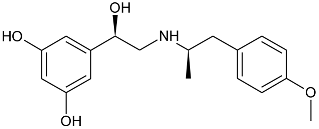 (R,R)-02 (R,R)-02 | 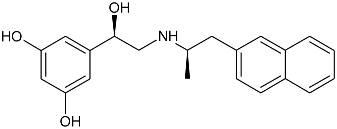 (R,R)-06 (R,R)-06 |
 (R,R)-03 (R,R)-03 | 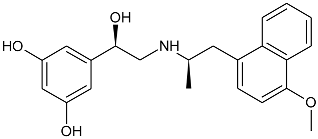 (R,R)-07 (R,R)-07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak, K.; Płazińska, A. Structural Insights into Ligand—Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors. Molecules 2021, 26, 851. https://doi.org/10.3390/molecules26040851
Jóźwiak K, Płazińska A. Structural Insights into Ligand—Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors. Molecules. 2021; 26(4):851. https://doi.org/10.3390/molecules26040851
Chicago/Turabian StyleJóźwiak, Krzysztof, and Anita Płazińska. 2021. "Structural Insights into Ligand—Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors" Molecules 26, no. 4: 851. https://doi.org/10.3390/molecules26040851
APA StyleJóźwiak, K., & Płazińska, A. (2021). Structural Insights into Ligand—Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors. Molecules, 26(4), 851. https://doi.org/10.3390/molecules26040851







