Neurotoxic Effect of Flavonol Myricetin in the Presence of Excess Copper
Abstract
1. Introduction
2. Results
2.1. Treatment with Myricetin Exacerbated Copper-Induced Decrease of Viability in SH-SY5Y Cells
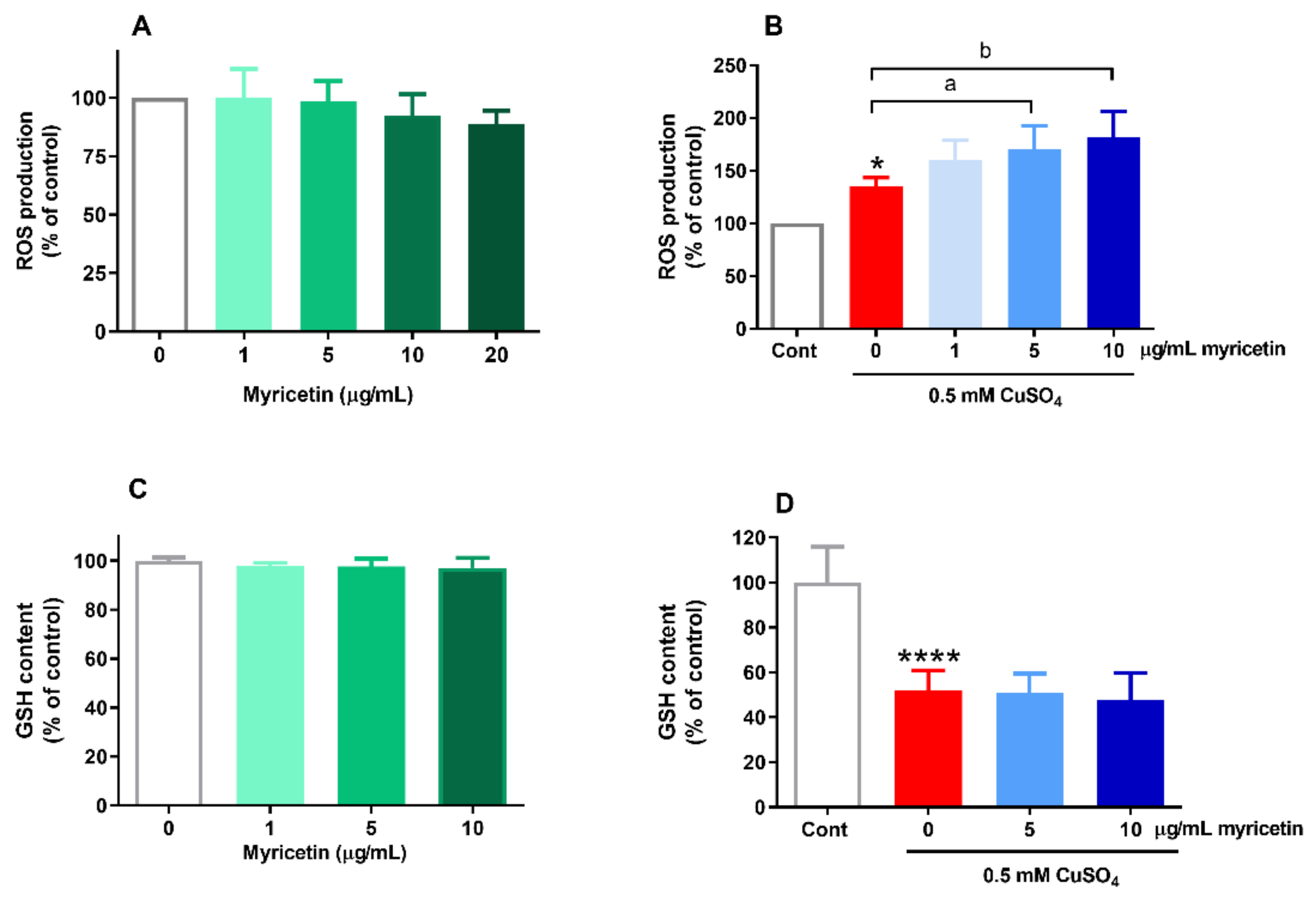
2.2. Effects of Myricetin on ROS Generation and GSH Content in the Presence of Excess Copper
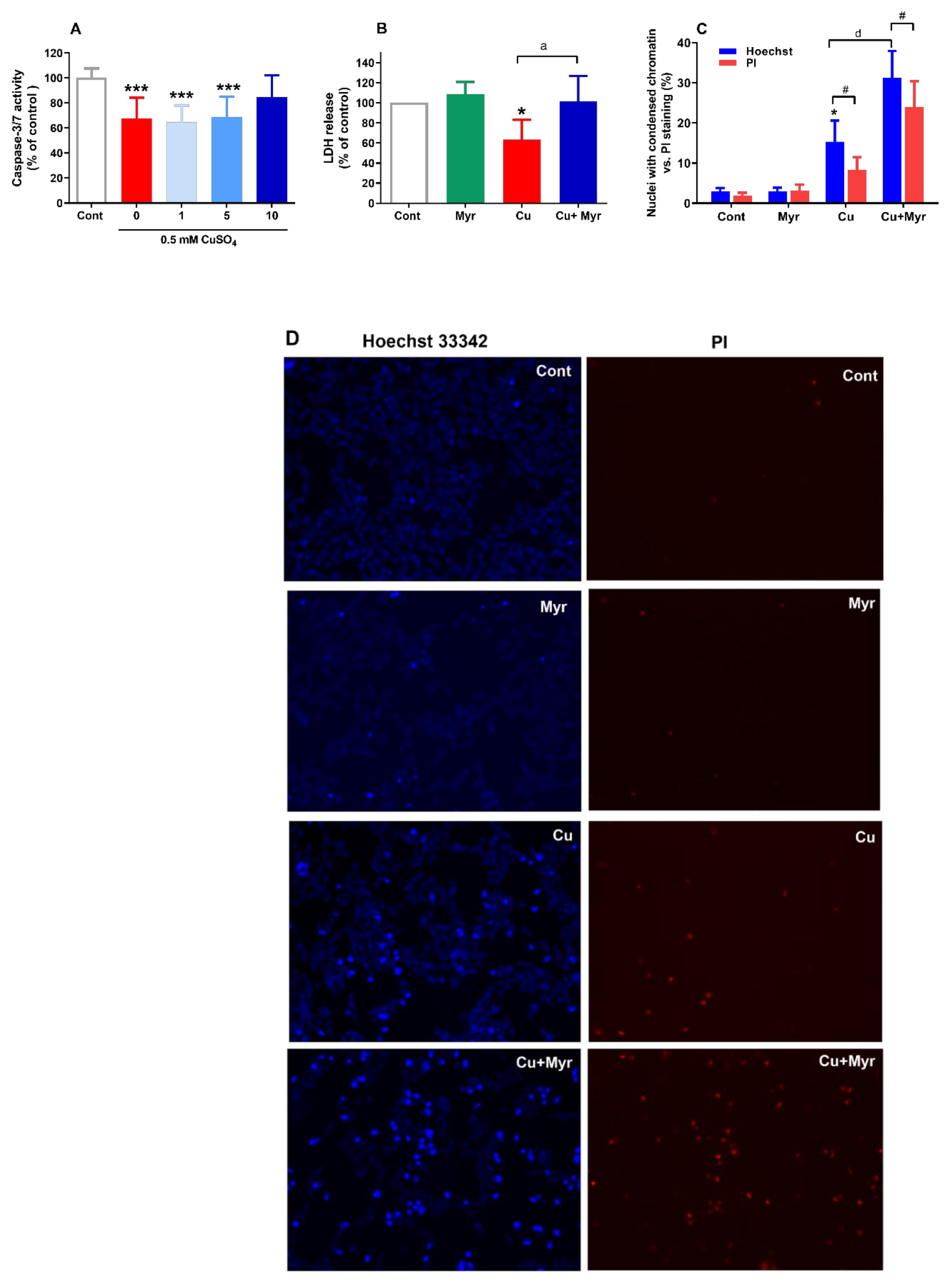
2.3. Myricetin Promoted Copper-Induced Changes in Chromatin Condensation and Stimulated LDH Release
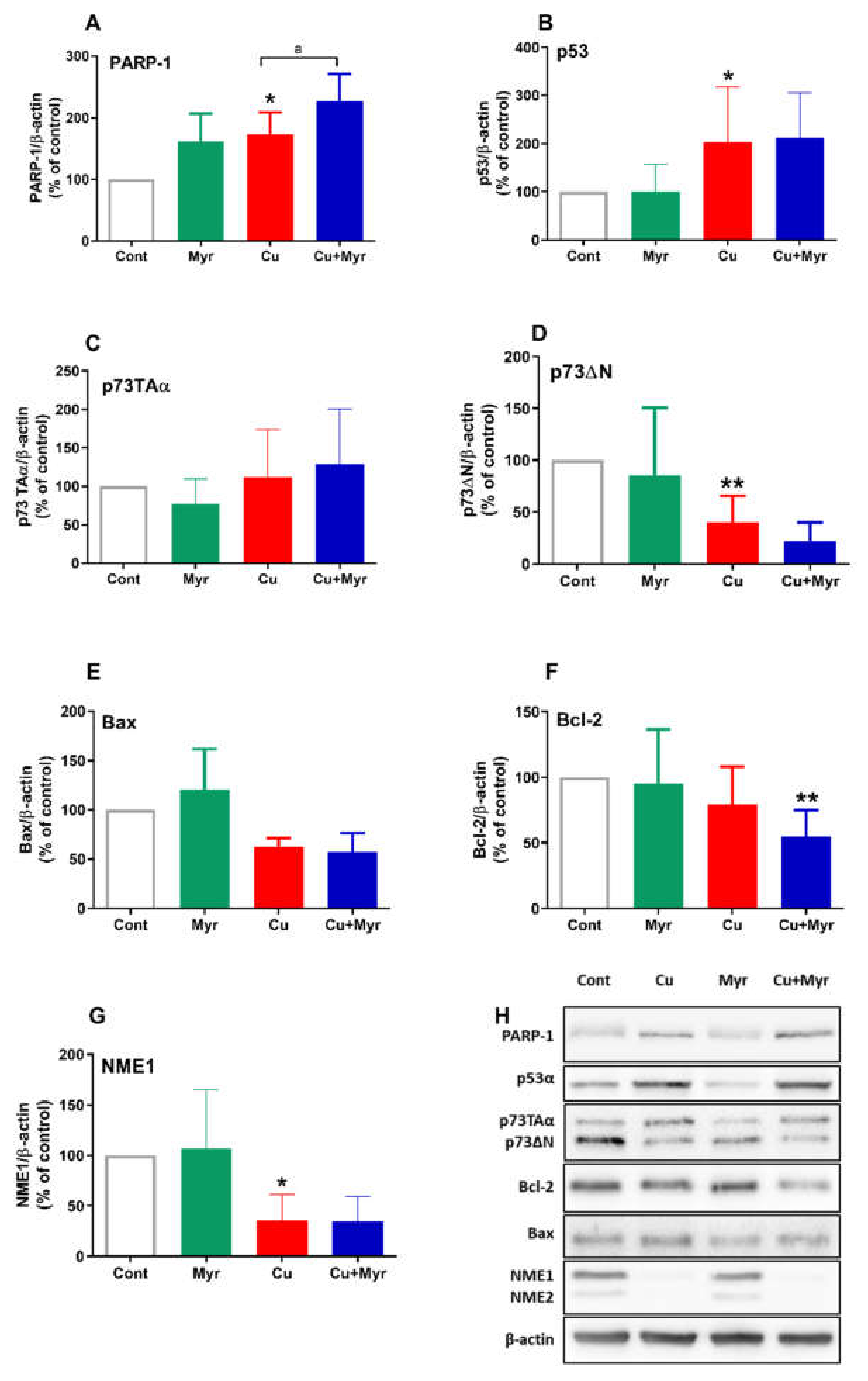
2.4. Effects of Copper and Myricetin on the Expression of Proteins Involved in Oxidative Response and Cell Death
2.5. Effects of the Inhibitors of OS-Related Signaling Pathways on Copper and Myricetin-Induced Neuronal Death
2.6. Effects of Myricetin and Copper on the Morphological Properties of SH-SY5Y Cells at the Nanoscale
2.7. Effects of Myricetin and Copper on the Nanomechanical Properties of Neuroblastoma SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Culturing of SH-SY5Y Cells
4.3. Drug Treatment
4.4. Assessment of Cell Viability
4.4.1. MTT Assay
4.4.2. Trypan Exclusion Assay
4.4.3. Determination of ATP Level
4.5. Measurement of Lactate Dehydrogenase (LDH) Release from SH-sY5Y Cells Exposed to Copper and Myricetin
4.6. Measurement of Intracellular ROS Accumulation
4.7. Determination of Reduced Glutathione (GSH) Levels
4.8. Determination of Caspase -3/7 Activity
4.9. Nuclear Staining with Hoechst 33342 and Propidium Iodide (PI)
4.10. Western Blot Analysis of PARP-1, p53, p73, Bcl-2, Bax and NME1/2 Protein Expression
4.11. AFM Measurements
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017, 2525967. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, Q.; Xu, H.; Du, X.; Shi, L.; Jia, F.; Jiang, H. Biometal dyshomeostasis and toxic metal accumulations in the development of Alzheimer’s disease. Front. Mol. Neurosci. 2017, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The role of copper in tau-related pathology in Alzheimer’s disease. Front. Mol. Neurosci. 2020, 13, 572308. [Google Scholar] [CrossRef]
- Zubčić, K.; Radovanović, V.; Vlainić, J.; Hof, P.R.; Oršolić, N.; Šimić, G.; Jazvinšćak Jembrek, M. PI3K/Akt and ERK1/2 signalling are involved in quercetin-mediated neuroprotection against copper-induced injury. Oxid. Med. Cell. Longev. 2020, 9834742. [Google Scholar] [CrossRef]
- Ventriglia, M.; Bucossi, S.; Panetta, V.; Squitti, R. Copper in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimers Dis. 2012, 30, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak Jembrek, M.; Vlainić, J.; Radovanović, V.; Erhardt, J.; Oršolić, N. Effects of copper overload in P19 neurons: Impairment of glutathione redox homeostasis and crosstalk between caspase and calpain protease systems in ROS-induced apoptosis. Biometals 2014, 27, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Mercer, J.F.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, M.E.; López-Alarcón, C.; Bridi, R.; Speisky, H. Redox-implications associated with the formation of complexes between copper ions and reduced or oxidized glutathione. J. Inorg. Biochem. 2016, 154, 78–88. [Google Scholar] [CrossRef]
- Alexandrova, A.; Kebis, A.; Misl’anová, C.; Kukan, M. Copper impairs biliary epithelial cells and induces protein oxidation and oxidative DNA damage in the isolated perfused rat liver. Exp. Toxicol. Pathol 2007, 58, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Saporito-Magriñá, C.M.; Musacco-Sebio, R.N.; Andrieux, G.; Kook, L.; Orrego, M.T.; Tuttolomondo, M.V.; Desimone, M.F.; Boerries, M.; Borner, C.; Repetto, M.G. Copper-induced cell death and the protective role of glutathione: The implication of impaired protein folding rather than oxidative stress. Metallomics 2018, 10, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Santoro, A.M.; Lanza, V.; Sbardella, D.; Tundo, G.R.; Ciaccio, C.; Marini, S.; Coletta, M.; Milardi, D. The double faced role of copper in Aβ homeostasis: A survey on the interrelationship between metal dyshomeostasis, UPS functioning and autophagy in neurodegeneration. Coord. Chem. Rev. 2017, 347, 1–22. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Slade, N.; Hof, P.R.; Šimić, G. The interactions of p53 with tau and Aß as potential therapeutic targets for Alzheimer’s disease. Prog. Neurobiol. 2018, 168, 104–127. [Google Scholar] [CrossRef]
- Alvarez-Arellano, L.; Salazar-García, M.; Corona, J.C. Neuroprotective effects of quercetin in pediatric neurological diseases. Molecules 2020, 25, 5597. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A switch between antioxidant and prooxidant properties of the phenolic compounds myricetin, morin, 3’,4’-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper(II) ions: A spectroscopic, absorption titration and DNA damage study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef]
- Radovanović, V.; Vlainić, J.; Hanžić, N.; Ukić, P.; Oršolić, N.; Baranović, G.; Jazvinšćak Jembrek, M. Neurotoxic effect of ethanolic extract of propolis in the presence of copper ions is mediated through enhanced production of ROS and stimulation of caspase-3/7 activity. Toxins 2019, 11, 273. [Google Scholar] [CrossRef]
- Sadžak, A.; Mravljak, J.; Maltar-Strmečki, N.; Arsov, Z.; Baranović, G.; Erceg, I.; Kriechbaum, M.; Strasser, V.; Přibyl, J.; Šegota, S. The structural integrity of the model lipid membrane during induced lipid peroxidation: The role of flavonols in the inhibition of lipid peroxidation. Antioxidants 2020, 9, 430. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Z.; Wang, J.; Xie, A.; Xie, J. Myricetin attenuated MPP(+)-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology 2011, 61, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Chobot, V.; Hadacek, F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 2011, 16, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Three distinct neuroprotective functions of myricetin against glutamate-induced neuronal cell death: Involvement of direct inhibition of caspase-3. J. Neurosci. Res. 2008, 86, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Multifunction of myricetin on Aβ: Neuroprotection via a conformational change of Aβ and reduction of Aβ via the interference of secretases. Neurosci. Res. 2008, 86, 368–377. [Google Scholar] [CrossRef]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate-induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Šimić, G.; Hof, P.R.; Šegota, S. Atomic force microscopy as an advanced tool in neuroscience. Transl. Neurosci. 2015, 6, 117–130. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Vlainić, J.; Čadež, V.; Šegota, S. Atomic force microscopy reveals new biophysical markers for monitoring subcellular changes in oxidative injury: Neuroprotective effects of quercetin at the nanoscale. PLoS ONE 2018, 13, e0200119. [Google Scholar] [CrossRef]
- Pamp, K.; Bramey, T.; Kirsch, M.; De Groot, H.; Petrat, F. NAD(H) enhances the Cu(II)-mediated inactivation of lactate dehydrogenase by increasing the accessibility of sulfhydryl groups. Free Radic. Res. 2005, 39, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.B.; Kinoshita, C.; Kinoshita, Y.; Morrison, R.S. p53 and mitochondrial function in neurons. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 1186–1197. [Google Scholar] [CrossRef]
- Puts, G.S.; Leonard, M.K.; Pamidimukkala, N.V.; Snyder, D.E.; Kaetzel, D.M. Nuclear functions of NME proteins. Lab. Investig. 2018, 98, 211–218. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Vuković, L.; Puhović, J.; Erhardt, J.; Oršolić, N. Neuroprotective effect of quercetin against hydrogen peroxide-induced oxidative injury in P19 neurons. J. Mol. Neurosci. 2012, 47, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: Role of ERK1/2 in H2O2-induced cell death. Eur. J. Pharmacol. 2004, 483, 163–173. [Google Scholar] [CrossRef]
- Cater, M.A.; Materia, S.; Xiao, Z.; Wolyniec, K.; Ackland, S.M.; Yap, Y.W.; Cheung, N.S.; La Fontaine, S. Glutaredoxin1 protects neuronal cells from copper-induced toxicity. Biometals 2014, 27, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Borchard, S.; Bork, F.; Rieder, T.; Eberhagen, C.; Popper, B.; Lichtmannegger, J.; Schmitt, S.; Adamski, J.; Klingenspor, M.; Weiss, K.H.; et al. The exceptional sensitivity of brain mitochondria to copper. Toxicol. In Vitro 2018, 51, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Gray, G.C. Interactions of flavonoids, trace metals, and oxygen: Nuclear DNA damage and lipid peroxidation induced by myricetin. Cancer Lett. 1993, 70, 73–79. [Google Scholar] [CrossRef]
- Yoshino, M.; Haneda, M.; Naruse, M.; Murakami, K. Prooxidant activity of flavonoids: Copper-dependent strand breaks and the formation of 8-hydroxy-2’-deoxyguanosine in DNA. Mol. Genet. Metab. 1999, 68, 468–472. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Glutathione in cellular redox homeostasis: Association with the excitatory amino acid carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Boots, A.W.; Li, H.; Schins, R.P.; Duffin, R.; Heemskerk, J.W.; Bast, A.; Haenen, G.R. The quercetin paradox. Toxicol. Appl. Pharmacol. 2007, 222, 89–96. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.E. Biological effects of myricetin. Gen. Pharmacol. 1997, 29, 121–126. [Google Scholar] [CrossRef]
- Trejo-Solís, C.; Jimenez-Farfan, D.; Rodriguez-Enriquez, S.; Fernandez-Valverde, F.; Cruz-Salgado, A.; Ruiz-Azuara, L.; Sotelo, J. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer 2012, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper induces oxidative stress and apoptosis in the mouse liver. Oxid. Med. Cell. Longev. 2020, 1359164. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.L.; Chan, K.M. Oxidative stress and apoptotic effects of copper and cadmium in the zebrafish liver cell line ZFL. Toxicol. Rep. 2020, 7, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhao, J.; Bulek, K.; Tang, F.; Chen, X.; Cai, G.; Jia, S.; Fox, P.L.; Huang, E.; Pizarro, T.T.; et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat. Commun. 2020, 11, 900. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Marchenko, N.D.; Ji, K.; Tsirka, S.E.; Holzmann, S.; Moll, U.M. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 2012, 149, 1536–1548. [Google Scholar] [CrossRef]
- Schwerdtle, T.; Hamann, I.; Jahnke, G.; Walter, I.; Richter, C.; Parsons, J.L.; Dianov, G.L.; Hartwig, A. Impact of copper on the induction and repair of oxidative DNA damage, poly(ADP-ribosyl)ation and PARP-1 activity. Mol. Nutr. Food Res. 2007, 51, 201–210. [Google Scholar] [CrossRef]
- Ko, H.L.; Ren, E.C. Functional aspects of PARP1 in DNA repair and transcription. Biomolecules 2012, 2, 524–548. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Umanah, G.K.; Chang, C.; Stevens, D.A.; Karuppagounder, S.S.; Gagné, J.P.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 10209–10214. [Google Scholar] [CrossRef]
- Tajuddin, N.; Kim, H.Y.; Collins, M.A. PARP inhibition prevents ethanol-induced neuroinflammatory Signaling and neurodegeneration in rat adult-age brain slice cultures. J. Pharmacol. Exp. Ther. 2018, 365, 117–126. [Google Scholar] [CrossRef]
- Dong, K.; Yan, Y.; Lu, L.; Wang, Y.; Li, J.; Zhang, M.; Ding, J. PJ34 protects photoreceptors from cell death by inhibiting PARP-1 induced parthanatos after experimental retinal detachment. Curr. Eye Res. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Gerace, E.; Masi, A.; Resta, F.; Felici, R.; Landucci, E.; Mello, T.; Pellegrini-Giampietro, D.E.; Mannaioni, G.; Moroni, F. PARP-1 activation causes neuronal death in the hippocampal CA1 region by increasing the expression of Ca(2+)-permeable AMPA receptors. Neurobiol. Dis. 2014, 70, 43–52. [Google Scholar] [CrossRef]
- Yang, J.L.; Lin, Y.T.; Chen, W.Y.; Yang, Y.R.; Sun, S.F.; Chen, S.D. The neurotrophic function of glucagon-like peptide-1 promotes human neuroblastoma differentiation via the PI3K-AKT axis. Biology 2020, 9, 348. [Google Scholar] [CrossRef]
- Culmsee, C.; Mattson, M.P. p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 2005, 331, 761–777. [Google Scholar] [CrossRef]
- Salech, F.; Ponce, D.P.; SanMartín, C.D.; Rogers, N.K.; Chacón, C.; Henríquez, M.; Behrens, M.I. PARP-1 and p53 regulate the increased susceptibility to oxidative death of lymphocytes from MCI and AD patients. Front. Aging Neurosci. 2017, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Abrahamse, H. Increased oxidative stress induced by Rubus bioactive compounds induce apoptotic cell death in human breast cancer cells. Oxid. Med. Cell. Longev. 2019, 6797921. [Google Scholar] [CrossRef] [PubMed]
- Vetrivel, P.; Kim, S.M.; Saralamma, V.; Ha, S.E.; Kim, E.H.; Min, T.S.; Kim, G.S. Function of flavonoids on different types of programmed cell death and its mechanism: A review. J. Biomed. Res. 2019, 33, 363–370. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Pozniak, C.D.; Radinovic, S.; Yang, A.; McKeon, F.; Kaplan, D.R.; Miller, F.D. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 2000, 289, 304–306. [Google Scholar] [CrossRef]
- Maisse, C.; Munarriz, E.; Barcaroli, D.; Melino, G.; De Laurenzi, V. DNA damage induces the rapid and selective degradation of the DeltaNp73 isoform, allowing apoptosis to occur. Cell Death Differ. 2004, 11, 685–6877. [Google Scholar] [CrossRef] [PubMed]
- Pozniak, C.D.; Barnabé-Heider, F.; Rymar, V.V.; Lee, A.F.; Sadikot, A.F.; Miller, F.D. p73 is required for survival and maintenance of CNS neurons. J. Neurosci. 2002, 22, 9800–9809. [Google Scholar] [CrossRef]
- Peuchant, E.; Bats, M.L.; Moranvillier, I.; Lepoivre, M.; Guitton, J.; Wendum, D.; Lacombe, M.L.; Moreau-Gaudry, F.; Boissan, M.; Dabernat, S. Metastasis suppressor NM23 limits oxidative stress in mammals by preventing activation of stress-activated protein kinases/JNKs through its nucleoside diphosphate kinase activity. FASEB J. 2017, 31, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.; Boulos, S.; Chieng, J.; Knuckey, N.W.; Meloni, B.P. Erythropoietin increases neuronal NDPKA expression, and NDPKA up-regulation as well as exogenous application protects cortical neurons from in vitro ischemia-related insults. Cell. Mol. Neurobiol. 2014, 34, 379–392. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal. Transduct. 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Aihara, K.; Kuroda, S.; Kanayama, N.; Matsuyama, S.; Tanizawa, K.; Horie, M. A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res. Mol. Brain Res. 2003, 116, 86–93. [Google Scholar] [CrossRef]
- Zheng, L.; Ishii, Y.; Tokunaga, A.; Hamashima, T.; Shen, J.; Zhao, Q.L.; Ishizawa, S.; Fujimori, T.; Nabeshima, Y.; Mori, H.; et al. Neuroprotective effects of PDGF against oxidative stress and the signaling pathway involved. J. Neurosci. Res. 2010, 88, 1273–1284. [Google Scholar] [CrossRef]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, X.; Mo, S.; Qin, J.; Li, W.; Liu, P.; Han, Y.; Pi, R. p38 and ERK, but not JNK, are involved in copper-induced apoptosis in cultured cerebellar granule neurons. Biochem. Biophys. Res. Commun. 2009, 379, 944–948. [Google Scholar] [CrossRef]
- Grewal, S.S.; Fass, D.M.; Yao, H.; Ellig, C.L.; Goodman, R.H.; Stork, P.J. Calcium and cAMP signals differentially regulate cAMP-responsive element-binding protein function via a Rap1-extracellular signal-regulated kinase pathway. J. Biol. Chem. 2000, 275, 34433–34441. [Google Scholar] [CrossRef]
- Kumamoto, T.; Fujii, M.; Hou, D.X. Akt is a direct target for myricetin to inhibit cell transformation. Mol. Cell. Biochem. 2009, 332, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Rotsch, C.; Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys. J. 2000, 78, 520–535. [Google Scholar] [CrossRef]
- Vileno, B.; Sienkiewicz, A.; Lekka, M.; Kulik, A.; Forro, L. In vitro assay of singlet oxygen generation in the presence of water-soluble derivatives of C60. Carbon 2004, 42, 1195–1198. [Google Scholar] [CrossRef]
- Fang, Y.; Iu, C.Y.; Lui, C.N.; Zou, Y.; Fung, C.K.; Li, H.W.; Xi, N.; Yung, K.K.; Lai, K.W. Investigating dynamic structural and mechanical changes of neuroblastoma cells associated with glutamate-mediated neurodegeneration. Sci. Rep. 2014, 4, 7074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Stamm, A.; Lee, J.S.; Gruverman, A.; Lim, J.Y.; Gu, L. Elasticity of differentiated and undifferentiated human neuroblastoma cells characterized by atomic force microscopy. J. Mech. Med. Biol. 2015, 15, 1550069. [Google Scholar] [CrossRef]
- Pastrana, H.F.; Cartagena-Rivera, A.X.; Raman, A.; Ávila, A. Evaluation of the elastic Young’s modulus and cytotoxicity variations in fibroblasts exposed to carbon-based nanomaterials. J. Nanobiotechnol. 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S.; Schnellmann, R.G. Measurement of cell death in mammalian cells. Curr. Protoc. Pharmacol. 2013. Chapter 12: Unit 12.8. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Clifford, C.A.; Seah, M.P. Improved methods and uncertainty analysis in the calibration of the spring constant of an atomic force microscope cantilever using static experimental methods. Meas. Sci. Technol. 2009, 20, 125501. [Google Scholar] [CrossRef]
- Roa, J.J.; Oncins, G.; Diaz, J.; Sanz, F.; Segarra, M. Calculation of Young’s modulus value by means of AFM. Recent Pat. Nanotechmol. 2011, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]



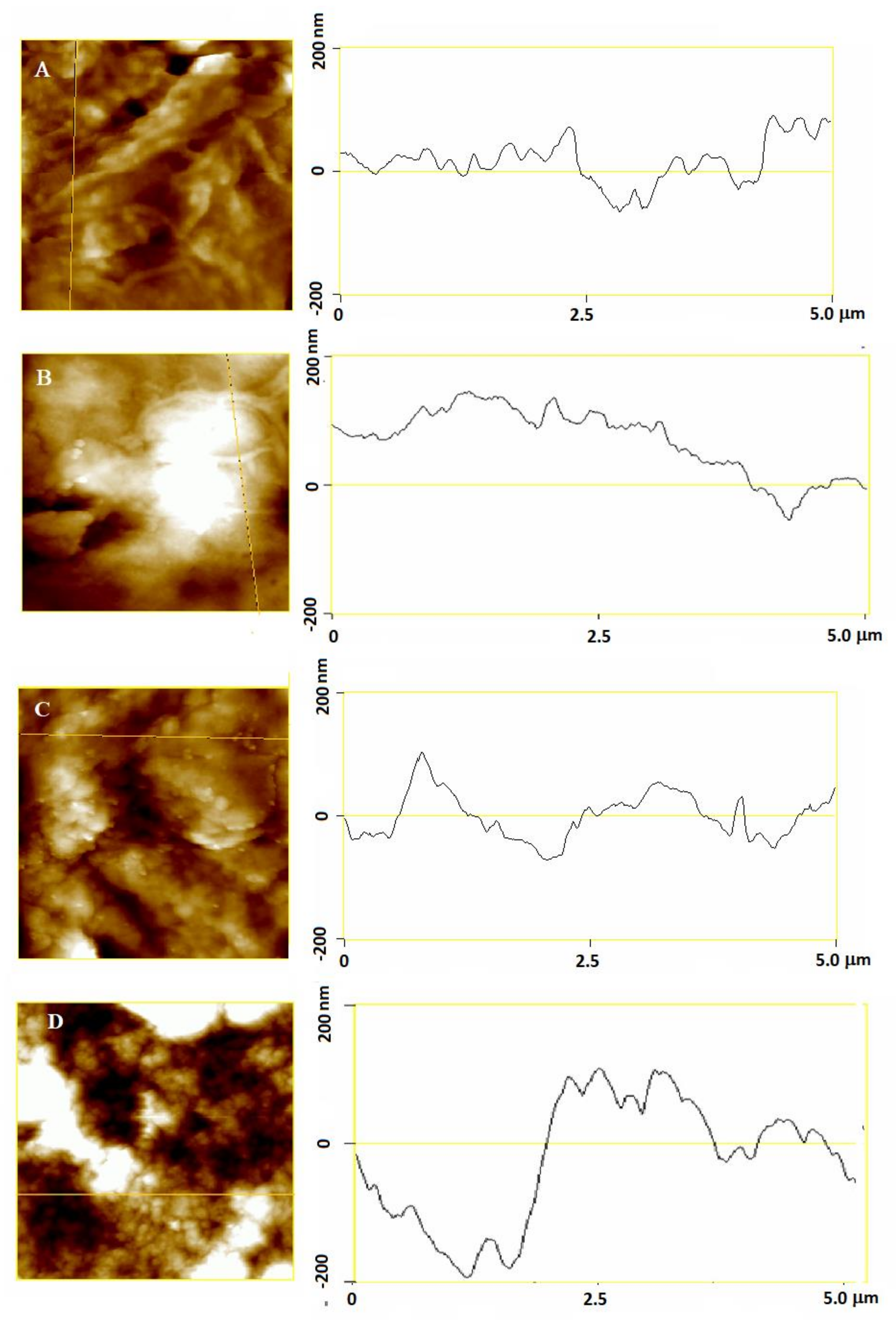

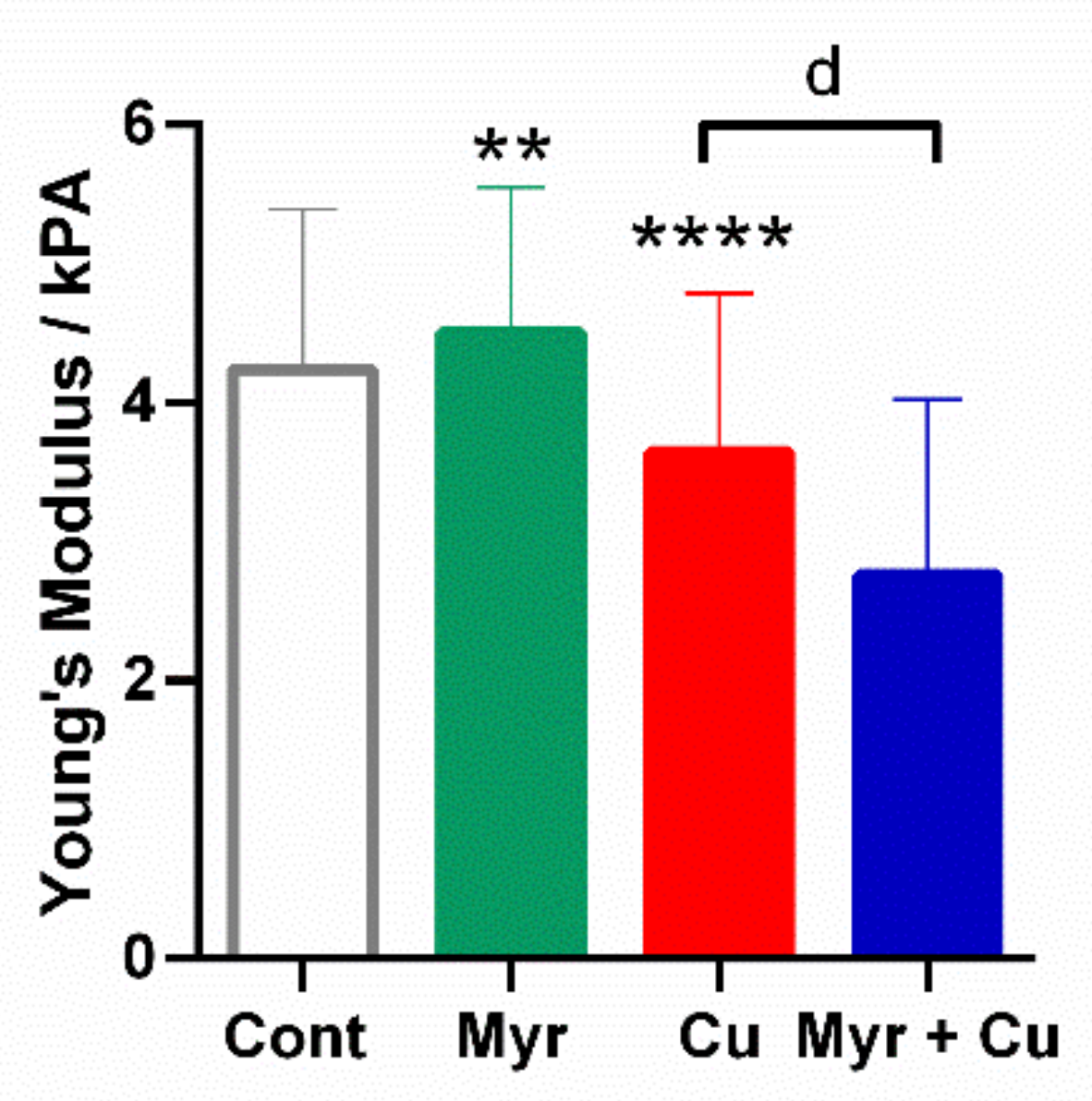
| Treatment | ||||
|---|---|---|---|---|
| Control Cells | Myricetin (10 μg/mL) | Cu2+ (0.5 mM CuSO4) | Myricetin (10 μg/mL) + Cu2+ (0.5 mM CuSO4) | |
| Z range (Ncell = 8) | 353 ± 227 | 389 ± 190 | 514 ± 123 | 540 ± 196 |
| Rq/nm (Ncell = 8) | 47 ± 27 | 44 ± 34 | 66 ± 27 | 89 ± 45 |
| Ra/nm (Ncell = 8) | 33 ± 16 | 35 ± 28 | 54 ± 23 | 71 ± 35 |
| E (Ncel L = 8)/kPa | 4.2 ± 1.1 | 4.5 ± 1.1 | 3.7 ± 1.1 | 2.7 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadžak, A.; Vlašić, I.; Kiralj, Z.; Batarelo, M.; Oršolić, N.; Jazvinšćak Jembrek, M.; Kušen, I.; Šegota, S. Neurotoxic Effect of Flavonol Myricetin in the Presence of Excess Copper. Molecules 2021, 26, 845. https://doi.org/10.3390/molecules26040845
Sadžak A, Vlašić I, Kiralj Z, Batarelo M, Oršolić N, Jazvinšćak Jembrek M, Kušen I, Šegota S. Neurotoxic Effect of Flavonol Myricetin in the Presence of Excess Copper. Molecules. 2021; 26(4):845. https://doi.org/10.3390/molecules26040845
Chicago/Turabian StyleSadžak, Anja, Ignacija Vlašić, Zoran Kiralj, Marijana Batarelo, Nada Oršolić, Maja Jazvinšćak Jembrek, Ines Kušen, and Suzana Šegota. 2021. "Neurotoxic Effect of Flavonol Myricetin in the Presence of Excess Copper" Molecules 26, no. 4: 845. https://doi.org/10.3390/molecules26040845
APA StyleSadžak, A., Vlašić, I., Kiralj, Z., Batarelo, M., Oršolić, N., Jazvinšćak Jembrek, M., Kušen, I., & Šegota, S. (2021). Neurotoxic Effect of Flavonol Myricetin in the Presence of Excess Copper. Molecules, 26(4), 845. https://doi.org/10.3390/molecules26040845










