Recent Advances in Scaffolding from Natural-Based Polymers for Volumetric Muscle Injury
Abstract
1. Introduction
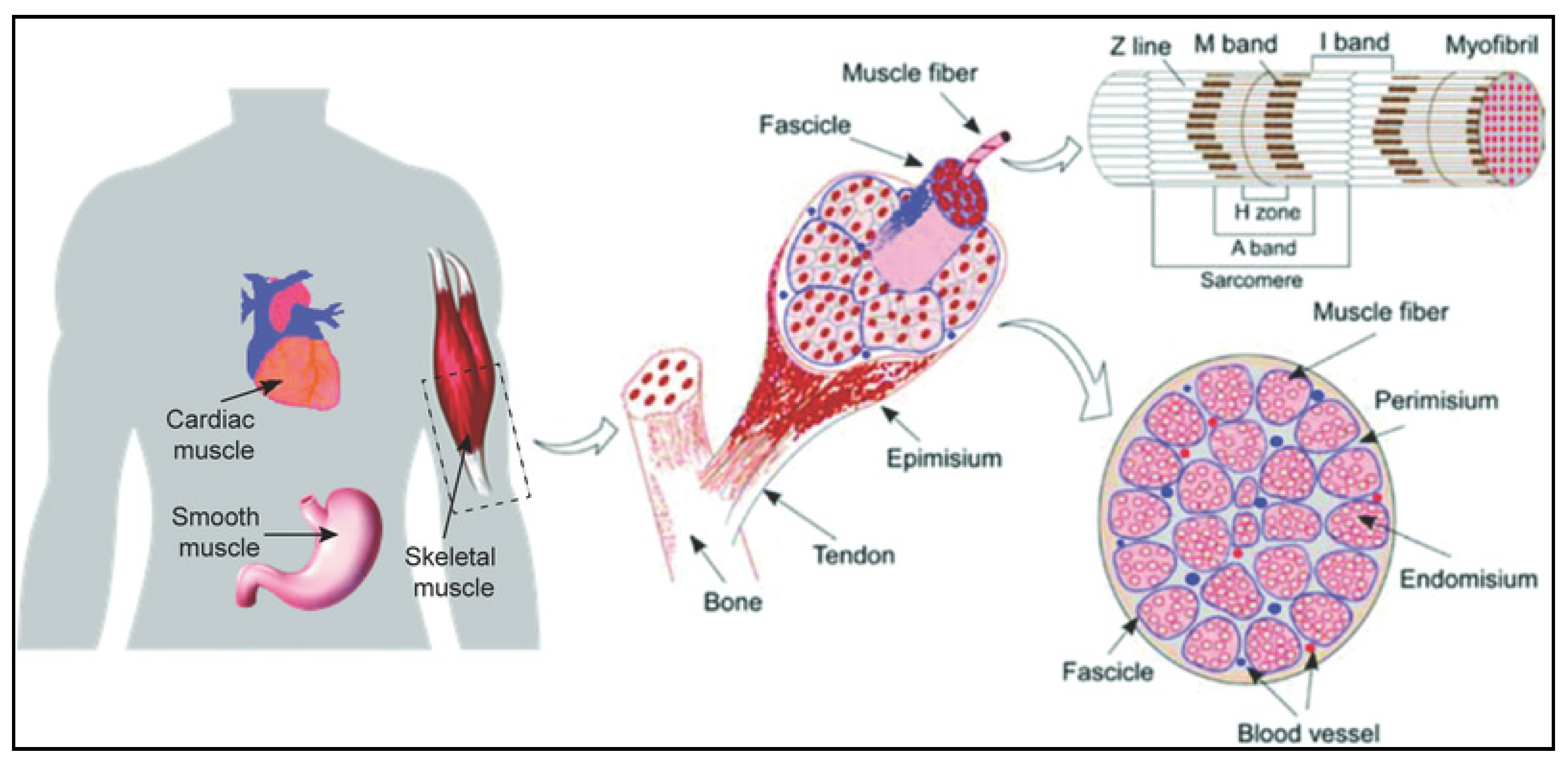
2. Structure and Organization of Skeletal Muscle Tissue
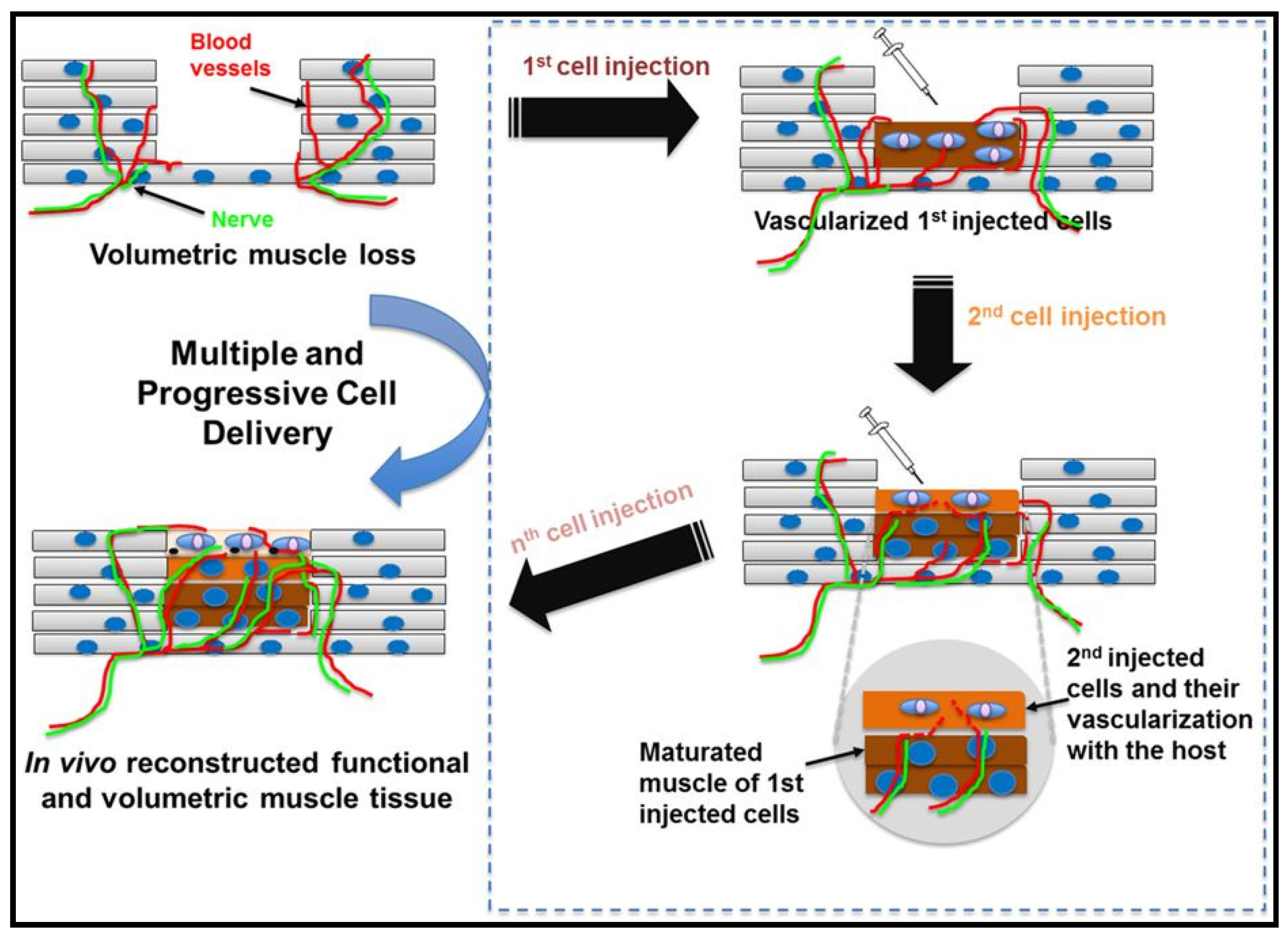
3. Volumetric Muscle Loss (VML)
4. Tissue Engineering Approaches for VML
4.1. Cell Based Therapies for Muscle Injury
4.1.1. Embryonic Stem Cells (ESCs)
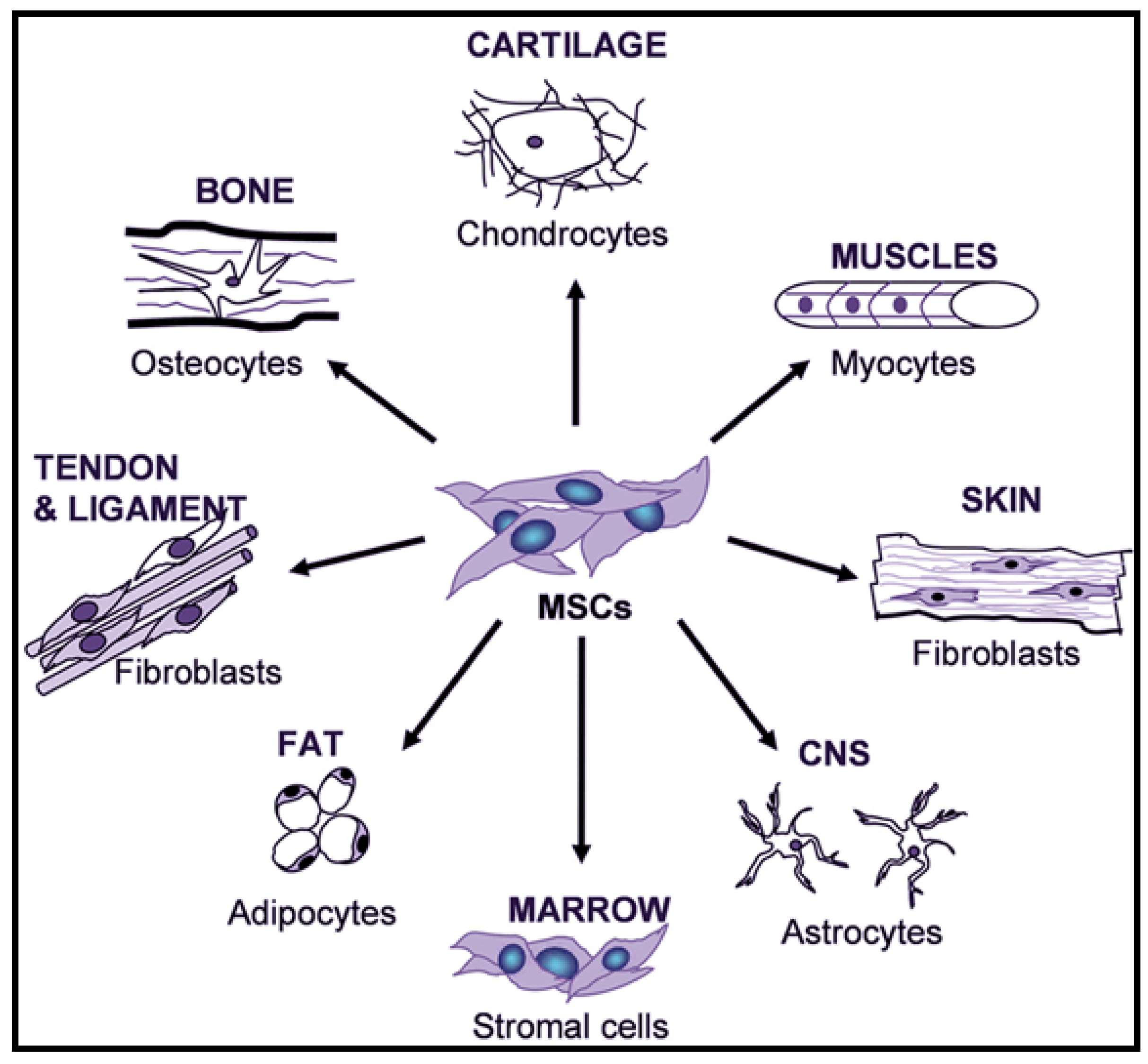
4.1.2. Adult Mesenchymal Stem Cells (MSCs)
4.1.3. Amniotic Fluid Stem Cells (AFSCs)
4.1.4. Induced Pluripotent Stem Cells (iPSC)
4.2. Tissue Engineering Scaffolding
5. Engineered Natural Polymeric Scaffolds for Muscle Regeneration
- Biocompatible
- Good mechanical properties to resist cells microenvironment stress
- Bioresorbable
- Tuneable Degradation
- Highly porous to allow cell infiltration and nutrient delivery
- Appropriate pore size for cell growth
- Conducive surface for cell attachment
- Able to functionalize to bioactive signals for favorable cellular interactions.
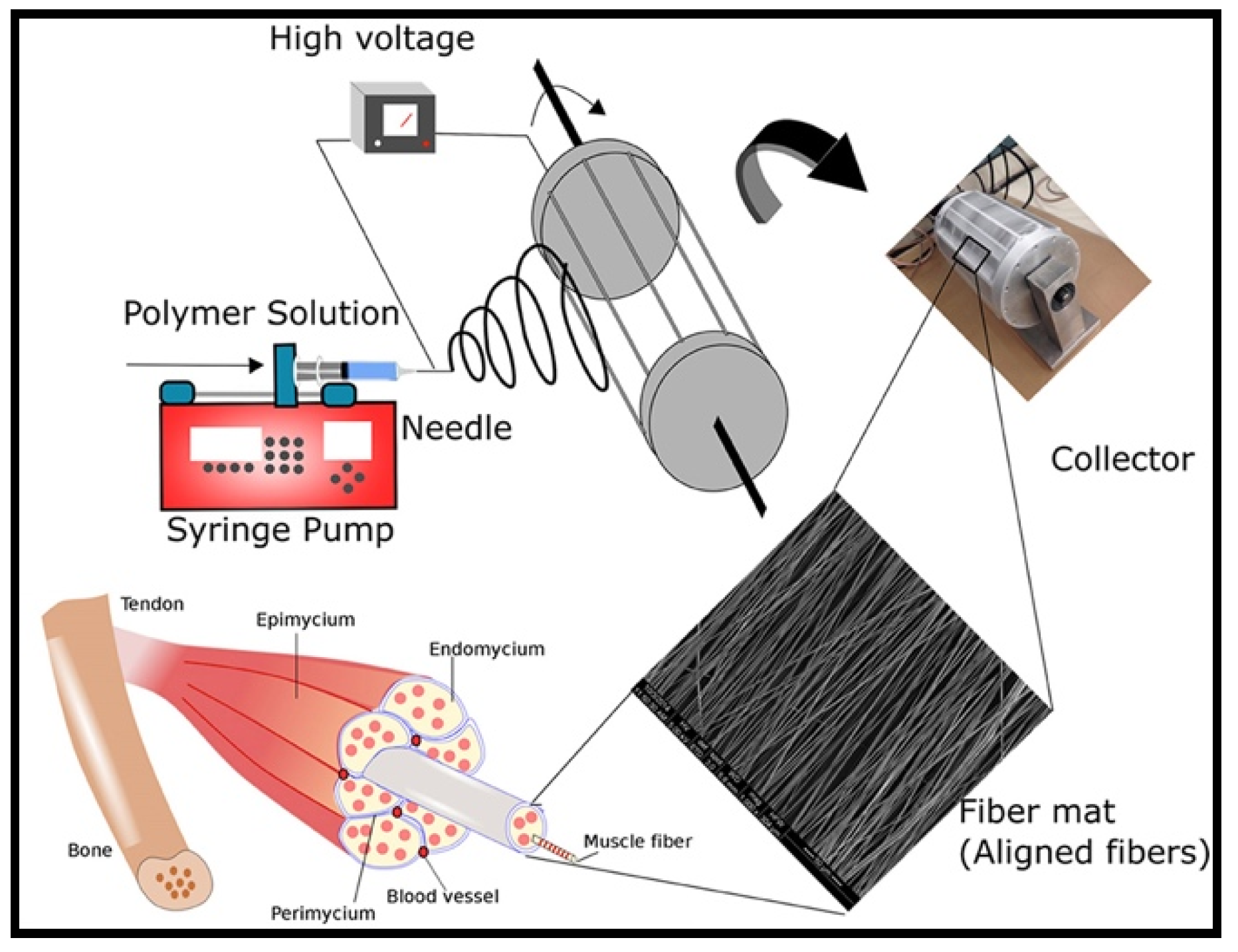
5.1. Electrospun Scaffolds
5.2. Hydrogels
5.3. Acellular Scaffolds
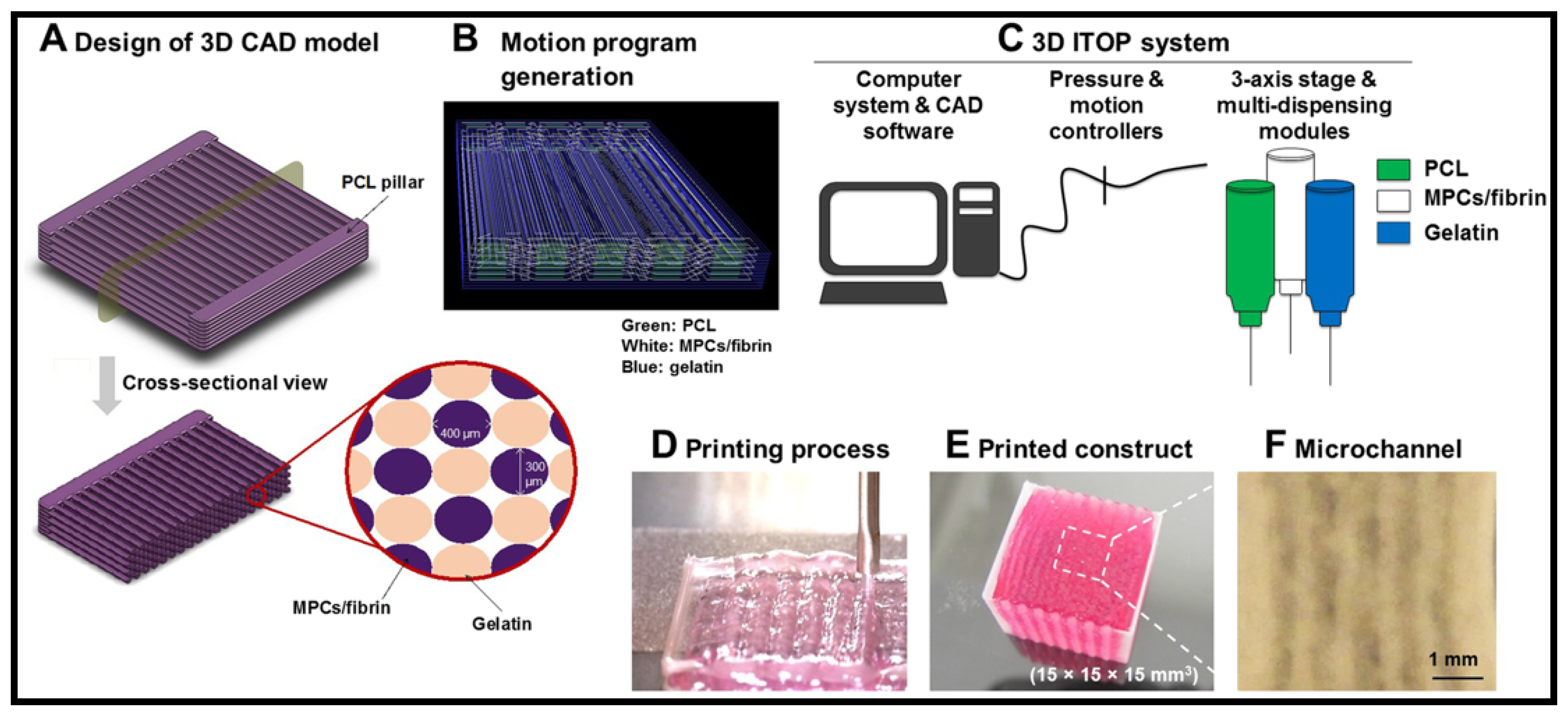
5.4. D Bioprinting
6. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cossu, G.; Sampaolesi, M. New therapies for Duchenne muscular dystrophy: Challenges, prospects and clinical trials. Trends Mol. Med. 2007, 13, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Kheradmandi, M.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Ganji, F. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res. Part A 2016, 104, 1720–1727. [Google Scholar] [CrossRef]
- Zammit, P. Kinetics of Myoblast Proliferation Show That Resident Satellite Cells Are Competent to Fully Regenerate Skeletal Muscle Fibers. Exp. Cell Res. 2002, 281, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ko, I.K.; Atala, A.; Yoo, J.J. Progressive muscle cell delivery as a solution for volumetric muscle defect repair. Sci. Rep. 2016, 6, 38754. [Google Scholar] [CrossRef]
- Greising, S.M.; Rivera, J.C.; Goldman, S.M.; Watts, A.; Aguilar, C.A.; Corona, B.T. Unwavering Pathobiology of Volumetric Muscle Loss Injury. Sci. Rep. 2017, 7, 13179. [Google Scholar] [CrossRef]
- Corona, B.T.; Flanagan, K.E.; Brininger, C.M.; Goldman, S.M.; Call, J.A.; Greising, S.M. Impact of volumetric muscle loss injury on persistent motoneuron axotomy. Muscle Nerve 2018, 57, 799–807. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Ward, C.L.; Garg, K.; Pollot, B.E.; Goldma, S.M.; McKinley, T.O.; Wenke, J.C.; Corona, B.T. Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing. J. Musculoskelet. Neuronal Interact. 2016, 16, 122–134. [Google Scholar]
- Corona, B.T.; Rivera, J.C.; Owens, J.G.; Wenke, J.C.; Rathbone, C.R. Volumetric muscle loss leads to permanent disability following extremity trauma. J. Rehabil. Res. Dev. 2015, 52, 785–792. [Google Scholar] [CrossRef]
- Lam, C.X.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 34108. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfreund-Kleinman, T.; Golenser, J.; Domb, A. Polysaccharide scaffolds for tissue engineering. In Scaffolding in Tissue Engineering; Ma, P., Elisseeff, J., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 27–44. [Google Scholar]
- Bove, A.A.; Lowenthal, D.T. Exercise Medicine: Physiological Principles and Clinical Applications; Academic Press, Inc.: New York, NY, USA, 1983; ISBN 0121197204. [Google Scholar]
- Relaix, F.; Zammit, P.S. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development 2012, 139, 2845–2856. [Google Scholar] [CrossRef]
- Tortora, G.; Derrickson, B. Principles of Anatory and Physiology, 9th ed.; John Wiley & Sons: New York, NY, USA, 2000; ISBN 9788578110796. [Google Scholar]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Jana, S.; Levengood, S.K.L.; Zhang, M. Anisotropic Materials for Skeletal-Muscle-Tissue Engineering. Adv. Mater. 2016, 28, 10588–10612. [Google Scholar] [CrossRef]
- McKeon-Fischer, K.D.; Browe, D.P.; Olabisi, R.M.; Freeman, J.W. Poly(3,4-ethylenedioxythiophene) nanoparticle and poly(ε-caprolactone) electrospun scaffold characterization for skeletal muscle regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 3633–3641. [Google Scholar] [CrossRef]
- Garg, K.; Ward, C.L.; Hurtgen, B.J.; Wilken, J.M.; Stinner, D.J.; Wenke, J.C.; Owens, J.G.; Corona, B.T. Volumetric muscle loss: Persistent functional deficits beyond frank loss of tissue. J. Orthop. Res. 2015, 33, 40–46. [Google Scholar] [CrossRef]
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Verhelst, P.; Dons, F.; Van Bever, D.D.S.P.; Schoenaers, D.D.S.J.; Nanhekhan, L.; Politis, C. Fibula Free Flap in Head and Neck Reconstruction: Identifying Risk Factors for Flap Failure and Analysis of Postoperative Complications in a Low Volume Setting. Craniomaxillofac. Trauma Reconstr. 2018, 1. [Google Scholar] [CrossRef]
- Novakovic, D.; Patel, R.S.; Goldstein, D.P.; Gullane, P.J. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009, 1, 33. [Google Scholar] [CrossRef]
- Vogt, P.R.; Brunner-LaRocca, H.P.; Lachat, M.; Ruef, C.; Turina, M.I. Technical details with the use of cryopreserved arterial allografts for aortic infection: Influence on early and midterm mortality. J. Vasc. Surg. 2002, 35, 80–86. [Google Scholar] [CrossRef]
- Horch, R.E.; Weigand, A.; Wajant, H.; An, R.; Sun, J.-M.; Arkudas, A. Towards the future of plastic surgery: From flaps to microsurgery and regenerative medicine and biofabrication? Plast. Aesthetic Res. 2017, 4, 185. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed]
- Buzhor, E.; Leshansky, L.; Blumenthal, J.; Barash, H.; Warshawsky, D.; Mazor, Y.; Shtrichman, R. Cell-based therapy approaches: The hope for incurable diseases. Regen. Med. 2014, 9, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Rippon, H.J.; Bishop, A.E. Embryonic stem cells. Cell Prolif. 2004, 37, 23–34. [Google Scholar] [CrossRef]
- Caspi, O.; Lesman, A.; Basevitch, Y.; Gepstein, A.; Arbel, G.; Huber, I.; Habib, M.; Gepstein, L.; Levenberg, S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ. Res. 2007, 100, 263–272. [Google Scholar] [CrossRef]
- Cananzi, M.; Atala, A.; De Coppi, P. Stem cells derived from amniotic fluid: New potentials in regenerative medicine. Reprod. Biomed. Online 2009, 18 (Suppl. 1), 17–27. [Google Scholar] [CrossRef]
- Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J.P.; Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J.P. Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Li, T.-S.; Shi, H.; Wang, L.; Yan, C. Effect of Bone Marrow Mesenchymal Stem Cells on Satellite Cell Proliferation and Apoptosis in Immobilization-Induced Muscle Atrophy in Rats. Med. Sci. Monit. 2016, 22, 4651–4660. [Google Scholar] [CrossRef]
- Grassel, S. Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front. Biosci. 2007, 12, 4946. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; De Angelis, G.C.; Coletta, M.; Paolucci, E.; Stornaiuolo, A.; Cossu, G.; Mavilio, F. Muscle Regeneration by Bone Marrow—Derived Myogenic Progenitors. Science 1998, 279, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- LaBarge, M.A.; Blau, H.M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 2002, 111, 589–601. [Google Scholar] [CrossRef]
- Winkler, T.; von Roth, P.; Matziolis, G.; Mehta, M.; Perka, C.; Duda, G.N. Dose–Response Relationship of Mesenchymal Stem Cell Transplantation and Functional Regeneration After Severe Skeletal Muscle Injury in Rats. Tissue Eng. Part A 2009, 15, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Von Roth, P.; Radojewski, P.; Urbanski, A.; Hanh, S.; Preininger, B.; Duda, G.; Perka, C. Immediate and delayed transplantation of mesenchymal stem cells improve muscle force after skeletal muscle injury in rats. J. Tissue Eng. Regen. Med. 2012, 4, 524–531. [Google Scholar] [CrossRef]
- Arinzeh, T.; Peter, S.; Archambault, M.; Van Den Bos, C.; Gordon, S.; Kraus, K.; Smith, A.; Kadiyala, S. Allogenic Mesenchymal stem cells regenerate bone in a critical sized canine segmental defect. J. Bone Jt. Surg. 2003, 85 A, 1927–1935. [Google Scholar] [CrossRef]
- Shayan, M.; Huang, N.F. Pre-clinical cell therapeutic approaches for repair of volumetric muscle loss. Bioengineering 2020, 7, 97. [Google Scholar] [CrossRef]
- Law, S.; Chaudhuri, S. Mesenchymal stem cell and regenerative medicine: Regeneration versus immunomodulatory challenges. Am. J. Stem Cells 2013, 2, 22–38. [Google Scholar]
- Zhang, Y.; Liang, X.; Lian, Q.; Tse, H.F. Perspective and challenges of mesenchymal stem cells for cardiovascular regeneration. Expert Rev. Cardiovasc. Ther. 2013, 11, 505–517. [Google Scholar] [CrossRef]
- Mun-Fun, H.; Ferdaos, N.; Hamzah, S.N.; Ridzuan, N.; Hisham, N.A.; Abdullah, S.; Ramasamy, R.; Cheah, P.S.; Thilakavathy, K.; Yazid, M.N.; et al. Rat full term amniotic fluid harbors highly potent stem cells. Res. Vet. Sci. 2015, 102, 89–99. [Google Scholar] [CrossRef]
- Sessarego, N.; Parodi, A.; Podestà, M.; Benvenuto, F.; Mogni, M.; Raviolo, V.; Lituania, M.; Kunkl, A.; Ferlazzo, G.; Dagna Bricarelli, F.; et al. Multipotent mesenchymal stromal cells from amniotic fluid: Solid perspectives for clinical application. Haematologica 2008, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Cananzi, M.; De Coppi, P. CD117 amniotic fluid stem cells. State of the art and future perspectives. Organogenesis 2012, 8, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Quattrocelli, M.; Sara, E.; Filippo, D.; Sindhwani, N.; Bosisio, F.; Sampaolesi, M.; Deprest, J. Human Amniotic Fluid Stem Cells Modulate Muscle Regeneration After Cardiotoxin Injury in Mice. J. Stem Cell Res. Ther. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Dai, W.; Kloner, R.A. Myocardial regeneration by human amniotic fluid stem cells: Challenges to be overcome. J. Mol. Cell. Cardiol. 2007, 42, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Bollini, S.; Pozzobon, M.; Nobles, M.; Riegler, J.; Dong, X.; Piccoli, M.; Chiavegato, A.; Price, A.N.; Ghionzoli, M.; Cheung, K.K.; et al. In Vitro and In Vivo Cardiomyogenic Differentiation of Amniotic Fluid Stem Cells. Stem Cell Rev. Rep. 2011, 7, 364–380. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Roca, I.; Requena, J.; Edel, M.; Alvarez-Palomo, A. Myogenic Precursors from iPS Cells for Skeletal Muscle Cell Replacement Therapy. J. Clin. Med. 2015, 4, 243–259. [Google Scholar] [CrossRef]
- Miyagoe-Suzuki, Y.; Takeda, S. Skeletal muscle generated from induced pluripotent stem cells-induction and application. World J. Stem Cells 2017, 9, 89–97. [Google Scholar] [CrossRef]
- van der Wal, E.; Herrero-Hernandez, P.; Wan, R.; Broeders, M.; in ’t Groen, S.L.M.; van Gestel, T.J.M.; van IJcken, W.F.J.; Cheung, T.H.; van der Ploeg, A.T.; Schaaf, G.J.; et al. Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Rep. 2018, 10, 1975–1990. [Google Scholar] [CrossRef]
- Chen, P.H.; Liao, H.C.; Hsu, S.H.; Chen, R.S.; Wu, M.C.; Yang, Y.F.; Wu, C.C.; Chen, M.H.; Su, W.F. A novel polyurethane/cellulose fibrous scaffold for cardiac tissue engineering. RSC Adv. 2015, 5, 6932–6939. [Google Scholar] [CrossRef]
- Lim, S.H.; Mao, H.Q. Electrospun scaffolds for stem cell engineering. Adv. Drug Deliv. Rev. 2009, 61, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M. Bone Marrow Stromal Cells Generate Muscle Cells and Repair Muscle Degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yin, Z.; Liu, H.; Chen, X.; Feng, B.; Yuan, H.; Su, B.; Ouyang, H.; Zhang, Y. Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs. Nanotechnology 2012, 23, 485102. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Shin, J.Y.; Kim, J.H.; Kim, J.G.; Beak, C.H.; Kim, D.-I.; Kim, H.J.; Jeon, S.S.; Choo, I.-W. Effect of Different Particles on Cell Proliferation in Polymer Scaffolds Using a Solvent-Casting and Particulate Leaching Technique. Am. Soc. Artif. Intern. Organs 2002, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, J.M.; Ma, P.X. 3D nanofibrous scaffolds for tissue engineering. J. Mater. Chem. 2011, 21, 10243–10251. [Google Scholar] [CrossRef]
- Gelain, F.; Bottai, D.; Vescovi, A.; Zhang, S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE 2006, 1, e119. [Google Scholar] [CrossRef]
- Hoque, M.E.; Chuan, Y.L.; Pashby, I. Extrusion based rapid prototyping technique: An advanced platform for tissue engineering scaffold fabrication. Biopolymers 2011, 97, 83–93. [Google Scholar] [CrossRef]
- Nuge, T.; Tshai, K.Y.; Lim, S.S.; Nordin, N.; Hoque, M.E. Preparation and Characterization of Cu-, Fe-, Ag-, Zn- and Ni- Doped Gelatin Nanofibers for Possible Applications in Antibacterial Nanomedicine. J. Eng. Sci. Technol. 2017, 12, 68–81. [Google Scholar]
- Salerno, A.; Oliviero, M.; Di Maio, E.; Iannace, S.; Netti, P.A. Design of porous polymeric scaffolds by gas foaming of heterogeneous blends. J. Mater. Sci. Mater. Med. 2009, 20, 2043–2051. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials 2010, 30, 4094–4103. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Tuzlakoglu, K.; Alves, C.M.; Mano, J.F.; Reis, R.L. Production and characterization of chitosan fibers and 3-D fiber mesh scaffolds for tissue engineering applications. Macromol. Biosci. 2004, 4, 811–819. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Mikos, A.G.; Temenoff, J.S. Formation of highly porous biodegradable scaffolds for tissue engineering. EJB Electron. J. Biotechnol. 2000, 3, 23–24. [Google Scholar] [CrossRef]
- Hoque, M.; Chuan, Y.L.; Pashby, I.; Aini, S.S.; Ng, A.; Hwei, M. Single and Hybrid Design Polycaprolactone (PCL) Scaffolds: Cell Culture Study. J. Mater. Sci. Eng. 2013, 3, 315–320. [Google Scholar]
- Chong, L.H.; Lim, M.M.; Sultana, N. Fabrication and Evaluation of Polycaprolactone/Gelatin-Based Electrospun Nanofibers with Antibacterial Properties. J. Nanomater. 2015, 2015, 970542. [Google Scholar] [CrossRef]
- Beigi, M.-H.; Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Karbalaie, K.; Azadeh, H.; Ramakrishna, S.; Baharvand, H.; Nasr-Esfahani, M.-H. In vivo integration of poly (ε-caprolactone)/gelatin nanofibrous nerve guide seeded with teeth derived stem cells for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2014. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Gelatin Based Scaffolds for Tissue Engineering—A Review. Polym. Res. J. 2014, 9, 15–32. [Google Scholar]
- Woodhead-Galloway, J. Collagen: The Anatomy of Protein (Studies in Biology); Hodder & Stoughton Educational: London, UK, 1980. [Google Scholar]
- Wess, T.J. Collagen Fibril Form and Function. In Fibrous Proteins: Coiled-Coils, Collagen and Elastomers; Parry, D., Squire, J., Eds.; Academic Press: San Diego, CA, USA, 2005; Volume 70, pp. 341–374. ISBN 0065-3233. [Google Scholar]
- Brodsky, B.; Persikov, A.V. Molecular Structure of the Collagen Triple Helix. In Fibrous Proteins: Coiled-Coils, Collagen and Elastomers; Parry, D., Squire, J., Eds.; Academic Press: San Diego, CA, USA, 2005; Volume 70, pp. 301–339. ISBN 0065-3233. [Google Scholar]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Merrett, K.; Fagerholm, P.; McLaughlin, C.R.; Dravida, S.; Lagali, N.; Shinozaki, N.; Watsky, M.A.; Munger, R.; Kato, Y.; Li, F.; et al. Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: Performance of type I versus type III collagen. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.; Collin, E.; Velasco-Bayon, D.; Murphy, M.; O’Halloran, D.; Pandit, A. Type II collagen-hyaluronan hydrogel--a step towards a scaffold for intervertebral disc tissue engineering. Eur. Cell. Mater. 2010, 20, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Jiang, C.; Wang, C.; Uzunalli, G.; Whittern, N.; Chen, D.; Jones, O.G.; Kuang, S.; Deng, M. Harnessing Fiber Diameter-Dependent Effects of Myoblasts Toward Biomimetic Scaffold-Based Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 203. [Google Scholar] [CrossRef]
- Matthews, J.A.; Boland, E.D.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Compatible Polymers Electrospinning of Collagen. J. Bioact. Compat. Polym. 2003, 18, 125–134. [Google Scholar] [CrossRef]
- Joshi, J.; Mahajan, G.; Kothapalli, C.R. Three-dimensional collagenous niche and azacytidine selectively promote time-dependent cardiomyogenesis from human bone marrow-derived MSC spheroids. Biotechnol. Bioeng. 2018, 115, 2013–2026. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.M.; Cai, A.; Dippold, D.; Boccaccini, A.R.; Schubert, D.W.; Hardt, M.; Lange, C.; Arkudas, A.; et al. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.; Ramakrishna, S.; Lim, C. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer (Guildf.) 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Hoque, M.; Nuge, T.; Yeow, T.K.; Nordin, N. Electrospinning of Gelatin Nanofibre: Current Trends in Tissue Engineering Applications. J. Appl. Mech. Eng. 2013, 2, 9873. [Google Scholar] [CrossRef]
- Zha, Z.; Teng, W.; Markle, V.; Dai, Z.; Wu, X. Fabrication of gelatin nanofibrous scaffolds using ethanol/phosphate buffer saline as a benign solvent. Biopolymers 2012, 97, 1026–1036. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer (Guildf.) 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Jameela, S.R. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials 1996, 17, 471–484. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer (Guildf.) 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Correia, D.M.; Padrão, J.; Rodrigues, L.R.; Dourado, F.; Lanceros-Méndez, S.; Sencadas, V. Thermal and hydrolytic degradation of electrospun fish gelatin membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar] [CrossRef]
- Nuge, T.; Tshai, K.Y.; Lim, S.S.; Nordin, N.; Hoque, M.E. Characterization and optimization of the mechanical properties of electrospun gelatin nanofibrous scaffolds. World J. Eng. 2020, 17, 12–20. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Mahto, S.K.; Mahanta, A.K.; Singh, R.; Vijayakumar, M.R.; Ray, B.; Misra, N. Cell proliferation influenced by matrix compliance of gelatin grafted poly(D,L-Lactide) three dimensional scaffolds. Colloids Surf. B Biointerfaces 2018, 166, 170–178. [Google Scholar] [CrossRef]
- Guo, Y.; Gilbert-honick, J.; Somers, S.M.; Mao, H. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fi brin microfiber bundles. Biochem. Biophys. Res. Commun. 2019, 516, 558–564. [Google Scholar] [CrossRef]
- Gilbert-honick, J.; Ginn, B.; Zhang, Y.; Salehi, S.; Wagner, K.R.; Mao, H.; Grayson, W.L. Adipose-derived Stem/Stromal Cells on Electrospun Fibrin Microfiber Bundles Enable Moderate Muscle Reconstruction in a Volumetric Muscle Loss Model. Cell Transplant. 2018, 27, 1644–1656. [Google Scholar] [CrossRef]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1019–E1031. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun antibacterial chitosan-based fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, K.; Cha, D.; Kim, H.; Nishida, A.; Yamamoto, H. Electrospinning of Chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. [Google Scholar] [CrossRef]
- De Vrieze, S.; Westbroek, P.; Van Camp, T.; Van Langenhove, L. Electrospinning of chitosan nanofibrous structures: Feasibility study. J. Mater. Sci. 2007, 42, 8029–8034. [Google Scholar] [CrossRef]
- Shim, I.K.; Suh, W.H.; Lee, S.Y.; Lee, S.H.; Heo, S.J.; Lee, M.C.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. A 2009, 90, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, H.; Liu, Y.; Xiao, D.; Li, W.; Wang, Q. Poly (-Caprolactone)/Chitosan Scaffold Improves Bladder Regeneration in a Rat Model. Regen. Med. 2018, 13, 331–342. [Google Scholar] [CrossRef]
- Ronchi, G.; Fornasari, B.E.; Crosio, A.; Budau, C.A.; Tos, P.; Perroteau, I.; Battiston, B.; Geuna, S.; Raimondo, S.; Gambarotta, G. Chitosan Tubes Enriched with Fresh Skeletal Muscle Fibers for Primary Nerve Repair. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Li, J.; He, A.; Han, C.C.; Fang, D.; Hsiao, B.S.; Chu, B. Electrospinning of Hyaluronic Acid (HA) and HA/Gelatin Blends. Macromol. Rapid Commun. 2006, 27, 114–120. [Google Scholar] [CrossRef]
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008, 4, 1611–1619. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, G.; Fang, D.; Xu, J.; Zhang, H.; Nie, J. Effects of solution properties and electric field on the electrospinning of hyaluronic acid. Carbohydr. Polym. 2011, 83, 1011–1015. [Google Scholar] [CrossRef]
- Lev, R.; Seliktar, D. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef]
- Pollot, B.E.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Marcinczyk, M.; Elmashhady, H.; Talovic, M.; Dunn, A. Laminin-111 enriched fibrin hydrogels for skeletal muscle regeneration. Biomaterials 2017. [Google Scholar] [CrossRef] [PubMed]
- Matthias, N.; Hunt, S.D.; Wu, J.; Lo, J.; Smith, L.A.; Li, Y.; Huard, J.; Darabi, R. Volumetric muscle loss injury repair using in situ fi brin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res. 2018, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Xue, B.; Gao, X.; Qin, M.; Wang, W.; Chen, B.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; De Maria, C.; Rimessi, A.; Donà, S.; Braghetta, P.; Pinton, P.; Vozzi, G.; Bonaldo, P. Gelatin-genipin-based biomaterials for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 2763–2777. [Google Scholar] [CrossRef]
- Tijore, A.; Behr, J.M.; Irvine, S.A.; Baisane, V.; Venkatraman, S. Bioprinted gelatin hydrogel platform promotes smooth muscle cell contractile phenotype maintenance. Biomed. Microdevices 2018, 20. [Google Scholar] [CrossRef]
- Dong, G.; Lian, Q.; Yang, L.; Mao, W.; Liu, S.; Xu, C. Preparation and Endothelialization of Multi-level Vessel-like Network in Enzymated Gelatin Scaffolds. J. Bionic Eng. 2018, 15, 673–681. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Chen, Q.; Fu, L.; Tao, L.; Wei, Y. Injectable and Self-Healing Chitosan Hydrogel Based on Imine Bond: Design and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 2198. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Napiwocki, B.N.; Peng, X.F.; Turng, L.S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon N. Y. 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Samadi, N.; Sabzi, M.; Babaahmadi, M. Self-healing and tough hydrogels with physically cross-linked triple networks based on Agar/PVA/Graphene. Int. J. Biol. Macromol. 2018, 107, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Song, F.; Qian, D.; He, Y.D.; Nie, W.C.; Wang, X.L.; Wang, Y.Z. Strong and tough fully physically crosslinked double network hydrogels with tunable mechanics and high self-healing performance. Chem. Eng. J. 2018, 349, 588–594. [Google Scholar] [CrossRef]
- Starnecker, F.; König, F.; Hagl, C.; Thierfelder, N. Tissue-engineering acellular scaffolds—The significant influence of physical and procedural decellularization factors. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 106, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Junka, R.; Yu, X. Novel Acellular Scaffold Made from Decellularized Schwann Cell Sheets for Peripheral Nerve Regeneration. Regen. Eng. Transl. Med. 2015, 1, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Tseng, F.W.; Chang, W.H.; Peng, I.C.; Hsieh, D.J.; Wu, S.W.; Yeh, M.L. Preparation of acellular scaffold for corneal tissue engineering by supercritical carbon dioxide extraction technology. Acta Biomater. 2017, 58, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Pashos, N.; Scarritt, M.; Eagle, Z.; Gimble, J.; Chaffin, A.; Bunnell, B. Characterization of an Acellular Scaffold for a TE approach to NAC reconstruction. Cells Tissues Organs 2017, 203, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, S.E.; Wagner, D.E. Acellular human lung scaffolds to model lung disease and tissue regeneration. Eur. Respir. Rev. 2018, 27, 180021. [Google Scholar] [CrossRef] [PubMed]
- Wilczek, P.; Paulina, G.; Karolina, J.; Martyna, M.; Grazyna, W.; Roman, M.; Aldona, M.; Anna, S.; Aneta, S. Biomechanical and morphological stability of acellular scaffolds for tissue-engineered heart valves depends on different storage conditions. J. Mater. Sci. Mater. Med. 2018, 29, 106. [Google Scholar] [CrossRef]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef]
- Urciuolo, A.; Urbani, L.; Perin, S.; Maghsoudlou, P.; Scottoni, F.; Gjinovci, A.; Collins-Hooper, H.; Loukogeorgakis, S.; Tyraskis, A.; Torelli, S.; et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci. Rep. 2018, 8, 8398. [Google Scholar] [CrossRef]
- Urciuolo, A.; De Coppi, P. Decellularized Tissue for Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2392. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Badylak, J.S.; Weber, D.J.; Badylak, S.F. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J. Surg. Res. 2012, 176, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Svystonyuk, D.A.; Mewhort, H.E.M.; Fedak, P.W.M. Using Acellular Bioactive Extracellular Matrix Scaffolds to Enhance Endogenous Cardiac Repair. Front. Cardiovasc. Med. 2018, 5, 35. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2012, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, C.; Hou, X. Bladder Acellular Matrix Conjugated with Basic Fibroblast Growth Factor for Bladder Regeneration. Tissue Eng. Part A 2014, 20, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.H.; Shayan, M.; Huang, N.F. Engineering Biomimetic Materials for Skeletal Muscle Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, e1801168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ito, Y. Inorganic material surfaces made bioactive by immobilizing growth factors for hard tissue engineering. RSC Adv. 2013, 3, 11095–11106. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Święszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef]
- Kim, J.H.; Seol, Y.J.; Ko, I.K.; Kang, H.W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef]
- Bour, R.; Presnell, S.; Shepherd, B.; Christ, G.; Peirce, S. Using Bioprinting to Tissue Engineer Microvascularized Constructs for Skeletal Muscle Repair. FASEB J. 2019, 33, lb449. [Google Scholar] [CrossRef]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qian, Z.; Tahtinen, M.; Qi, S.; Zhao, F. Prevascularization of natural nanofibrous extracellular matrix for engineering completely biological three-dimensional prevascularized tissues for diverse applications. J. Tissue Eng. Regen. Med. 2018, 12, e1325–e1336. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Blaker, J.; Brown, D.; Weinstein-Oppenheimer, C.; Pepczynska, M.; Olguin, Y.; Sanchez, E.; Acevedo, C. Edible scaffolds based on non mamalian biopolymers for myoblast. Materials (Basel) 2017, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Siltanen, A.; Nuutila, K.; Imanishi, Y.; Uenaka, H.; Mäkelä, J.; Pätilä, T.; Vento, A.; Miyagawa, S.; Sawa, Y.; Harjula, A.; et al. The Paracrine Effect of Skeletal Myoblasts is Cardioprotective against Oxidative Stress and Involves EGFR-ErbB4 Signaling, Cystathionase, and the Unfolded Protein Response. Cell Transplant. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers (Basel) 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Aguilar-Agon, K.W.; Capel, A.J.; Martin, N.R.W.; Player, D.J.; Lewis, M.P. Mechanical loading stimulates hypertrophy in tissue-engineered skeletal muscle: Molecular and phenotypic responses. J. Cell. Physiol. 2019, 234, 23547–23558. [Google Scholar] [CrossRef]
- Kluger, R.; Alagic, A. Chemical cross-linking and protein-protein interactions-a review with illustrative protocols. Bioorg. Chem. 2004, 32, 451–472. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Tandon, B.; Blaker, J.J.; Cartmell, S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018, 73, 1–20. [Google Scholar] [CrossRef]
- Kloth, L.C. Electrical Stimulation Technologies for Wound Healing. Adv. Wound Care 2014, 3, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric Nano-Biomaterials for Biomedicine and Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Yuan, H.; Lei, T.; Qin, Y.; He, J.-H.; Yang, R. Design and application of piezoelectric biomaterials. J. Phys. D Appl. Phys. 2019, 52, 194002. [Google Scholar] [CrossRef]
- Yoon, J.-K.; Misra, M.; Yu, S.J.; Kim, H.Y.; Bhang, S.H.; Song, S.Y.; Lee, J.-R.; Ryu, S.; Choo, Y.W.; Jeong, G.-J.; et al. Thermosensitive, Stretchable, and Piezoelectric Substrate for Generation of Myogenic Cell Sheet Fragments from Human Mesenchymal Stem Cells for Skeletal Muscle Regeneration. Adv. Funct. Mater. 2017, 27, 1703853. [Google Scholar] [CrossRef]


| Technique | Advantages | Disadvantages | Example | Ref. |
|---|---|---|---|---|
| Solvent casting/Particulate Leaching techniques | Control over porosity, pore size and crystallinity | Use of highly toxic solvents Labor intensive fabrication process Residual particles in the polymer matrix Irregular shaped pores Insufficient interconnectivity | PLGA a/Gelatin as scaffolds for cell-based artificial organs. Findings: Enhanced cells adhesion and proliferation of chondrocytes and smooth muscles. | [58] |
| Gas foaming | Free of harsh organic solvent Control over porosity, pore size and fiber diameter | Formation of a non-porous matrix resulted from rapid diffusion of gas away from the surface Lack of interconnectivity between pores. | PCL a/Gelatin as scaffolds for new tissue regeneration Findings: The human mesenchymal stem cells (hMSCs) were able to colonize the outer and inner regions of the scaffolds. | [63] |
| Thermally-induced Phase separation/Porogen leaching | Versatile Control over pore size when combined with other techniques Great control over the 3D shape | Little control over fiber diameter and orientation Time-consuming | Pure gelatin based scaffold for Tissue engineering applications Findings: The 3D shape with porous and nanofibrous scaffolds has induced a higher level of osteocalcin and bone sialoprotein expression (bone markers). | [59,64] |
| Wet spinning | Large surface area for cell attachment and rapid diffusion of the nutrients in favor of cell survival and growth | Poor mechanical properties. | Chitosan based scaffolds for bone tissue engineering Finding: The scaffolds allowed significant cell proliferation of osteoblast and exhibited good attachment and developed bridging between cells via filopodia structures. | [65,66] |
| Fiber bonding | Produce highly porous scaffolds with interconnected pores | The solvent used could be toxic to the cells if not completely remove | PGA a/PLLA a as polymeric scaffolds for Cell-based artificial liver Findings: A higher degree of interaction between hepatocytes and porous scaffolds after 18 hours of cultivation. Major interaction between cell-cell rather than cell-polymer was observed after 1week of cultivation. | [67,68] |
| Self-assembly | The scaffolds can be modified. Do not produce synthetic degradation by-products The scaffolds provide the opportunity to incorporate modified variants containing quite large bioactive motifs or domain | Expensive material and complex design parameters. | Peptide as natural based scaffolds for promising scaffolds for the study of cell signal pathway Findings: The functionalized peptides that underwent self-assembly into nanofiber structures have significantly enhanced the neural cell survival without additional extra growth factors. | [60] |
| Rapid prototyping | Produce scaffolds with a fully interconnected pore structure. Full control over porosity, pore size, pore shape and permeability. | Highly expensive equipment | HA a/PCL a as scaffolds for bone tissue engineering Findings: The high surface area of the scaffolds favors the adhesion and growth of the osteoblast. | [69] |
| Electrospinning | Inexpensive. Simple set-up. High surface area to volume ratio. Ease of fiber functionalization.Ease of material hybridization. Possibility of scaling–up the process for mass production. | The solvents used could be toxic to the cells if not completely removed. The process depends on many variables. | PCL a/Gelatin hybrid scaffolds for peripheral nerve regeneration Findings: The scaffolds offered a more mimicking micro and macro environment for peripheral nerve regeneration by providing excellent substrate delivery to guide axons regeneration. | [70,71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuge, T.; Liu, Z.; Liu, X.; Ang, B.C.; Andriyana, A.; Metselaar, H.S.C.; Hoque, M.E. Recent Advances in Scaffolding from Natural-Based Polymers for Volumetric Muscle Injury. Molecules 2021, 26, 699. https://doi.org/10.3390/molecules26030699
Nuge T, Liu Z, Liu X, Ang BC, Andriyana A, Metselaar HSC, Hoque ME. Recent Advances in Scaffolding from Natural-Based Polymers for Volumetric Muscle Injury. Molecules. 2021; 26(3):699. https://doi.org/10.3390/molecules26030699
Chicago/Turabian StyleNuge, Tamrin, Ziqian Liu, Xiaoling Liu, Bee Chin Ang, Andri Andriyana, Hendrik Simon Cornelis Metselaar, and Md Enamul Hoque. 2021. "Recent Advances in Scaffolding from Natural-Based Polymers for Volumetric Muscle Injury" Molecules 26, no. 3: 699. https://doi.org/10.3390/molecules26030699
APA StyleNuge, T., Liu, Z., Liu, X., Ang, B. C., Andriyana, A., Metselaar, H. S. C., & Hoque, M. E. (2021). Recent Advances in Scaffolding from Natural-Based Polymers for Volumetric Muscle Injury. Molecules, 26(3), 699. https://doi.org/10.3390/molecules26030699







