Pharmacological Extracts and Molecules from Virola Species: Traditional Uses, Phytochemistry, and Biological Activity
Abstract
1. Introduction
2. Botany
2.1. Taxonomy
2.2. Morphology and Anatomy
2.3. Geographic Distribution
3. Ethnopharmacological Uses
4. Phytochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Drašar, P.B.; Rimpelová, S.; Christensen, S.B.; Khripach, V.A.; Jurášek, M. Large Scale Conversion of Trilobolide into the Payload of Mipsagargin: 8-O-(12-Aminododecanoyl)-8-O-Debutanoylthapsigargin. Biomolecules 2020, 10, 1640. [Google Scholar] [CrossRef] [PubMed]
- Santamaría-Aguilar, D.; Aguilar, R.; Lagomarsino, L.P. A taxonomic synopsis of Virola (Myristicaceae) in Mesoamerica, including six new species. PhytoKeys 2019, 134, 1–82. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J.; Towers, G.; Abbott, F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and β-carboline constituents of Ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. [Google Scholar] [CrossRef]
- ITIS Standard Report Page: Lycopodiophytina. 2019. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=18122#null) (accessed on 16 October 2020).
- De Almeida, G.V.B.; Arunachalam, K.; Balogun, S.O.; Pavan, E.; Ascêncio, S.D.; Soares, I.M.; Zanatta, A.C.; Vilegas, W.; Macho, A.; Martins, D.T.D.O. Chemical characterization and evaluation of gastric antiulcer properties of the hydroethanolic extract of the stem bark of Virola elongata (Benth.) Warb. J. Ethnopharmacol. 2019, 231, 113–124. [Google Scholar] [CrossRef]
- Ureta Adrianzén, M. Taxonomic Review of the Myristicaceae Family from Central Forest, Oxapampa-Perú. Intropica 2010, 5, 29–46. [Google Scholar]
- Plantes et Botanique. Available online: https://archive.is/20070519184701/http://www.plantes-botanique.be/e2-Myristicaceae-Virola-venosa (accessed on 16 October 2020).
- Rodrigues, W.A. Revisão taxonômica das espécies de Virola Aublet (Myristicaceae) do Brasil. Acta Amaz. 1980, 10, 3–127. [Google Scholar] [CrossRef]
- Tropical Plants Database, Ken Fern. Available online: http://tropical.theferns.info/viewtropical.php?id=Virola+oleifera (accessed on 16 October 2020).
- Tropical Plants Database, Ken Fern. Available online: http://tropical.theferns.info/viewtropical.php?id=Virola+surinamensis (accessed on 25 October 2020).
- Lai, A.; Tin-Wa, M.; Mika, E.S.; Persinos, G.J.; Farnsworth, N.R. Phytochemical Investigation of Virola peruviana, A New Hallucinogenic Plant. J. Pharm. Sci. 1973, 62, 1561–1563. [Google Scholar] [CrossRef]
- Roumy, V.; Macedo, J.C.R.; Bonneau, N.; Samaillie, J.; Azaroual, N.; Encinas, L.A.; Rivière, C.; Hennebelle, T.; Sahpaz, S.; Antherieu, S.; et al. Plant therapy in the Peruvian Amazon (Loreto) in case of infectious diseases and its antimicrobial evaluation. J. Ethnopharmacol. 2020, 249, 112411. [Google Scholar] [CrossRef]
- Castro, E.; Suarez, L.E.C.; Siengalewicz, P.; Gutmann, R.; Czermak, G.; Brueggeller, P. 3,6-Dihydroxy-2-(11-phenylundecanoyl)cyclohex-2-en-1-one from Virola venosa bark. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2004, 60, 467. [Google Scholar] [CrossRef]
- Fernandes, K.R.P.; Bittercourt, P.S.; De Souza, A.Q.L.; De Souza, A.Q.L.; Da Silva, F.M.A.; Lima, E.S.; Acho, L.D.R.; Nunomura, R.D.C.S.; Teixeira, A.F.; Koolen, H.H.F. Phenolic compounds from Virola venosa (Myristicaceae) and evaluation of their antioxidant and enzyme inhibition potential. Acta Amaz. 2019, 49, 48–53. [Google Scholar] [CrossRef]
- Pereira, A.C.H.; Lenz, D.; Nogueira, B.V.; Scherer, R.; Andrade, T.U.; Da Costa, H.B.; Romão, W.; Pereira, T.M.C.; Endringer, D.C. Gastroprotective activity of the resin from Virola oleifera. Pharm. Biol. 2016, 55, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Riba-Hernández, P.; Segura, J.L.; Muñoz-Valverde, J. Female fruit production depends on female flower production and crown size rather than male density in a continuous population of a tropical dioecious tree (Virola surinamensis). Am. J. Bot. 2016, 103, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Chabert, P.; Fougerousse, A.; Brouillard, R. Anti-mitotic properties of resveratrol analog (Z)-3,5,4′-trimethoxystilbene. BioFactors 2006, 27, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cassady, J.M.; E Blair, G.; Raffauf, R.F.; E Tyler, V. The isolation of 6-methoxyharmalan and 6-methoxyharman from Virola cuspidata. Lloydia 1971, 34, 161–162. [Google Scholar] [PubMed]
- Denny, C.; Zacharias, M.E.; Lúcia, A.; Gois, T.; Amaral, C.E.; Bittrich, V.; Kohn, L.K.; De Oliveira, I.M.; Alexandre, R.; Rodrigues, F.; et al. Antiproliferative Properties of Polyketides Isolated from Virola Sebifera Leaves. Phytother. Res. 2008, 130, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Pagnocca, F.C.; Ribeiro, S.B.; Torkomian, V.L.V.; Hebling, M.J.A.; Bueno, O.C.; Da Silva, O.A.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.D.G.F.D.; Ferreira, A.G. Toxicity of lignans to symbiotic fungus of leaf-cutting ants. J. Chem. Ecol. 1996, 22, 1325–1330. [Google Scholar] [CrossRef]
- Coutinho, P.N.; Pereira, B.P.; Pereira, A.C.H.; Porto, M.L.; De Assis, A.L.E.M.; Destefani, A.C.; Meyrelles, S.S.; Vasquez, E.C.; Nogueira, B.V.; De Andrade, T.U.; et al. Chronic administration of antioxidant resin from Virola oleifera attenuates atherogenesis in LDLr -/- mice. J. Ethnopharmacol. 2017, 206, 65–72. [Google Scholar] [CrossRef]
- Kuroshima, K.N.; De Campos, F.; De Souza, M.M.; Yunes, R.A.; Monache, F.D.; Filho, V.C. Phytochemical and Pharmacological Investigations of Virola oleifera Leaves. Z. Nat. C 2001, 56, 703–706. [Google Scholar] [CrossRef]
- Carvalho, A.A.V.; Galdino, P.M.; Nascimento, M.V.M.; Kato, M.J.; Valadares, M.C.; Cunha, L.C.; Costa, E.A. Antinociceptive and antiinflammatory activities of grandisin extracted fromVirola surinamensis. Phytother. Res. 2010, 24, 113–118. [Google Scholar] [CrossRef]
- Costa, E.S.; Hiruma-Lima, C.A.; Lima, E.O.; Sucupira, G.C.; Bertolin, A.O.; Lolis, S.F.; Andrade, F.D.P.; Vilegas, W.; Souza-Brito, A.R.M. Antimicrobial activity of some medicinal plants of the Cerrado, Brazil. Phytother. Res. 2008, 22, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Hiruma-Lima, C.A.; Batista, L.M.; De Almeida, A.B.A.; Magri, L.D.P.; Dos Santos, L.C.; Vilegas, W.; Brito, A.R.M.S. Antiulcerogenic action of ethanolic extract of the resin from Virola surinamensis Warb. (Myristicaceae). J. Ethnopharmacol. 2009, 122, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Barata, L.E.; Baker, P.M.; Gottlieb, O.R.; Rúveda, E.A. Neolignans of Virola surinamensis. Phytochemistry 1978, 17, 783–786. [Google Scholar] [CrossRef]
- Barata, L.E.S.; Santos, L.S.; Ferri, P.H.; Phillipson, J.; Paine, A.; Croft, S.L. Anti-leishmanial activity of neolignans from Virola species and synthetic analogues. Phytochemistry 2000, 55, 589–595. [Google Scholar] [CrossRef]
- Lopes, N.P.; Kato, M.J.; Eloısa, H.D.A.; Maia, J.G.; Yoshida, M.; Planchart, A.R.; Katzin, A.M. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiãpi Amazon Indians. J. Ethnopharmacol. 1999, 67, 313–319. [Google Scholar] [CrossRef]
- Lopes, N.P.; Chicaro, P.; Kato, M.J.; De Albuquerque, S.; Yoshida, M. Flavonoids and Lignans fromVirola surinamensisTwigs and theirin vitroActivity againstTrypanosoma cruzi. Planta Medica 1998, 64, 667–669. [Google Scholar] [CrossRef]
- Habenschus, M.; Moreira, F.D.L.; Lopes, N.P.; De Oliveira, A.R.M. In Vitro Inhibition of Human CYP450s 1A2, 2C9, 3A4/5, 2D6 and 2E1 by Grandisin. Planta Medica 2017, 83, 727–736. [Google Scholar] [CrossRef]
- Macre, W.D.; Towers, G.N. An ethnopharmacological examination ofVirola elongata bark: A South American arrow poison. J. Ethnopharmacol. 1984, 12, 75–92. [Google Scholar] [CrossRef]
- Bôa, I.S.F.; Porto, M.L.; Pereira, A.C.H.; Ramos, J.P.L.; Scherer, R.; Oliveira, J.P.; Nogueira, B.V.; Meyrelles, S.S.; Vasquez, E.C.; Endringer, D.C.; et al. Resin from Virola oleifera Protects Against Radiocontrast-Induced Nephropathy in Mice. PLOS ONE 2015, 10, e0144329. [Google Scholar] [CrossRef]
- Sarquis, R.D.S.F.R.; Sarquis, Í.R.; Sarquis, I.R.; Fernandes, C.P.; Da Silva, G.A.; E Silva, R.B.L.; Jardim, M.A.G.; Sánchez-Ortíz, B.L.; Carvalho, J.C.T. The Use of Medicinal Plants in the Riverside Community of the Mazagão River in the Brazilian Amazon, Amapá, Brazil: Ethnobotanical and Ethnopharmacological Studies. Evid. Based Complement. Altern. Med. 2019, 2019, 1–25. [Google Scholar] [CrossRef]
- Rezende, K.R.; Kato, M.J. Dibenzylbutane and aryltetralone lignans from seeds of Virola sebifera. Phytochemistry 2002, 61, 427–432. [Google Scholar] [CrossRef]
- Rezende, K.R.; Davino, S.C.; Barros, S.B.; Kato, M.J. Antioxidant activity of aryltetralone lignans and derivatives fromVirola sebifera(Aubl.). Nat. Prod. Res. 2005, 19, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.J.; Yoshida, M.; Gottlieb, O.R. Flavones and lignans in flowers, fruits and seedlings ofVirola venosa. Phytochemistry 1992, 31, 283–287. [Google Scholar] [CrossRef]
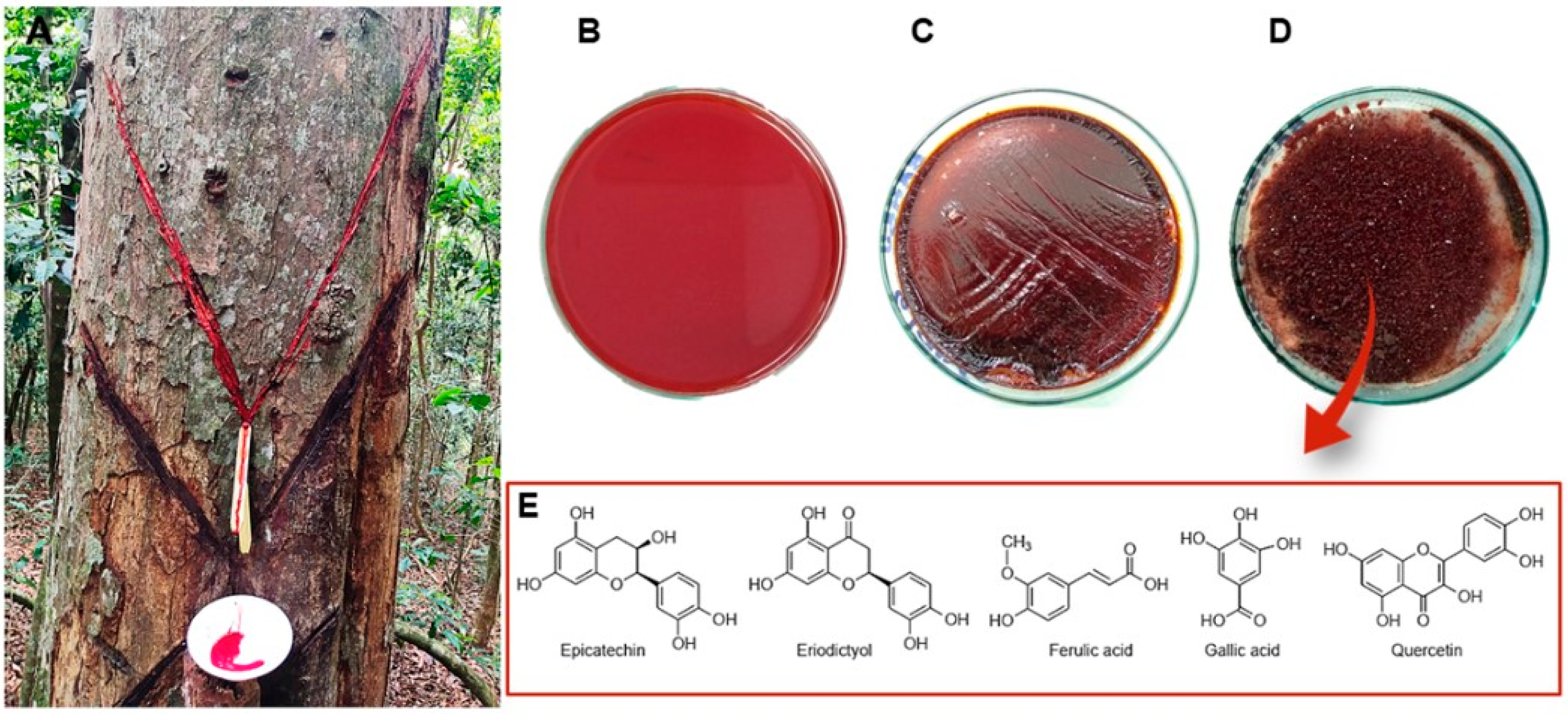
| Species | Plant Part Used | Type of Extract | Traditional Uses | Bioactivity and Mechanism of Action (In Vitro and In Vivo Models) | Type of Compound | Compound Identified | Ref. |
|---|---|---|---|---|---|---|---|
| V. elongata (or V. cuspidata) | Stem bark | Hydro- ethanolic | Stomach pain Indigestion Gastric ulcers | Gastroprotective/antiulcer: antioxidant, gastric secretion, and total acidity reduction (rodent models of acute and chronic gastric ulcers) | Phenolic acids | Gallic acid | [6] |
| Stilbenes | 3,3′,4-trihydroxystilbene | ||||||
| Flavonoids | Catechin Rutin | ||||||
| Neolignans | Arylnaphtalene Dibenzylbutane | ||||||
| Antiproliferative in colon adenocarcinoma cells: cell cycle arrest in G2/M, anti-mitotic activity through tubulin polymerization inhibition (Caco-2 cell line) | Stilbenes | (Z)-3,5,4′-trihydroxystilbene (resveratrol analog) | [18] | ||||
| Psychoactive | Alkaloids (β-carbolines) | 6-methoxyharmalan 6-methoxyharman | [19] | ||||
| V. peruviana | Coarse bark | Petroleum ether extraction | Hallucinogenic | Alkaloids | 5-methoxy-N,N-dimethyltryptamine N,N-dimethyltryptamine 5-methoxytryptamine | [12] | |
| V. sebifera | Leaves | Dichloromethane | Cytotoxic and antiproliferative in cancer cells (human ovarian OVCAR03 and multidrug-resistance-phenotype NCI-ADR tumor cell lines) | Polyketide | 3,5-dihydro-2-(1′-oxo-3′-hexadecenil)-2-cyclohexen-1-one | [20,21] | |
| Acylresorcinol | (4′Z)1-hexadec-4′-enoyl-2,6-dihydroxybenzene | ||||||
| V. oleifera | Seed | Oil | Rheumatic pain Bronchial asthma Joint tumors Intestinal worms Halitosis Hemorrhoids Skin diseases | [9] | |||
| Resin | Plant fluid exudate collection | Chronic wound healing Hemoptysis Leukorrhea Diarrhea | Gastroprotective/antiulcer: antioxidant activity (ethanol/HCl and indomethacin ulcer-induction mouse models) | Flavonoids | Epicatechin Eriodictyol | [16] | |
| Tannins | |||||||
| Atheroprotective: systemic and local antioxidant and anti-inflammatory activity (LDLr−/− mouse model) | |||||||
| Nephroprotective: antioxidant and antiapoptotic in renal glomerular and tubular cells (contrast-induced nephropathy mouse model) | Phenolic acids | Ferulic acid Gallic acid | [22] | ||||
| Flavonoids | Quercetin | ||||||
| Leaves | Methanolic | Analgesic | Analgesic (writhing test in mice) | Flavonoids | Quercitrin Astilbin | [23] | |
| Neolignans | Oleiferin-C | ||||||
| V. surinamensis | Resin | Ethanolic | Ulcer Gastritis Inflammation Cancer | Antiulcer: inhibition of gastric lesions (ethanol/HCl, indomethacin, stress, and pylorus ligature ulcer-induction mouse models) | Flavonoids | Epicatechin | [24,25,26] |
| Stem bark | Ethanolic | Inflammation Cancer | Antibacterial Antifungal (diameter of the inhibition zone test) | Phenolic compounds | |||
| Flavonoids | Catechin | ||||||
| Tannins | |||||||
| Leaves | Infusion | Colic Dyspepsia | Antinociceptive (acetic acid-induced abdominal writhing test and formalin test in mice) Anti-inflammatory (croton oil-induced ear oedema test in mice) | Neolignans | Grandisin | ||
| Hexane | Anti-schistosomal and anti-leishmanial activity (in vitro tests) | Neolignans | Surinamensin | [27,28] | |||
| Plantlet leaves | Essential oil | Malaria | Antimalarial (in vitro test) | Sesquiterpenes | Nerolidol | [29] | |
| Twig | Dichloromethane | Trypanosomicidal activity (in vitro test against Trypanosoma cruzi) | Lignans and neolignans | Veraguensin Grandisin | [30] | ||
| Inhibition of CYP2C9 and CYP3A4/5 | Neolignans | Grandisin | [31] | ||||
| V. venosa | Bark Leaves | Methanolic | Antioxidant | Antioxidant and α-glucosidase-inhibitory activity (DPPH and α-glucosidase-inhibition assay) | Phenolic acids | Ferulic acid Gallic acid ρ-coumaric acid | [15] |
| Flavonoids | Quercetin Quercitrin Kaempferol Catechin | ||||||
| Neolignans | Oleiferin-C | ||||||
| V. pavonis | Root Bark stem | Decoction | Skin infection Oral mycosis | Antimicrobial (in vitro tests) | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, M.; Ruiz-Fernández, C.; Francisco, V.; Ait Eldjoudi, D.; Farrag AbdElHafez, Y.R.; Cordero-Barreal, A.; Pino, J.; Lago, F.; Campos-Toimil, M.; Rocha Carvalho, G.; et al. Pharmacological Extracts and Molecules from Virola Species: Traditional Uses, Phytochemistry, and Biological Activity. Molecules 2021, 26, 792. https://doi.org/10.3390/molecules26040792
González-Rodríguez M, Ruiz-Fernández C, Francisco V, Ait Eldjoudi D, Farrag AbdElHafez YR, Cordero-Barreal A, Pino J, Lago F, Campos-Toimil M, Rocha Carvalho G, et al. Pharmacological Extracts and Molecules from Virola Species: Traditional Uses, Phytochemistry, and Biological Activity. Molecules. 2021; 26(4):792. https://doi.org/10.3390/molecules26040792
Chicago/Turabian StyleGonzález-Rodríguez, María, Clara Ruiz-Fernández, Vera Francisco, Djedjiga Ait Eldjoudi, Yousof Ramadan Farrag AbdElHafez, Alfonso Cordero-Barreal, Jesus Pino, Francisca Lago, Manuel Campos-Toimil, Glaucimeire Rocha Carvalho, and et al. 2021. "Pharmacological Extracts and Molecules from Virola Species: Traditional Uses, Phytochemistry, and Biological Activity" Molecules 26, no. 4: 792. https://doi.org/10.3390/molecules26040792
APA StyleGonzález-Rodríguez, M., Ruiz-Fernández, C., Francisco, V., Ait Eldjoudi, D., Farrag AbdElHafez, Y. R., Cordero-Barreal, A., Pino, J., Lago, F., Campos-Toimil, M., Rocha Carvalho, G., Melo Costa Pereira, T., & Gualillo, O. (2021). Pharmacological Extracts and Molecules from Virola Species: Traditional Uses, Phytochemistry, and Biological Activity. Molecules, 26(4), 792. https://doi.org/10.3390/molecules26040792












