RORα Regulates Odontoblastic Differentiation and Mediates the Pro-Odontogenic Effect of Melatonin on Dental Papilla Cells
Abstract
1. Introduction
2. Results
2.1. Expression of Nuclear Receptor RORs in Rat DPCs (rDPCs)
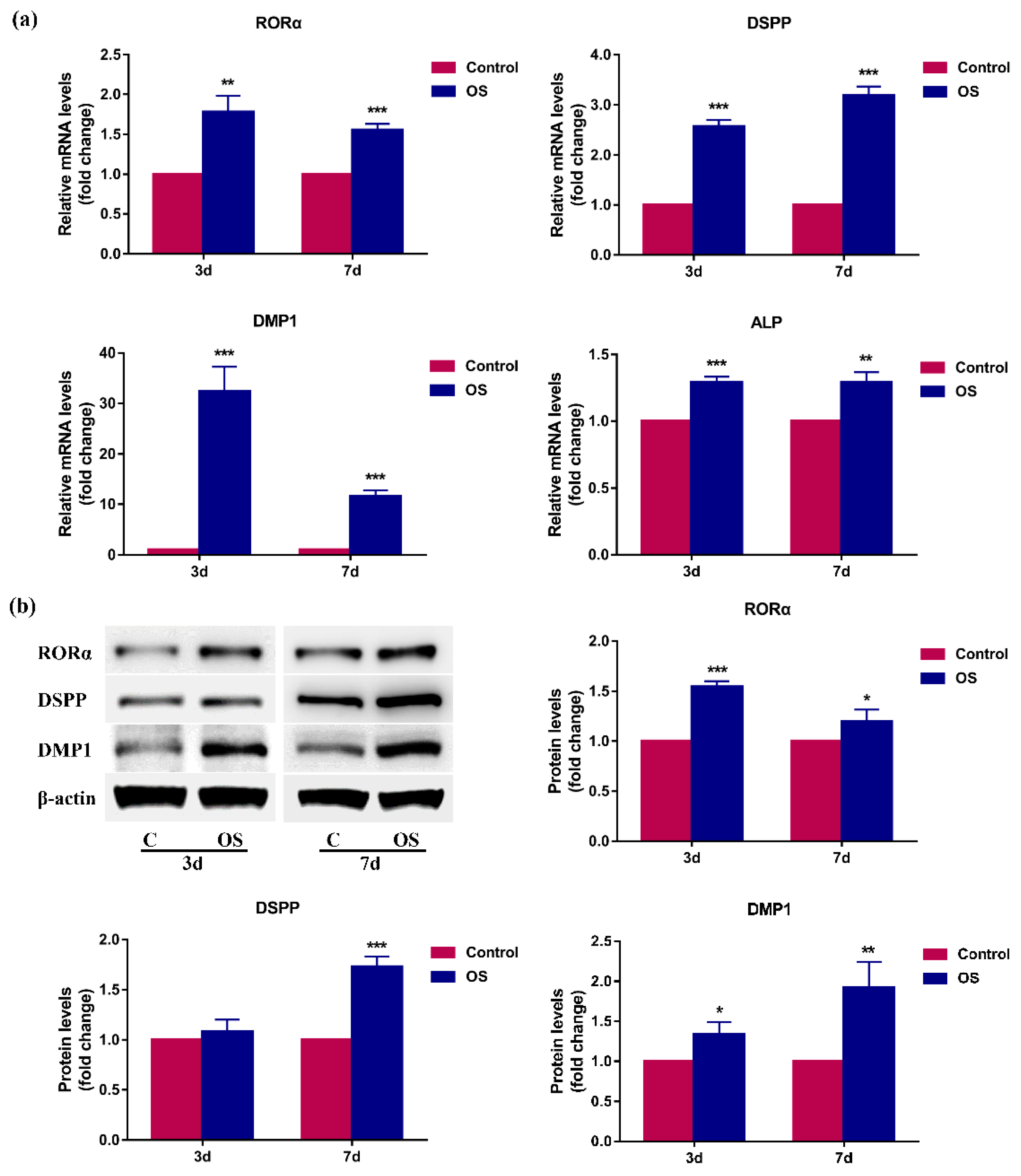
2.2. RORα Is Upregulated during Odontoblastic Differentiation of rDPCs In Vitro
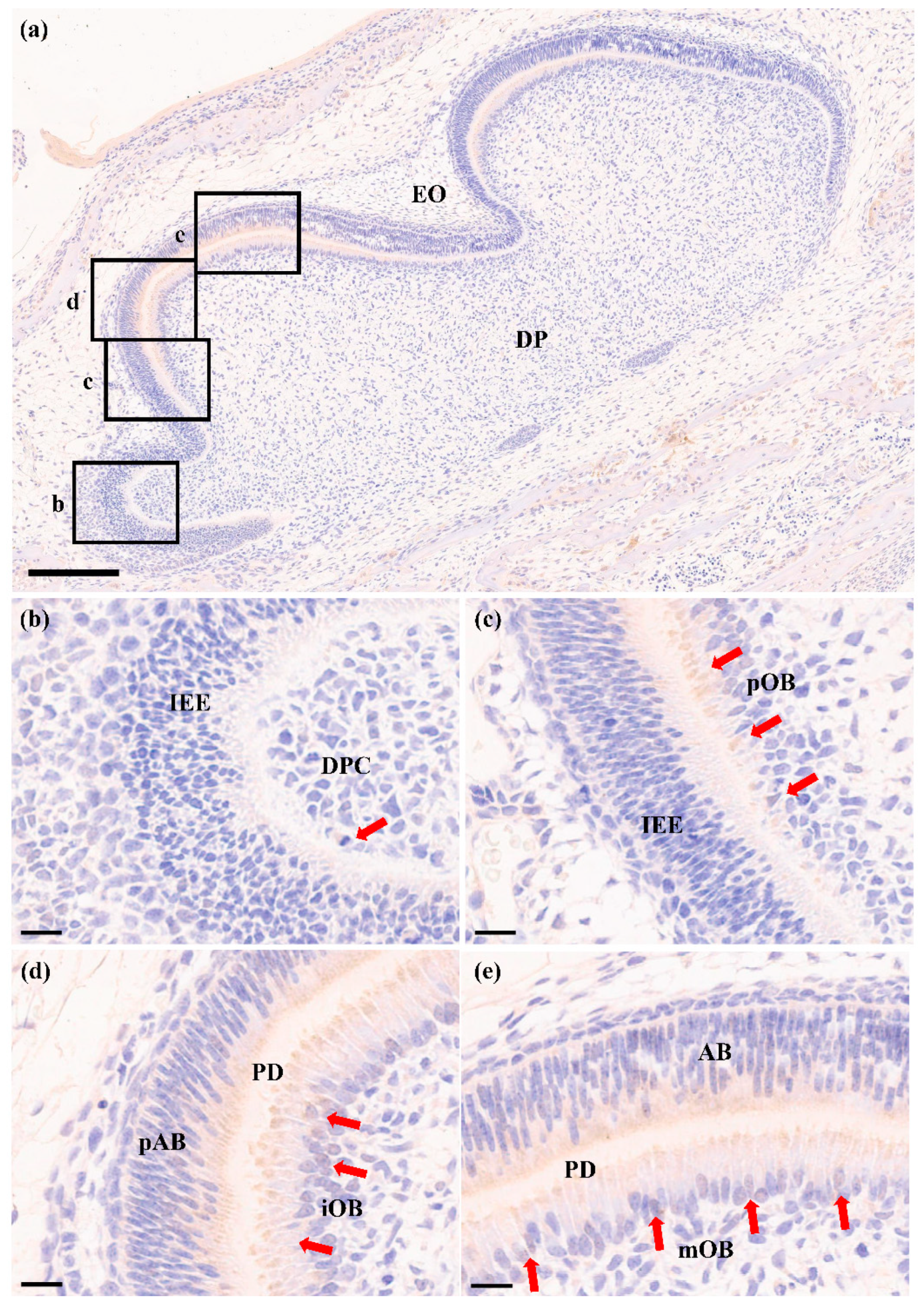
2.3. RORα Is Increased during Odontoblastic Differentiation In Vivo
2.4. Overexpression of RORα Promotes Odontoblastic Differentiation in rDPCs
2.5. Knockdown of RORα Inhibits Odontoblastic Differentiation in rDPCs
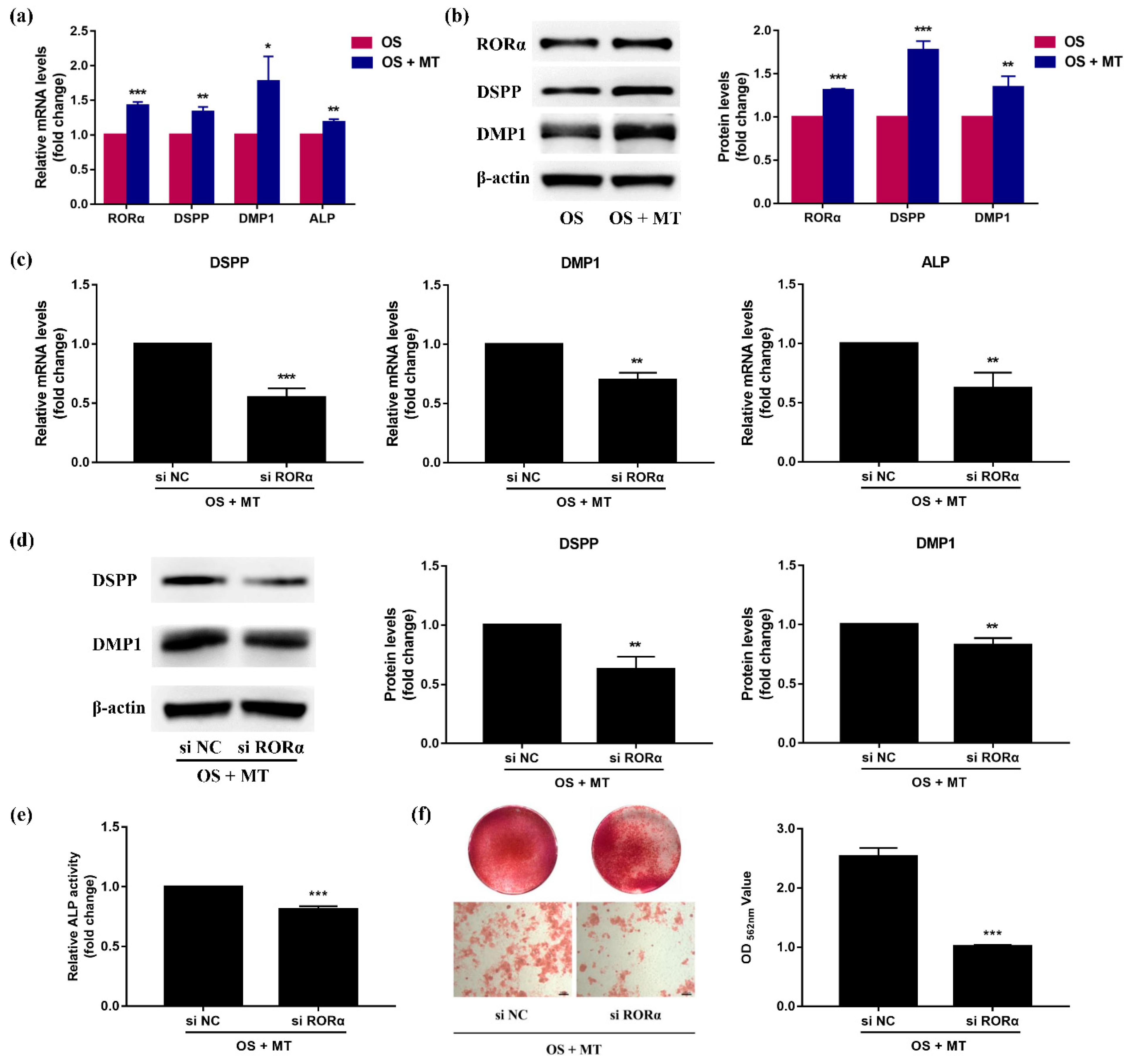
2.6. Melatonin Promotes Odontoblastic Differentiation of rDPCs in an RORα-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Cell Transfection
4.3. Immunofluorescence Staining
4.4. Immunohistochemistry
4.5. RNA Isolation, Quantitative Real-Time Polymerase Chain Reaction and Agarose Gel Electrophoresis
4.6. Western Blotting
4.7. Alkaline Phosphatase Activity
4.8. Alizarin Red Staining
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. WHO 2005, 83, 661–669. [Google Scholar]
- Smith, A.J.; Cooper, P.R. Regenerative Endodontics: Burning Questions. J. Endod. 2017, 43, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef]
- Bègue-Kirn, C.; Ruch, J.V.; Ridall, A.L.; Butler, W.T. Comparative analysis of mouse DSP and DPP expression in odontoblasts, preameloblasts, and experimentally induced odontoblast-like cells. Eur. J. Oral Sci. 1998, 106 (Suppl. 1), 254–259. [Google Scholar] [CrossRef]
- Kikuchi, H.; Suzuki, K.; Sakai, N.; Yamada, S. Odontoblasts induced from mesenchymal cells of murine dental papillae in three-dimensional cell culture. Cell Tissue Res. 2004, 317, 173–185. [Google Scholar] [CrossRef]
- Tziafas, D.; Kodonas, K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J. Endod. 2010, 36, 781–789. [Google Scholar] [CrossRef]
- Thesleff, I.; Nieminen, P. Tooth morphogenesis and cell differentiation. Curr. Opin. Cell Biol. 1996, 8, 844–850. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Z.; Sun, W.; Li, J.; Liang, Y.; Yang, X.; Xu, Y.; Yu, M.; Tian, W.; Chen, G.; et al. Inhibition of Ape1 Redox Activity Promotes Odonto/osteogenic Differentiation of Dental Papilla Cells. Sci. Rep. 2015, 5, 17483. [Google Scholar] [CrossRef]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8, 2041731417702531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, L.; He, Y.; Fan, W.; Guan, X.; Deng, Q.; Huang, F.; He, H. Changes of mitochondrial respiratory function during odontogenic differentiation of rat dental papilla cells. J. Mol. Histol. 2018, 49, 51–61. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, hormone of darkness and more—Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef]
- Raghavendra, V.; Singh, V.; Kulkarni, S.K.; Agrewala, J.N. Melatonin enhances Th2 cell mediated immune responses: Lack of sensitivity to reversal by naltrexone or benzodiazepine receptor antagonists. Mol. Cell. Biochem. 2001, 221, 57–62. [Google Scholar] [CrossRef]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J. Pineal Res. 2011, 50, 132–139. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin receptor genes in vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Mohebbi, I.; Rastegar, M.; Kaviani, M.; Darband, S.G.; Jahanban-Esfahlan, R.; Nabavi, S.M.; Yousefi, B. The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 2018, 45, 33–52. [Google Scholar] [CrossRef]
- Mendivil-Perez, M.; Soto-Mercado, V.; Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Shen, Y.Q.; Tejada, M.A.; Capilla-Gonzalez, V.; Rusanova, I.; Garcia-Verdugo, J.M.; et al. Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef]
- Slominski, A.; Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Semak, I.; Zbytek, B.; Slominski, R.M.; Tobin, D.J. On the role of melatonin in skin physiology and pathology. Endocrine 2005, 27, 137–148. [Google Scholar] [CrossRef]
- Solís-Chagoyán, H.; Domínguez-Alonso, A.; Valdés-Tovar, M.; Argueta, J.; Sánchez-Florentino, Z.A.; Calixto, E.; Benítez-King, G. Melatonin Rescues the Dendrite Collapse Induced by the Pro-Oxidant Toxin Okadaic Acid in Organotypic Cultures of Rat Hilar Hippocampus. Molecules 2020, 25, 5508. [Google Scholar] [CrossRef]
- Gómez-Moreno, G.; Guardia, J.; Ferrera, M.J.; Cutando, A.; Reiter, R.J. Melatonin in diseases of the oral cavity. Oral Dis. 2010, 16, 242–247. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Gómez-Moreno, G.; Barone, A.; Cutando, A.; Alcaraz-Baños, M.; Chiva, F.; López-Marí, L.; Guardia, J. Melatonin plus porcine bone on discrete calcium deposit implant surface stimulates osteointegration in dental implants. J. Pineal Res. 2009, 47, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Srinath, R.; Acharya, A.B.; Thakur, S.L. Salivary and gingival crevicular fluid melatonin in periodontal health and disease. J. Periodontol. 2010, 81, 277–283. [Google Scholar] [CrossRef]
- Cutando, A.; Aneiros-Fernández, J.; Aneiros-Cachaza, J.; Arias-Santiago, S. Melatonin and cancer: Current knowledge and its application to oral cavity tumours. J. Oral Pathol. Med. 2011, 40, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A Review of Its Potential Functions and Effects on Dental Diseases. Int. J. Mol. Sci. 2017, 18, 865. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Fan, W.; Dong, W.; Fu, S.; He, H.; Huang, F. Melatonin influences proliferation and differentiation of rat dental papilla cells in vitro and dentine formation in vivo by altering mitochondrial activity. J. Pineal Res. 2013, 54, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Zhang, F.P.; He, Y.F.; Fan, W.G.; Zheng, M.M.; Kang, J.; Huang, F.; He, H.W. Melatonin regulates mitochondrial function and biogenesis during rat dental papilla cell differentiation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5967–5979. [Google Scholar] [CrossRef]
- Solt, L.A.; Burris, T.P. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol. Metab. 2012, 23, 619–627. [Google Scholar] [CrossRef]
- Giguère, V.; McBroom, L.D.; Flock, G. Determinants of target gene specificity for ROR alpha 1: Monomeric DNA binding by an orphan nuclear receptor. Mol. Cell. Biol. 1995, 15, 2517–2526. [Google Scholar] [CrossRef]
- Medvedev, A.; Yan, Z.H.; Hirose, T.; Giguère, V.; Jetten, A.M. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene 1996, 181, 199–206. [Google Scholar] [CrossRef]
- Hamilton, B.A.; Frankel, W.N.; Kerrebrock, A.W.; Hawkins, T.L.; FitzHugh, W.; Kusumi, K.; Russell, L.B.; Mueller, K.L.; van Berkel, V.; Birren, B.W.; et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature 1996, 379, 736–739. [Google Scholar] [CrossRef]
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; Huidobro-Fernández, C.; Soria-Valles, C.; De Gonzalo-Calvo, D.; Tolivia, D.; Gutierrez-Cuesta, J.; Pallas, M.; Camins, A.; et al. Favorable effects of a prolonged treatment with melatonin on the level of oxidative damage and neurodegeneration in senescence-accelerated mice. J. Pineal Res. 2008, 45, 302–311. [Google Scholar] [CrossRef]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M.; Joo, J.H. Retinoid-related Orphan Receptors (RORs): Roles in Cellular Differentiation and Development. Adv. Dev. Biol. 2006, 16, 313–355. [Google Scholar] [CrossRef]
- Steinmayr, M.; André, E.; Conquet, F.; Rondi-Reig, L.; Delhaye-Bouchaud, N.; Auclair, N.; Daniel, H.; Crépel, F.; Mariani, J.; Sotelo, C.; et al. staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 3960–3965. [Google Scholar] [CrossRef]
- Dzhagalov, I.; Giguère, V.; He, Y.W. Lymphocyte development and function in the absence of retinoic acid-related orphan receptor alpha. J. Immunol. 2004, 173, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Duez, H.; Duhem, C.; Laitinen, S.; Patole, P.S.; Abdelkarim, M.; Bois-Joyeux, B.; Danan, J.L.; Staels, B. Inhibition of adipocyte differentiation by RORalpha. FEBS Lett. 2009, 583, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Bailey, P.; Dowhan, D.H.; Muscat, G.E. Exogenous expression of a dominant negative RORalpha1 vector in muscle cells impairs differentiation: RORalpha1 directly interacts with p300 and myoD. Nucleic Acids Res. 1999, 27, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Benderdour, M.; Fahmi, H.; Beaudet, F.; Fernandes, J.C.; Shi, Q. Nuclear receptor retinoid-related orphan receptor alpha1 modulates the metabolic activity of human osteoblasts. J. Cell. Biochem. 2011, 112, 2160–2169. [Google Scholar] [CrossRef]
- Delerive, P.; Monté, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J.C.; Staels, B. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. Embo Rep. 2001, 2, 42–48. [Google Scholar] [CrossRef]
- Miyamoto, S.; Cooper, L.; Watanabe, K.; Yamamoto, S.; Inoue, H.; Mishima, K.; Saito, I. Role of retinoic acid-related orphan receptor-alpha in differentiation of human mesenchymal stem cells along with osteoblastic lineage. Pathobiology 2010, 77, 28–37. [Google Scholar] [CrossRef]
- Meyer, T.; Kneissel, M.; Mariani, J.; Fournier, B. In vitro and in vivo evidence for orphan nuclear receptor RORalpha function in bone metabolism. Proc. Natl. Acad. Sci. USA 2000, 97, 9197–9202. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Li, Y.; Guo, X.; Li, J.; Zhong, R.; Zhang, X. Melatonin-induced demethylation of antioxidant genes increases antioxidant capacity through RORalpha in cumulus cells of prepubertal lambs. Free Radical Biol. Med. 2019, 131, 173–183. [Google Scholar] [CrossRef]
- Xu, L.; Su, Y.; Zhao, Y.; Sheng, X.; Tong, R.; Ying, X.; Gao, L.; Ji, Q.; Gao, Y.; Yan, Y.; et al. Melatonin differentially regulates pathological and physiological cardiac hypertrophy: Crucial role of circadian nuclear receptor RORalpha signaling. J. Pineal Res. 2019, 67, e12579. [Google Scholar] [CrossRef]
- García, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; López, L.C.; Acuña-Castroviejo, D. Disruption of the NF-κB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-α and blocks the septic response in mice. FASEB J. 2015, 29, 3863–3875. [Google Scholar] [CrossRef]
- Shajari, S.; Laliena, A.; Heegsma, J.; Tunon, M.J.; Moshage, H.; Faber, K.N. Melatonin suppresses activation of hepatic stellate cells through RORalpha-mediated inhibition of 5-lipoxygenase. J. Pineal Res. 2015, 59, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Chen, S.; Gluhak-Heinrich, J.; Wang, Y.H.; Wu, Y.M.; Chuang, H.H.; Chen, L.; Yuan, G.H.; Dong, J.; Gay, I.; MacDougall, M. Runx2, osx, and dspp in tooth development. J. Dent. Res. 2009, 88, 904–909. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Yuan, G.; Liu, P.; Fan, M. The effects of osterix on the proliferation and odontoblastic differentiation of human dental papilla cells. J. Endod. 2014, 40, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y.; Li, Z.; Zhou, Y.; Gu, Y.; Pang, X.; Wu, J.; Gobin, R.; Yu, J. Oestrogen receptor alpha regulates the odonto/osteogenic differentiation of stem cells from apical papilla via ERK and JNK MAPK pathways. Cell Prolif. 2018, 51, e12485. [Google Scholar] [CrossRef]
- Kim, J.W.; Choi, H.; Jeong, B.C.; Oh, S.H.; Hur, S.W.; Lee, B.N.; Kim, S.H.; Nör, J.E.; Koh, J.T.; Hwang, Y.C. Transcriptional factor ATF6 is involved in odontoblastic differentiation. J. Dent. Res. 2014, 93, 483–489. [Google Scholar] [CrossRef]
- Kuzynski, M.; Goss, M.; Bottini, M.; Yadav, M.C.; Mobley, C.; Winters, T.; Poliard, A.; Kellermann, O.; Lee, B.; Millan, J.L.; et al. Dual role of the Trps1 transcription factor in dentin mineralization. J. Biol. Chem. 2014, 289, 27481–27493. [Google Scholar] [CrossRef]
- André, E.; Gawlas, K.; Becker-André, M. A novel isoform of the orphan nuclear receptor RORbeta is specifically expressed in pineal gland and retina. Gene 1998, 216, 277–283. [Google Scholar] [CrossRef]
- André, E.; Conquet, F.; Steinmayr, M.; Stratton, S.C.; Porciatti, V.; Becker-André, M. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998, 17, 3867–3877. [Google Scholar] [CrossRef]
- Eberl, G.; Littman, D.R. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer’s patches. Immunol. Rev. 2003, 195, 81–90. [Google Scholar] [CrossRef]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept Signal 2009, 7, e003. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Unutmaz, D.; Zou, Y.R.; Sunshine, M.J.; Pierani, A.; Brenner-Morton, S.; Mebius, R.E.; Littman, D.R. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science 2000, 288, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jia, Q.; He, W.; Liu, J.; Hou, L.; Zhang, J.; Niu, Z.; Ni, L. Conditioned medium from periapical follicle cells induces the odontogenic differentiation of stem cells from the apical papilla in vitro. J. Endod. 2013, 39, 1015–1022. [Google Scholar] [CrossRef]
- Halling Linder, C.; Ek-Rylander, B.; Krumpel, M.; Norgård, M.; Narisawa, S.; Millán, J.L.; Andersson, G.; Magnusson, P. Bone Alkaline Phosphatase and Tartrate-Resistant Acid Phosphatase: Potential Co-regulators of Bone Mineralization. Calcif. Tissue Int. 2017, 101, 92–101. [Google Scholar] [CrossRef]
- Narayanan, K.; Srinivas, R.; Ramachandran, A.; Hao, J.; Quinn, B.; George, A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc. Natl. Acad. Sci. USA 2001, 98, 4516–4521. [Google Scholar] [CrossRef]
- MacDougall, M.; Gu, T.T.; Luan, X.; Simmons, D.; Chen, J. Identification of a novel isoform of mouse dentin matrix protein 1: Spatial expression in mineralized tissues. J. Bone Miner. Res. 1998, 13, 422–431. [Google Scholar] [CrossRef]
- Min, H.Y.; Son, H.E.; Jang, W.G. Estradiol-induced RORalpha expression positively regulates osteoblast differentiation. Steroids 2019, 149, 108412. [Google Scholar] [CrossRef] [PubMed]
- Ohoka, N.; Kato, S.; Takahashi, Y.; Hayashi, H.; Sato, R. The orphan nuclear receptor RORalpha restrains adipocyte differentiation through a reduction of C/EBPbeta activity and perilipin gene expression. Mol. Endocrinol. 2009, 23, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Haakonsson, A.K.; Madsen, J.G.S.; Larsen, M.; Forss, I.; Madsen, M.R.; Van Hauwaert, E.L.; Wiwie, C.; Jespersen, N.Z.; Tencerova, M.; et al. Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nat. Genet. 2019, 51, 716–727. [Google Scholar] [CrossRef]
- Farez, M.F.; Calandri, I.L.; Correale, J.; Quintana, F.J. Anti-inflammatory effects of melatonin in multiple sclerosis. Bioessays 2016, 38, 1016–1026. [Google Scholar] [CrossRef]
- Lardone, P.J.; Guerrero, J.M.; Fernandez-Santos, J.M.; Rubio, A.; Martin-Lacave, I.; Carrillo-Vico, A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid-related orphan receptor. J. Pineal Res. 2011, 51, 454–462. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Jetten, A.M. RORalpha is not a receptor for melatonin (response to DOI 10.1002/bies.201600018). Bioessays 2016, 38, 1193–1194. [Google Scholar] [CrossRef]
- Xiang, S.; Dauchy, R.T.; Hauch, A.; Mao, L.; Yuan, L.; Wren, M.A.; Belancio, V.P.; Mondal, D.; Frasch, T.; Blask, D.E.; et al. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 2015, 59, 60–69. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chiang, P.C.; Chen, Y.L.; Chien, Y.W. Effects of Melatonin on Lipid Metabolism and Circulating Irisin in Sprague-Dawley Rats with Diet-Induced Obesity. Molecules 2020, 25, 3329. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Witt-Enderby, P.A. Melatonin effects on bone: Potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Chen, H.H.; Lin, K.C.; Wallace, C.G.; Chen, Y.T.; Yang, C.C.; Leu, S.; Chen, Y.C.; Sun, C.K.; Tsai, T.H.; Chen, Y.L.; et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J. Pineal Res. 2014, 57, 16–32. [Google Scholar] [CrossRef] [PubMed]
- García-Bernal, D.; López-García, S.; Sanz, J.L.; Guerrero-Gironés, J.; García-Navarro, E.M.; Moraleda, J.M.; Forner, L.; Rodríguez-Lozano, F.J. Melatonin Treatment Alters Biological and Immunomodulatory Properties of Human Dental Pulp Mesenchymal Stem Cells via Augmented Transforming Growth Factor Beta Secretion. J. Endod. 2020. [Google Scholar] [CrossRef]
- Tachibana, R.; Tatehara, S.; Kumasaka, S.; Tokuyama, R.; Satomura, K. Effect of melatonin on human dental papilla cells. Int. J. Mol. Sci. 2014, 15, 17304–17317. [Google Scholar] [CrossRef] [PubMed]
- Beak, J.Y.; Kang, H.S.; Huang, W.; Myers, P.H.; Bowles, D.E.; Jetten, A.M.; Jensen, B.C. The nuclear receptor RORα protects against angiotensin II-induced cardiac hypertrophy and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H186–H200. [Google Scholar] [CrossRef]
- Montague, C.R.; Fitzmaurice, A.; Hover, B.M.; Salazar, N.A.; Fey, J.P. Screen for small molecules increasing the mitochondrial membrane potential. J. Biomol. Screen. 2014, 19, 387–398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.J.; Han, Y.H.; Na, H.; Kim, J.Y.; Kim, T.; Kim, H.J.; Shin, C.; Lee, J.W.; Lee, M.O. Liver-specific deletion of RORalpha aggravates diet-induced nonalcoholic steatohepatitis by inducing mitochondrial dysfunction. Sci. Rep. 2017, 7, 16041. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| RORα | CTACCAGAACAAGCAGAGA | CGAACTCCACCACATACT |
| RORβ | ATCCGCTAACAGGCACAGATG | AGGAAAGAAAGAAAGGCGGCA |
| RORγ | CTGGCTGCAAAGAAGACCCA | CCCGTAGTGGATGCCAGATG |
| DSPP | ACAGCGACAGCGACGATTC | CCTCCTACGGCTATCGACTC |
| DMP1 | CTGGTATCAGGTCGGAAGAATC | CTCTCATTAGACTCGCTGTCAC |
| ALP | GGAAGGAGGCAGGATTGA | TCAGCAGTAACCACAGTCA |
| GAPDH | TATGACTCTACCCACGGCAAGT | ATACTCAGCACCAGCATCACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Chen, H.; Zhang, F.; Yan, T.; Fan, W.; Jiang, L.; He, H.; Huang, F. RORα Regulates Odontoblastic Differentiation and Mediates the Pro-Odontogenic Effect of Melatonin on Dental Papilla Cells. Molecules 2021, 26, 1098. https://doi.org/10.3390/molecules26041098
Kang J, Chen H, Zhang F, Yan T, Fan W, Jiang L, He H, Huang F. RORα Regulates Odontoblastic Differentiation and Mediates the Pro-Odontogenic Effect of Melatonin on Dental Papilla Cells. Molecules. 2021; 26(4):1098. https://doi.org/10.3390/molecules26041098
Chicago/Turabian StyleKang, Jun, Haoling Chen, Fuping Zhang, Tong Yan, Wenguo Fan, Liulin Jiang, Hongwen He, and Fang Huang. 2021. "RORα Regulates Odontoblastic Differentiation and Mediates the Pro-Odontogenic Effect of Melatonin on Dental Papilla Cells" Molecules 26, no. 4: 1098. https://doi.org/10.3390/molecules26041098
APA StyleKang, J., Chen, H., Zhang, F., Yan, T., Fan, W., Jiang, L., He, H., & Huang, F. (2021). RORα Regulates Odontoblastic Differentiation and Mediates the Pro-Odontogenic Effect of Melatonin on Dental Papilla Cells. Molecules, 26(4), 1098. https://doi.org/10.3390/molecules26041098






