Optical Response Characteristics of Single-Walled Carbon Nanotube Chirality Exposed to Oxidants with Different Oxidizing Power
Abstract
1. Introduction
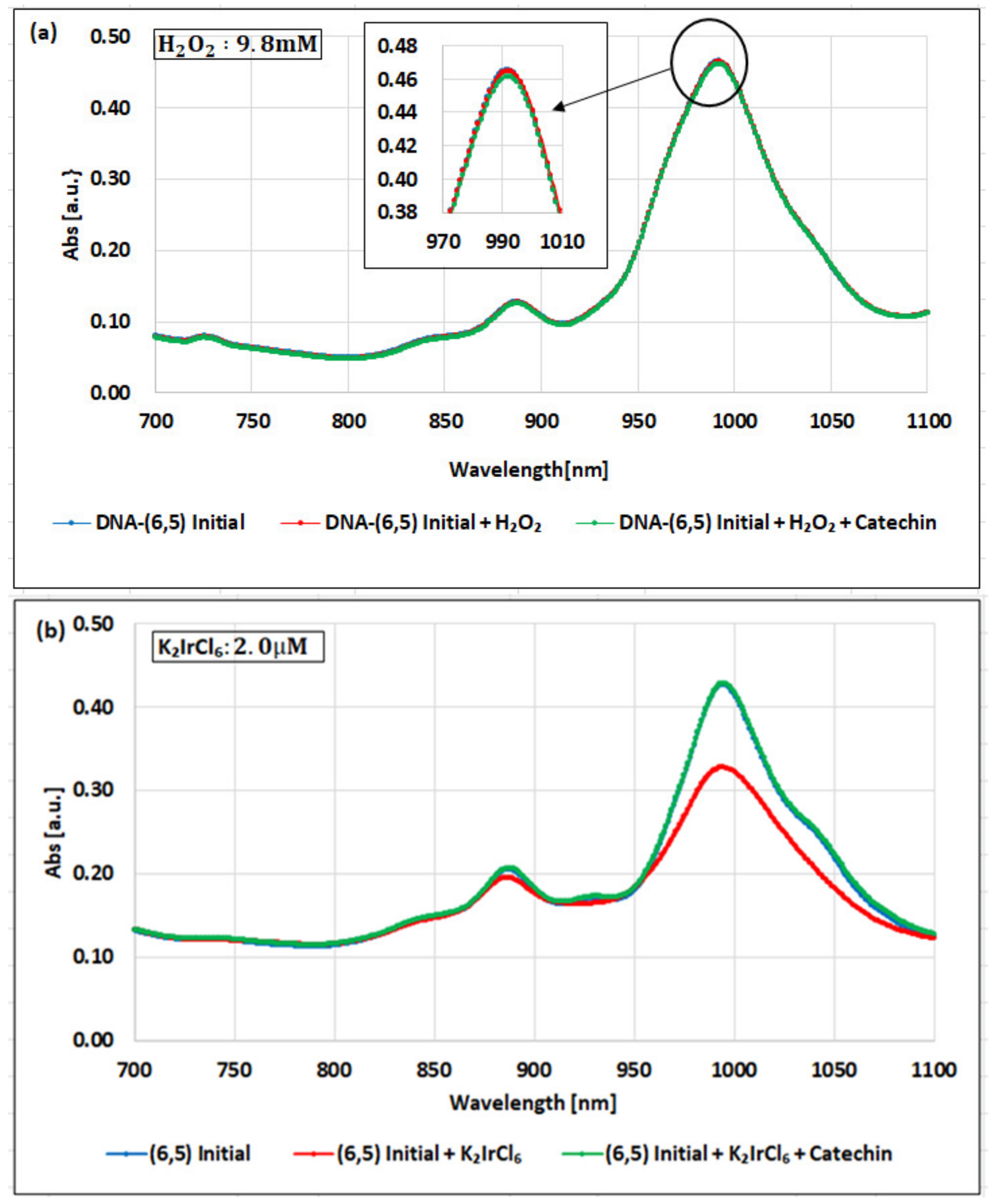
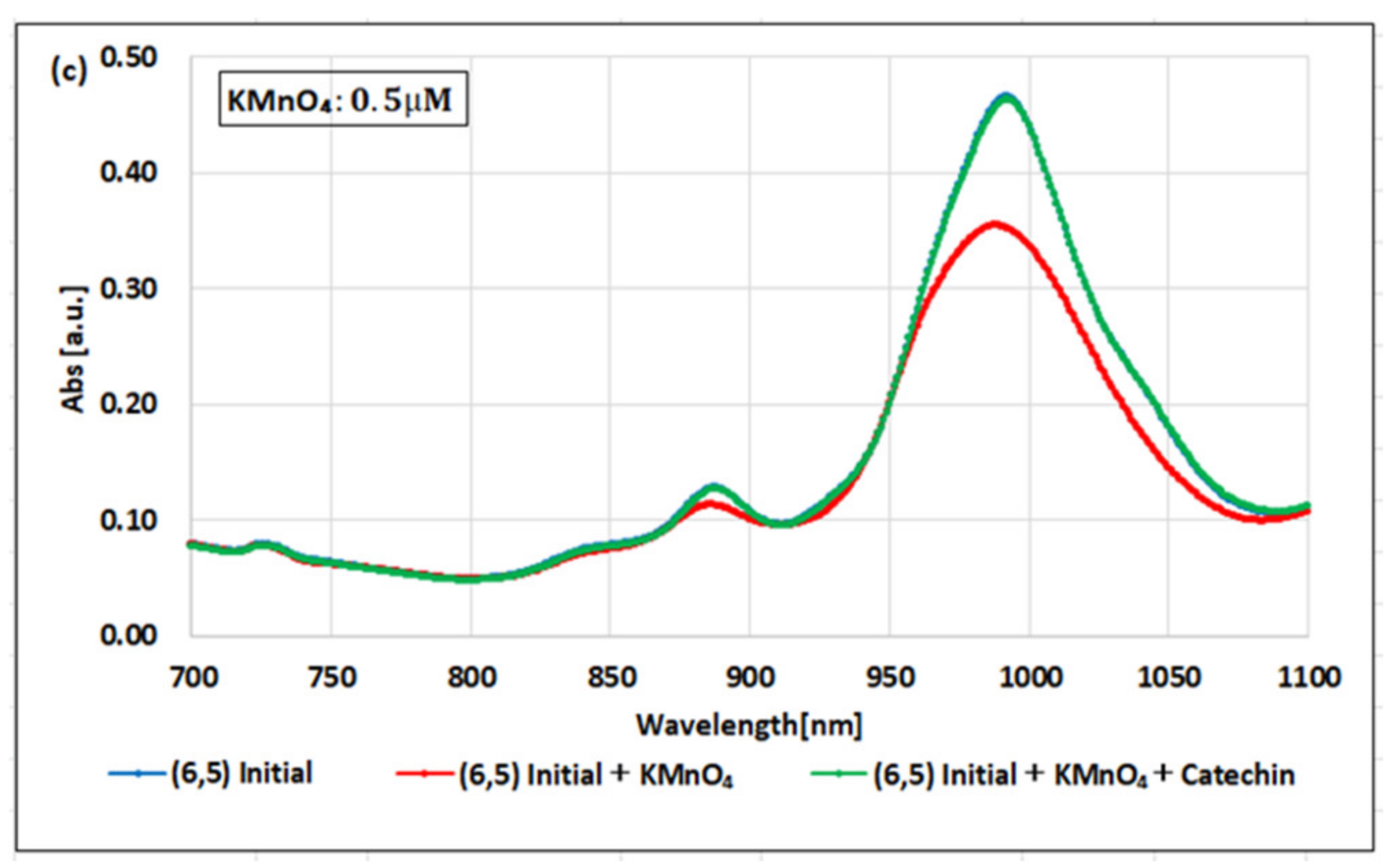
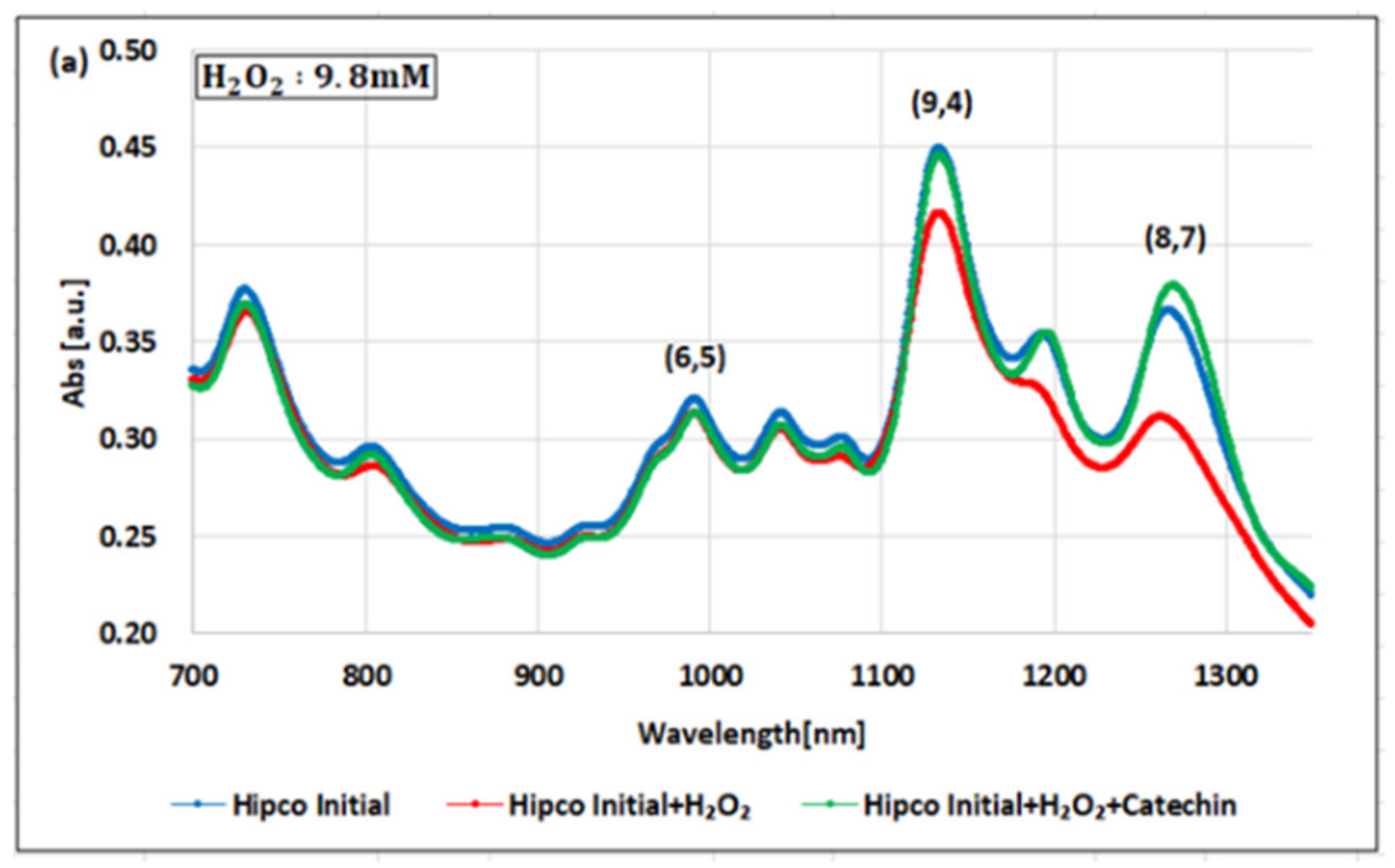
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nakashima, N.; Okuzono, S.; Murakami, H.; Nakai, T.; Yoshikawa, K. DNA dissolves single-walled carbon nanotubes in water. Chem. Lett. 2003, 32, 456–457. [Google Scholar] [CrossRef]
- Nakashima, N. Solubilization of single-walled carbon nanotubes with condensed aromatic compounds. Sci. Technol. Adv. Mater. 2006, 7, 609–616. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Gao, L.; Zheng, S.; Wang, Y.; Sun, J.; Kajiura, H.; Li, Y.; Noda, K. Debundling of single-walled carbon nanotubes by using natural polyelectrolytes. Nanotechnology 2007, 18, 6. [Google Scholar] [CrossRef]
- Umemura, K. Hybrids of Nucleic Acids and Carbon Nanotubes for Nanobiotechnology. Nanomaterials 2015, 5, 321–350. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Boul, P.; Ericson, L.M.; Huffman, C.; Wang, Y.H.; Haroz, E.; Kuper, C.; Tour, J.; Ausman, K.D.; Smalley, R.E. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 2001, 342, 265–271. [Google Scholar] [CrossRef]
- Star, A.; Stoddart, J.F.; Steuerman, D.; Diehl, M.; Boukai, A.; Wong, E.W.; Yang, X.; Chung, S.W.; Choi, H.; Heath, J.R. Preparation and properties of polymer-wrapped single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2001, 40, 1721–1725. [Google Scholar] [CrossRef]
- Dieckmann, G.R.; Dalton, A.B.; Johnson, P.A.; Razal, J.; Chen, J.; Giordano, G.M.; Munoz, E.; Musselman, I.H.; Baughman, R.H.; Draper, R.K. Controlled assembly of carbon nanotubes by designed amphiphilic peptide helices. J. Am. Chem. Soc. 2003, 125, 1770–1777. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Qin, L.C. Determination of the chiral indices (n,m) of carbon nanotubes by electron diffraction. Phys. Chem. Chem. Phys. 2007, 9, 31–48. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Umemura, K. Chirality luminescent properties of single-walled carbon nanotubes during redox reactions. Opt. Mater. 2021, 112, 110748. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; McLean, R.S.; Onoa, G.B.; et al. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 2003, 302, 1545–1548. [Google Scholar] [CrossRef]
- Bachilo, S.M.; Strano, M.S.; Kittrell, C.; Hauge, R.H.; Smalley, R.E.; Weisman, R.B. Structure-assigned optical spectra of single-walled carbon nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef]
- Berciaud, S.; Cognet, L.; Poulin, P.; Weisman, R.B.; Lounis, B. Absorption spectroscopy of individual single-walled carbon nanotubes. Nano Lett. 2007, 7, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujigaya, T.; Niidome, Y.; Nakashima, N. Fundamental properties of oligo double-stranded DNA/single-walled carbon nanotube nanobiohybrids. Nanoscale 2010, 2, 1767–1772. [Google Scholar] [CrossRef]
- Strano, M.S.; Huffman, C.B.; Moore, V.C.; O’Connell, M.J.; Haroz, E.H.; Hubbard, J.; Miller, M.; Rialon, K.; Kittrell, C.; Ramesh, S.; et al. Reversible, band-gap-selective protonation of single-walled carbon nanotubes in solution. J. Phys. Chem. B 2003, 107, 6979–6985. [Google Scholar] [CrossRef]
- Lee, A.J.; Wang, X.Y.; Carlson, L.J.; Smyder, J.A.; Loesch, B.; Tu, X.M.; Zheng, M.; Krauss, T.D. Bright Fluorescence from Individual Single-Walled Carbon Nanotubes. Nano Lett. 2011, 11, 1636–1640. [Google Scholar] [CrossRef]
- Hamon, M.A.; Sorci, G.A.; Sugar, M.A.; McVaugh, J.P.; Walker, T.D. Solution properties of single-walled carbon nanotubes. Abstr. Pap. Am. Chem. Soc. 2005, 230, U3674. [Google Scholar]
- Tu, X.M.; Manohar, S.; Jagota, A.; Zheng, M. DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature 2009, 460, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.M.; Zheng, M. A DNA-Based Approach to the Carbon Nanotube Sorting Problem. Nano Res. 2008, 1, 185–194. [Google Scholar] [CrossRef]
- Weisman, R.B.; Bachilo, S.M. Dependence of optical transition energies on structure for single-walled carbon nanotubes in aqueous suspension: An empirical Kataura plot. Nano Lett. 2003, 3, 1235–1238. [Google Scholar] [CrossRef]
- Choi, J.H.; Strano, M.S. Solvatochromism in single-walled carbon nanotubes. Appl. Phys. Lett. 2007, 90, 3. [Google Scholar] [CrossRef]
- Polo, E.; Kruss, S. Impact of Redox-Active Molecules on the Fluorescence of Polymer-Wrapped Carbon Nanotubes. J. Phys. Chem. C 2016, 120, 3061–3070. [Google Scholar] [CrossRef]
- Hain, T.C.; Kroker, K.; Stich, D.G.; Hertel, T. Influence of DNA conformation on the dispersion of SWNTs: Single-strand DNA vs. hairpin DNA. Soft Matter 2012, 8, 2820–2823. [Google Scholar] [CrossRef]
- Xu, Y.; Pehrsson, P.E.; Chen, L.W.; Zhang, R.; Zhao, W. Double-stranded DNA single-walled carbon nanotube hybrids for optical hydrogen peroxide and glucose sensing. J. Phys. Chem. C 2007, 111, 8638–8643. [Google Scholar] [CrossRef]
- Tu, X.M.; Pehrsson, P.E.; Zhao, W. Redox reaction of DNA-Encased HiPco carbon nanotubes with hydrogen peroxide: A near infrared optical sensitivity and kinetics study. J. Phys. Chem. C 2007, 111, 17227–17231. [Google Scholar] [CrossRef]
- Zhao, W.; Song, C.H.; Pehrsson, P.E. Water-soluble and optically pH-sensitive single-walled carbon nanotubes from surface modification. J. Am. Chem. Soc. 2002, 124, 12418–12419. [Google Scholar] [CrossRef]
- Song, C.H.; Pehrsson, P.E.; Zhao, W. Recoverable solution reaction of HiPco carbon nanotubes with hydrogen peroxide. J. Phys. Chem. B 2005, 109, 21634–21639. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.Q.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef]
- Zhao, E.H.; Ergul, B.; Zhao, W. Caffeine’s Antioxidant Potency Optically Sensed with Double-Stranded DNA-Encased Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2015, 119, 4068–4075. [Google Scholar] [CrossRef]
- Umemura, K.; Ishibashi, Y.; Ito, M.; Homma, Y. Quantitative Detection of the Disappearance of the Antioxidant Ability of Catechin by Near-Infrared Absorption and Near-Infrared Photoluminescence Spectra of Single-Walled Carbon Nanotubes. ACS Omega 2019, 4, 7750–7758. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A.; Santos, A.P.; Ribeiro, H.B.; Fantini, C.; Souza, M.; Vieira, J.P.M.; Furtado, C.A.; Jiang, J.; Saito, R.; Balzano, L.; et al. Quantifying carbon-nanotube species with resonance Raman scattering. Phys. Rev. B 2005, 72, 5. [Google Scholar] [CrossRef]
- Kato, Y.; Inoue, A.; Niidome, Y.; Nakashima, N. Thermodynamics on Soluble Carbon Nanotubes: How Do DNA Molecules Replace Surfactants on Carbon Nanotubes? Sci. Rep. 2012, 2, 7. [Google Scholar] [CrossRef]
- Knorr, F.J.; Hung, W.C.; Wai, C.M. Aromatic Electron Acceptors Change the Chirality Dependence of Single-Walled Carbon Nanotube Oxidation. Langmuir 2009, 25, 10417–10421. [Google Scholar] [CrossRef] [PubMed]
- Weisman, R.B.; Bachilo, S.M.; Tsyboulski, D. Fluorescence spectroscopy of single-walled carbon nanotubes in aqueous suspension. Appl. Phys. A-Mater. Sci. Process. 2004, 78, 1111–1116. [Google Scholar] [CrossRef]
- Hamano, R.; Miyashiro, D.; Umemura, K. Study on optical response sensitivity in hybrid of single-walled carbon nanotubes mixed with double-stranded DNA and carboxymethylcellulose. Opt. Mater. 2020, 109, 8. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Ito, M.; Homma, Y.; Umemura, K. Monitoring the antioxidant effects of catechin using single-walled carbon nanotubes: Comparative analysis by near-infrared absorption and near-infrared photoluminescence. Colloid Surf. B-Biointerfaces 2018, 161, 139–146. [Google Scholar] [CrossRef]
- Ming, Z.; Diner, B.A. Solution redox chemistry of carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 15490–15494. [Google Scholar]
- Matsukawa, Y.; Ohura, S.; Umemura, K. Differences in the response of the near-infrared absorbance spectra of single-walled carbon nanotubes; Effects of chirality and wrapping polymers. Colloid Surf. B-Biointerfaces 2018, 172, 684–689. [Google Scholar] [CrossRef]
- Hayashida, T.; Umemura, K. Surface morphology of hybrids of double-stranded DNA and single-walled carbon nanotubes studied by atomic force microscopy. Colloid Surf. B-Biointerfaces 2013, 101, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Nii, D.; Hayashida, T.; Yamaguchi, Y.; Ikawa, S.; Shibata, T.; Umemura, K. Selective binding of single-stranded DNA-binding proteins onto DNA molecules adsorbed on single-walled carbon nanotubes. Colloid Surf. B-Biointerfaces 2014, 121, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, T.; Kawashima, T.; Nii, D.; Ozasa, K.; Umemura, K. Kelvin Probe Force Microscopy of Single-walled Carbon Nanotubes Modified with DNA or Poly(ethylene glycol). Chem. Lett. 2013, 42, 666–668. [Google Scholar] [CrossRef]
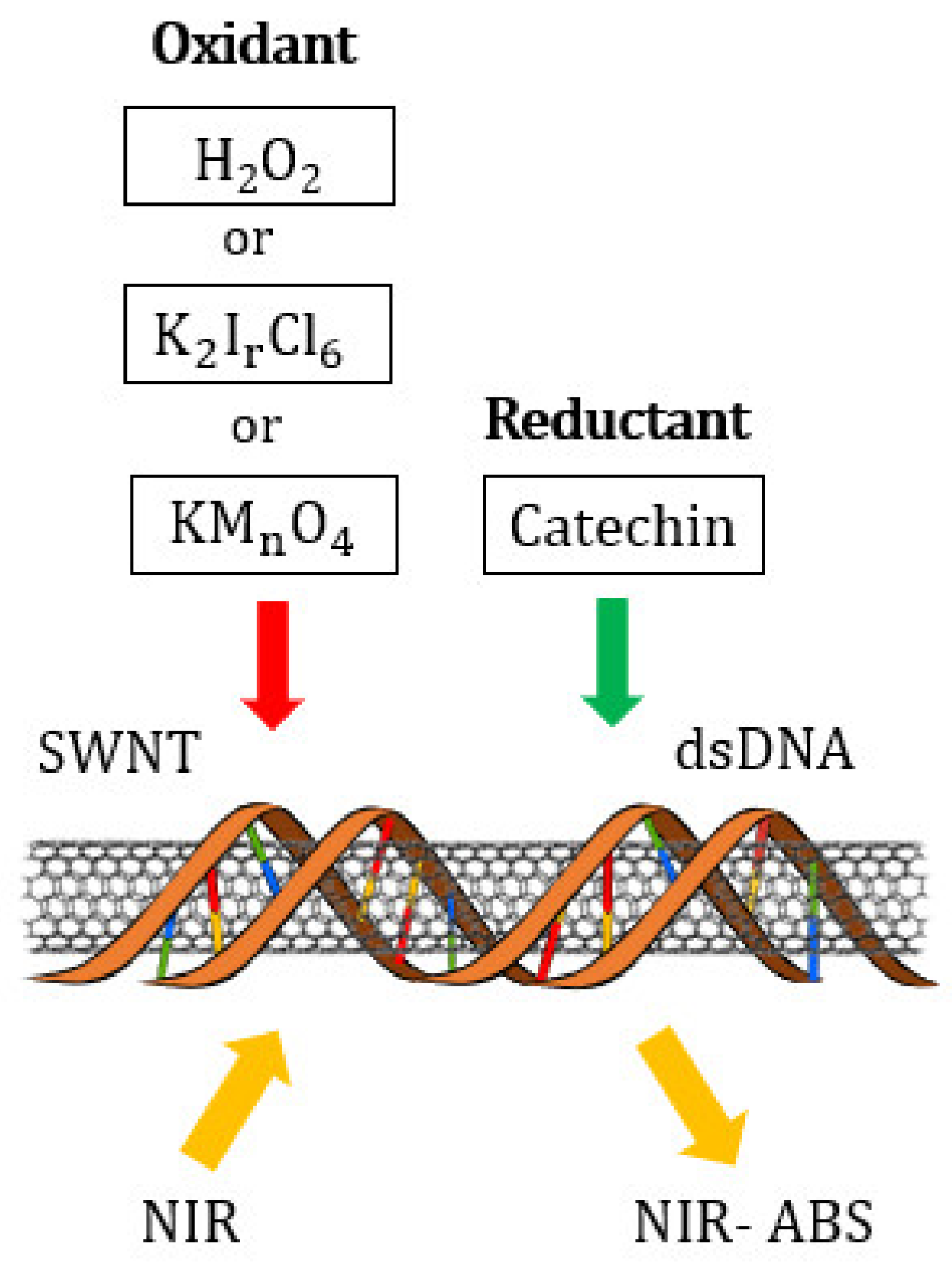
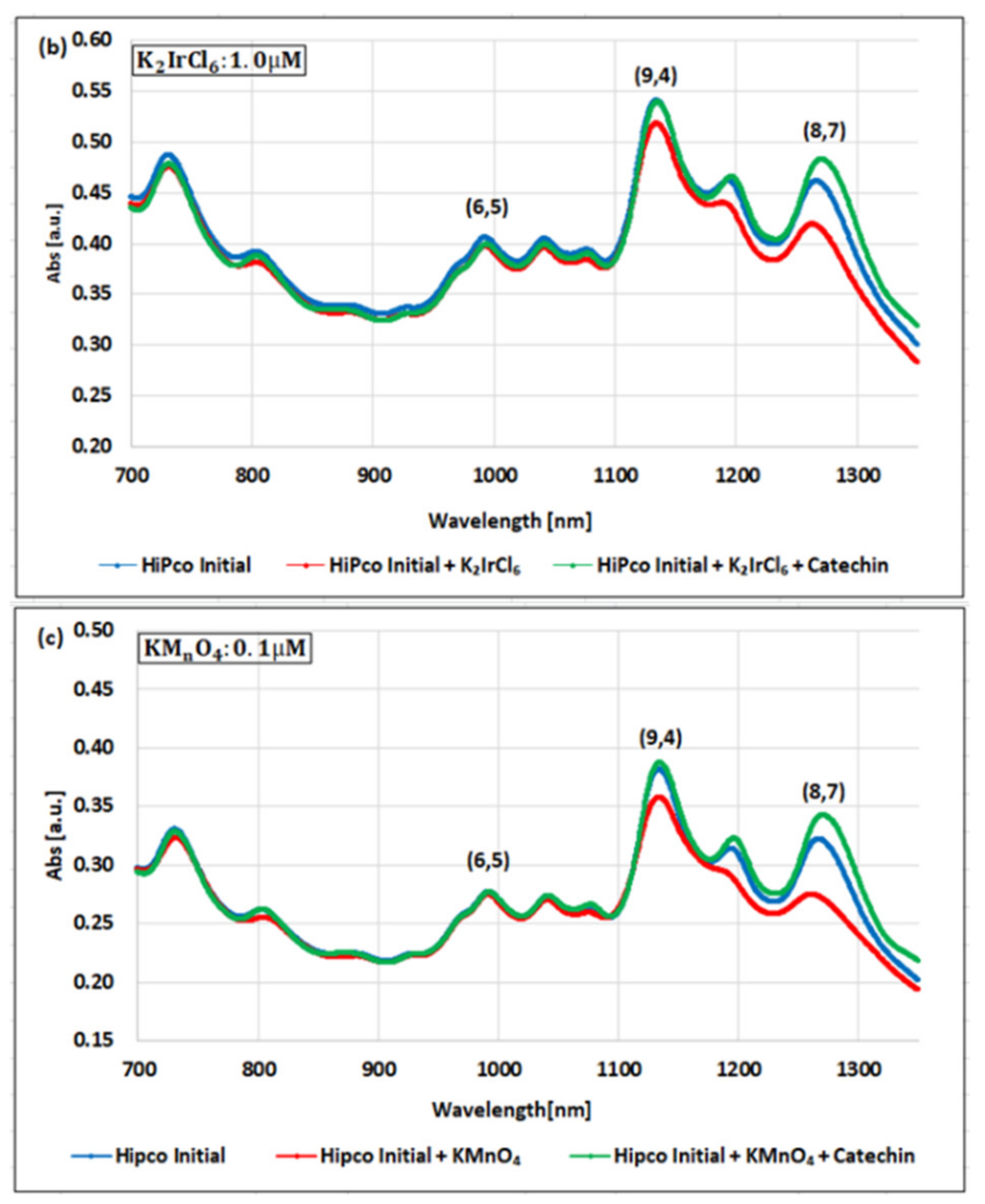
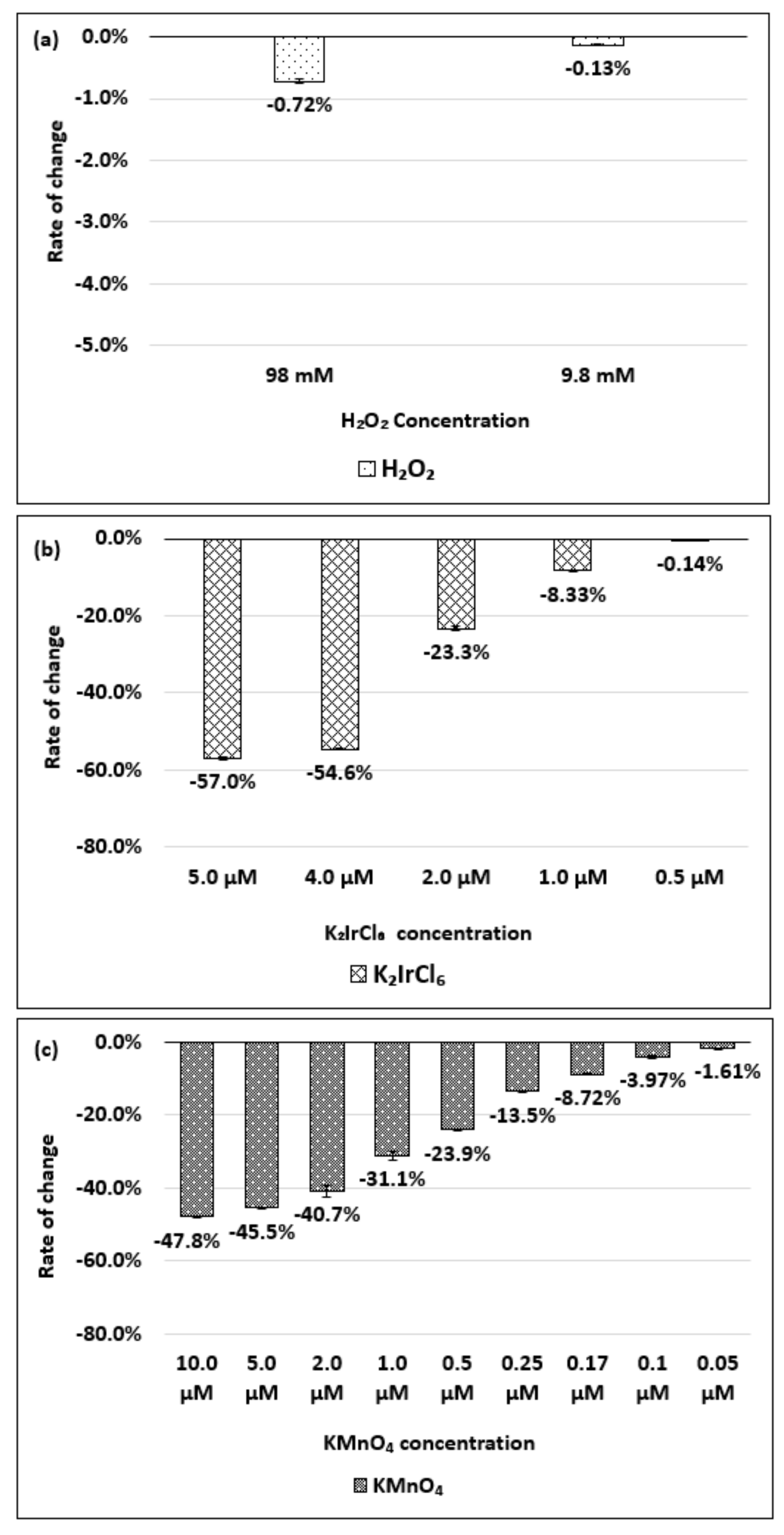
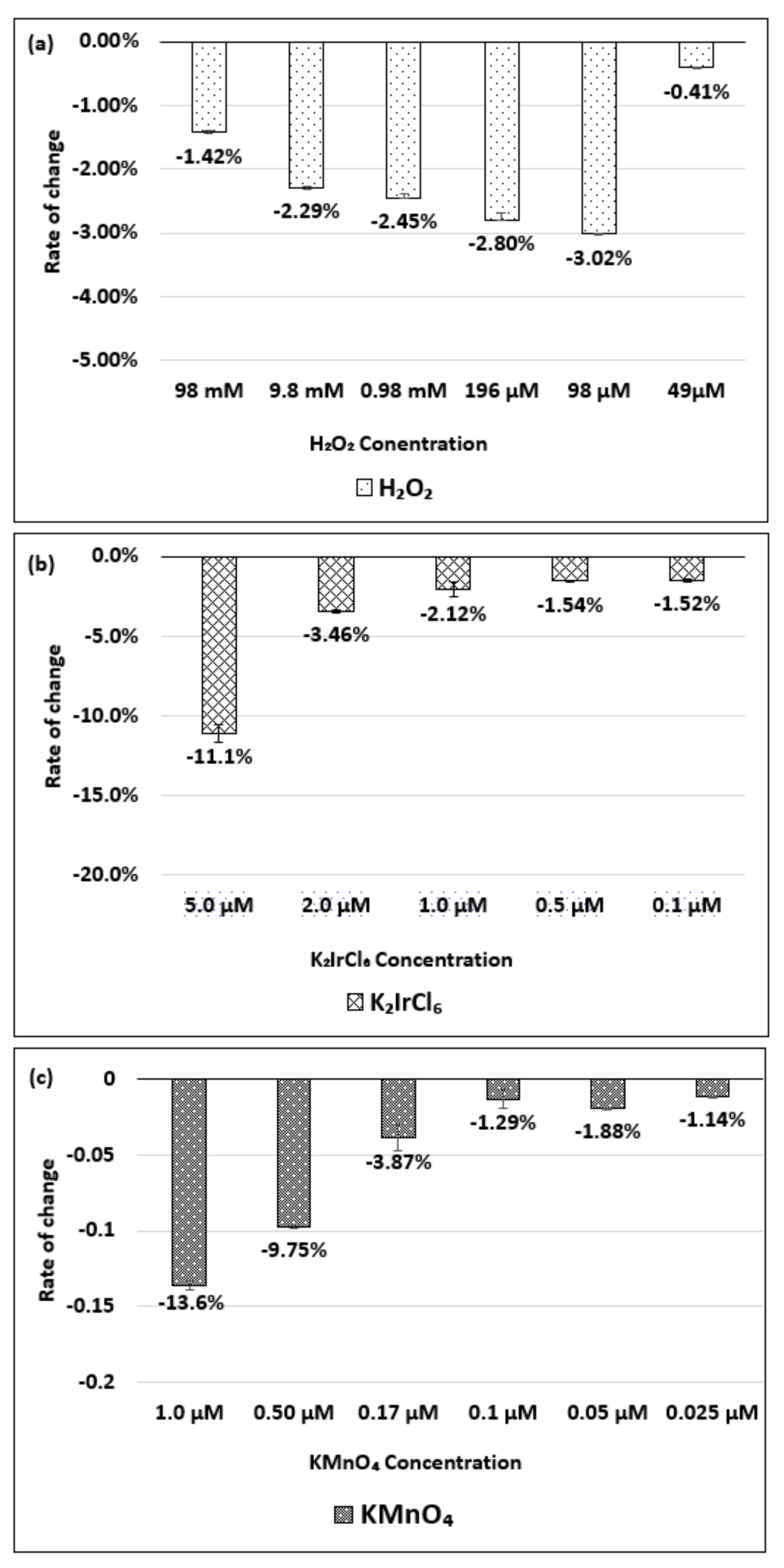
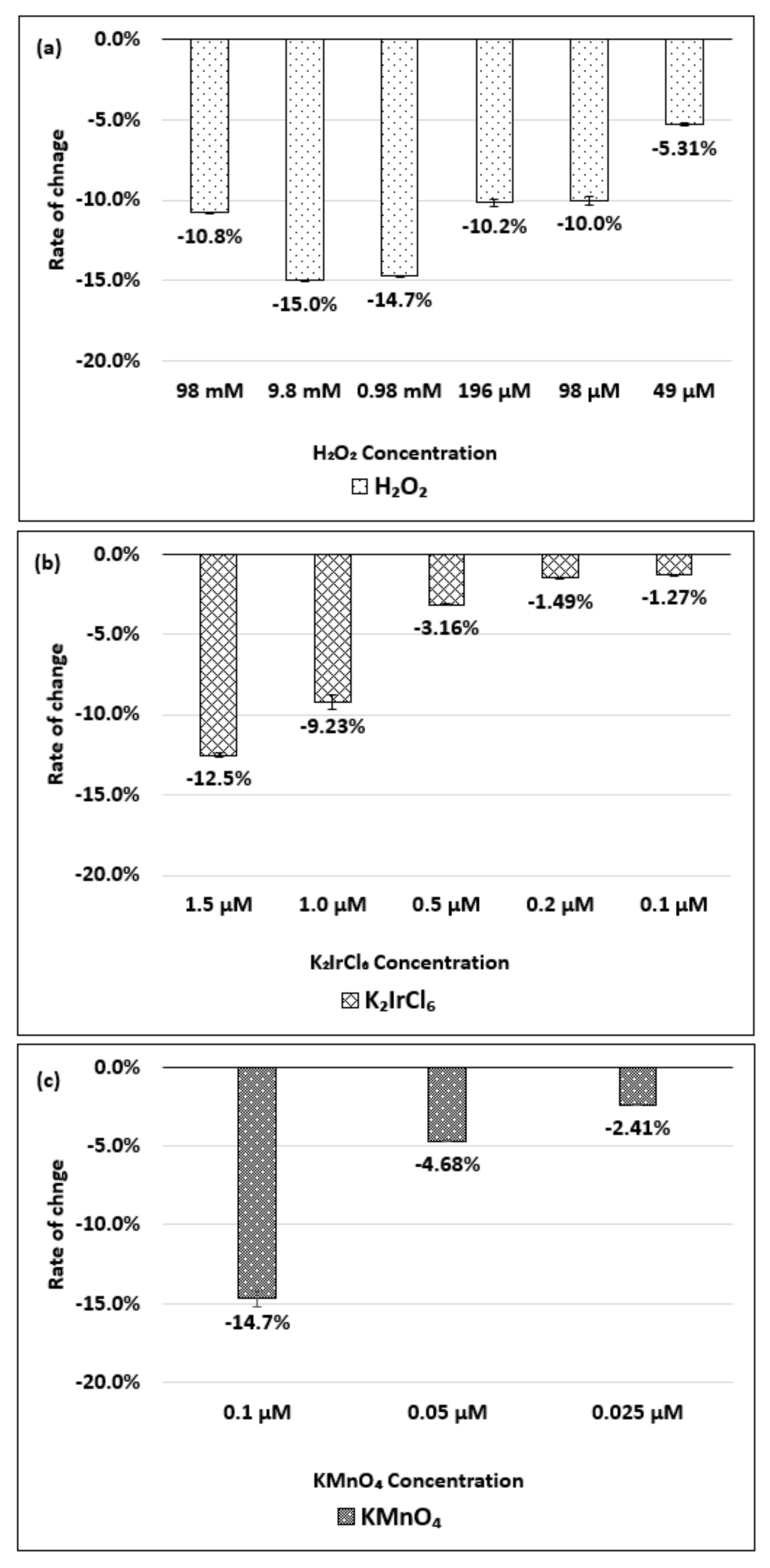
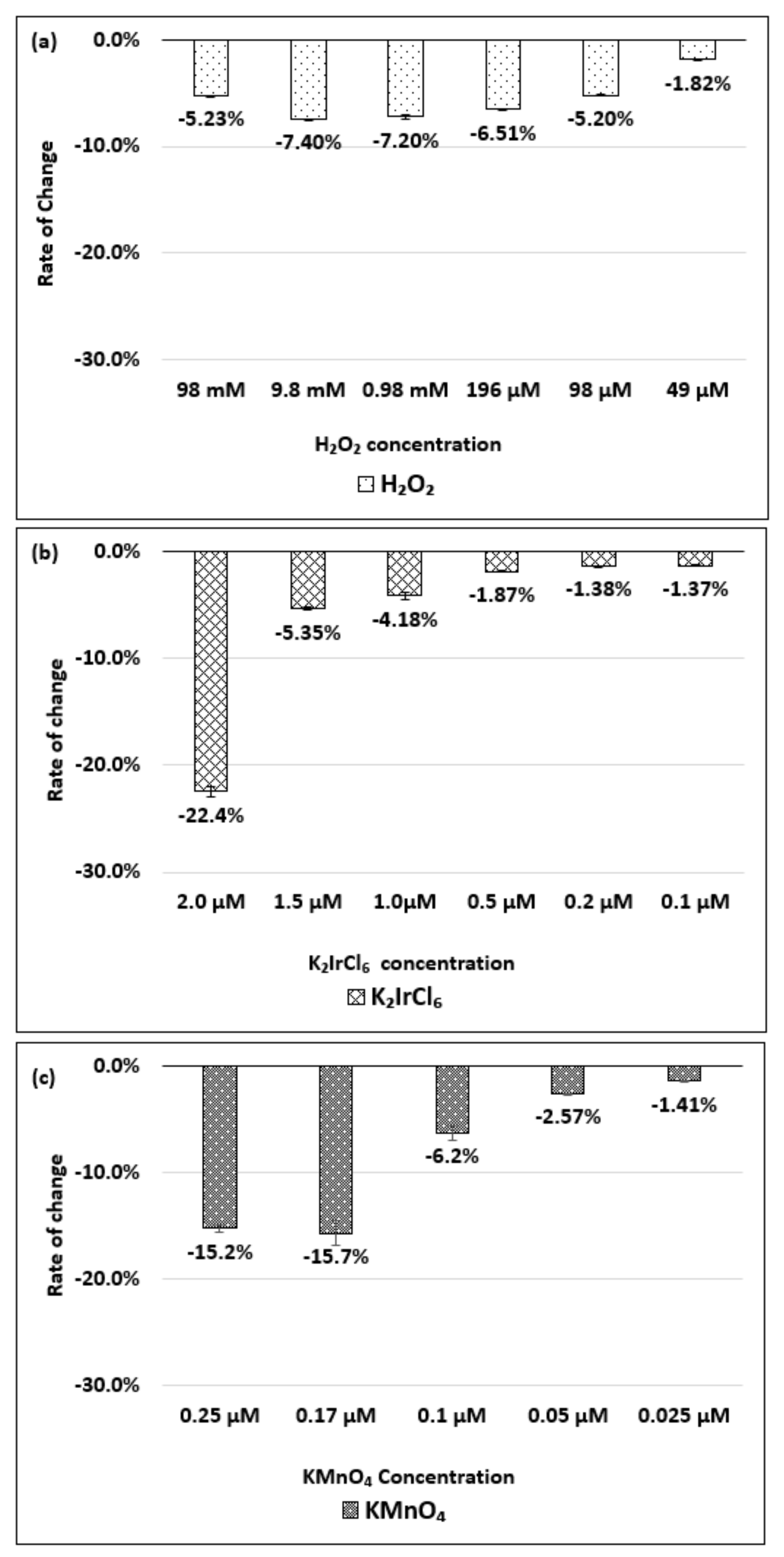
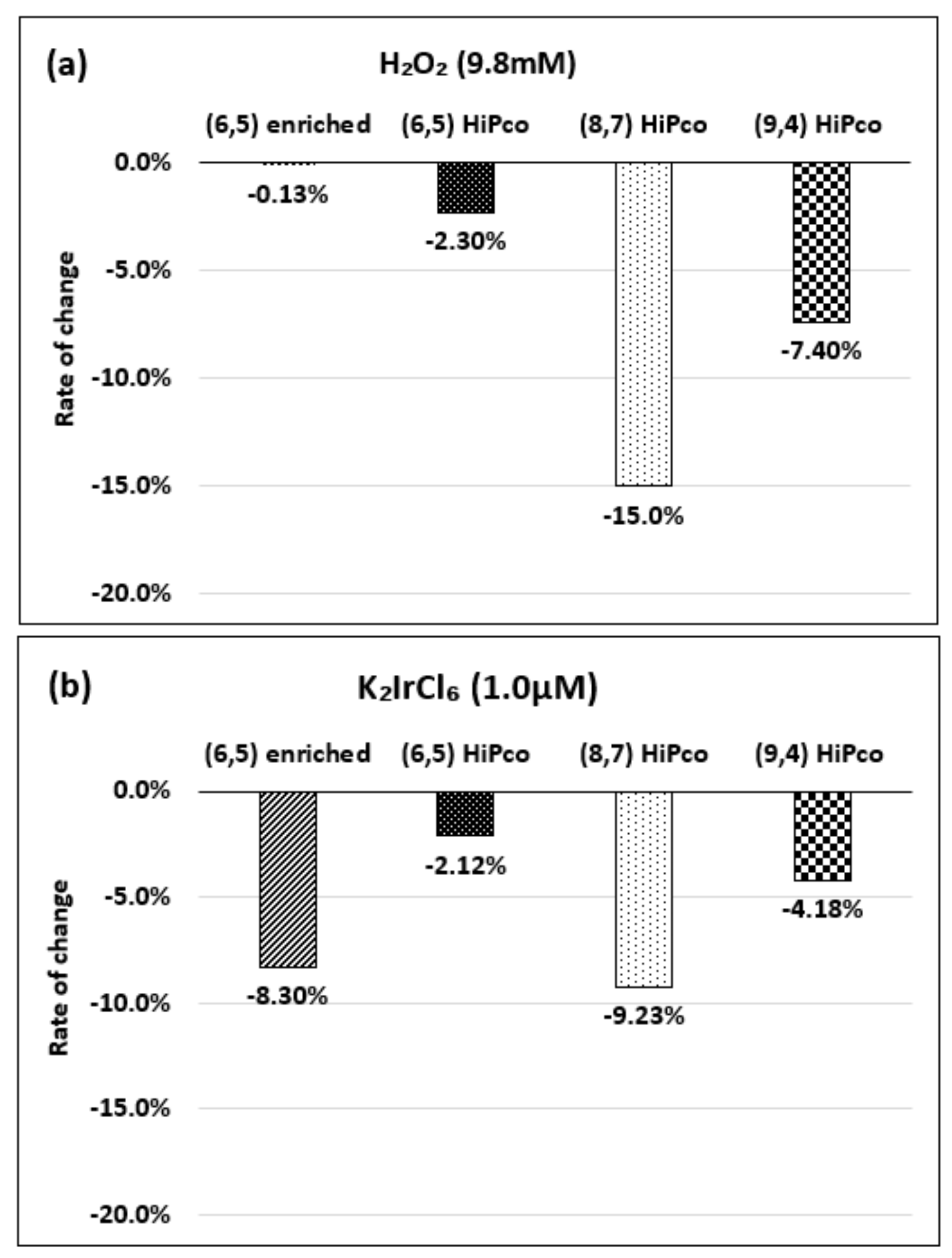
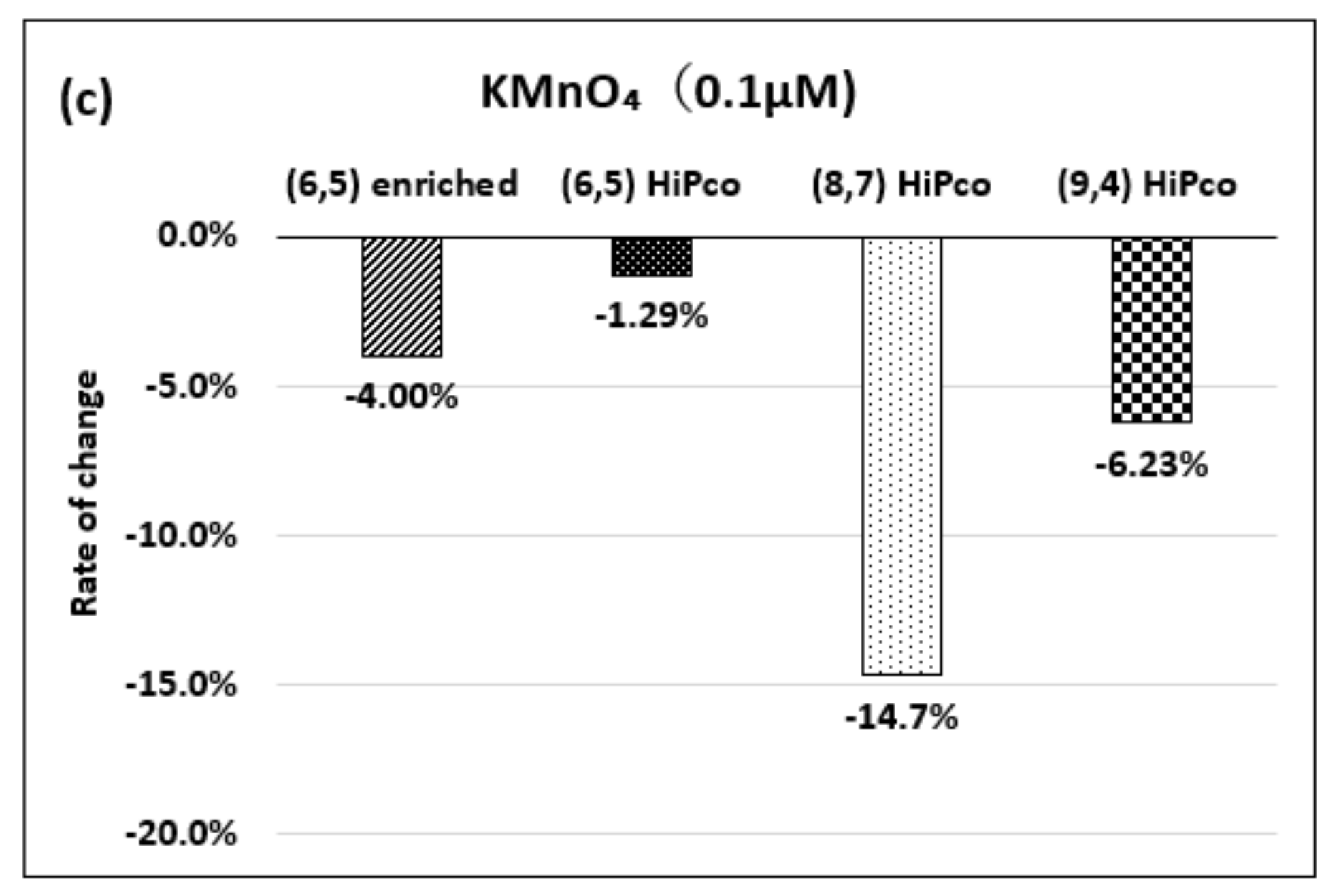
| Oxidizing Power Chirality | −1 (H2O2) | +4 (K2IrCl6) | +7 (KMnO4) | Concentration Ratio of Oxidizing Power | ||
|---|---|---|---|---|---|---|
| H2O2/K2IrCl6 | H2O2/KMnO4 | K2IrCl6/KMnO4 | ||||
| (6,5) (6,5)-enriched | 9800 µM | 0.5 µM | 0.05 µM | 1.9 × 104 | 1.9 × 105 | 10 |
| Absorbance Change | −0.13% | −0.10% | −1.60% | - | - | - |
| (6,5) HiPco | 9800 µM | 1.0 µM | 0.05 µM | 0.98 × 104 | 1.9 × 105 | 20 |
| Absorbance Change | −2.30% | −2.12% | −1.90% | - | - | - |
| (8,7) HiPco | 980 µM | 1.50 µM | 0.10 µM | 6.5 × 102 | 0.98 × 104 | 15 |
| Absorbance Change | −14.7% | −12.5% | −14.7% | - | - | - |
| (9,4) HiPco | 98 µM | 1.50 µM | 0.05 µM | 6.5 × 10 | 1.9×103 | 30 |
| Absorbance Change | −5.20% | −5.35% | −6.20% | - | - | - |
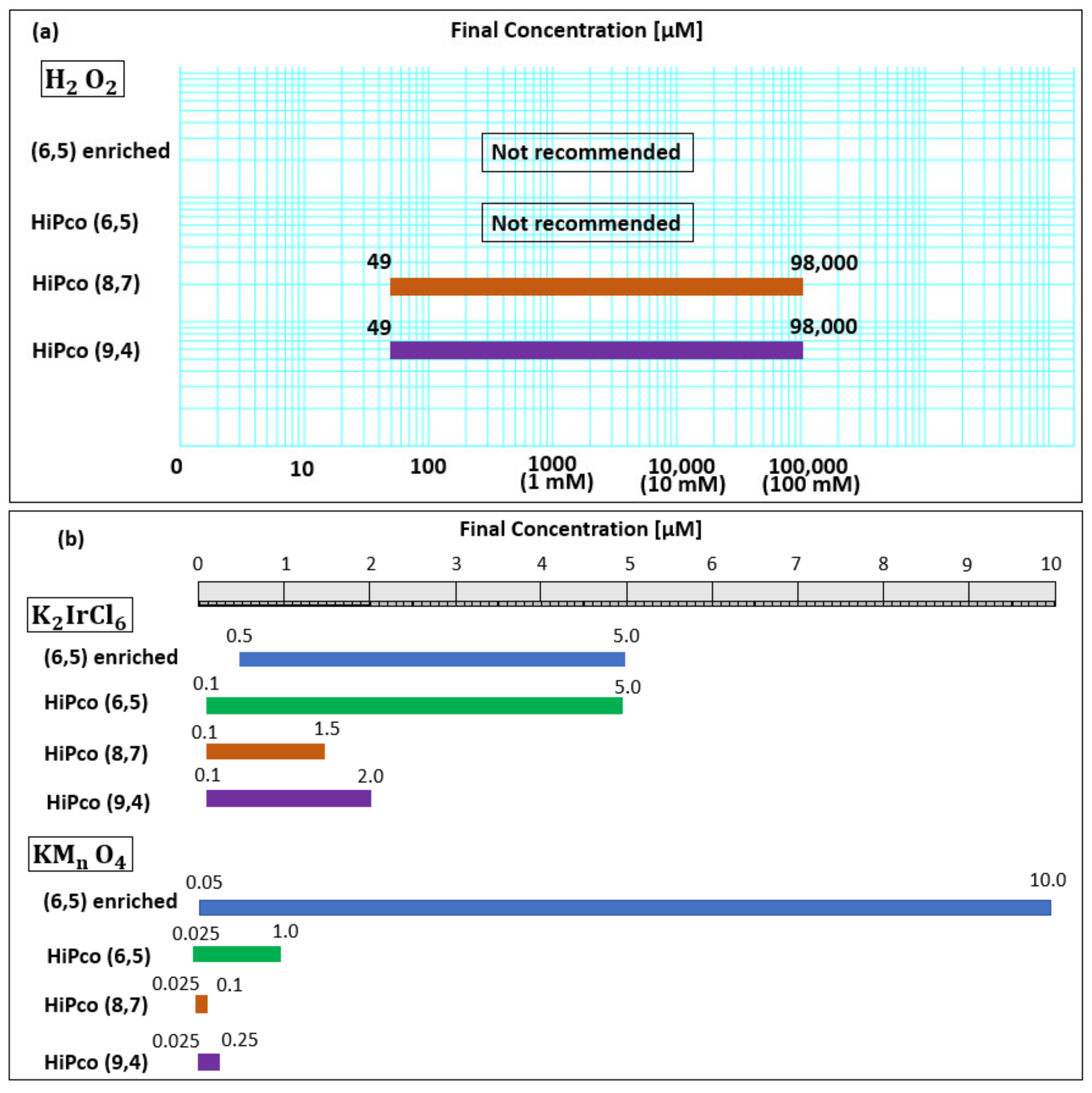
| Oxidant Chirality | H2O2 Concentration | K2IrCl6 Concentration | KMnO4 Concentration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49 µM | 98 µM | 196 µM | 0.98 mM | 0.98 mM | 98 mM | 0.5 µM | 1.0 µM | 2.0 µM | 5.0 µM | 0.1 µM | 0.25 µM | 0.5 µM | 1.0 µM | 5.0 µM | |
| (6,5) enriched | - | - | - | - | 0.0 | 0.0 | −0.3 | −0.3 | 0.0 | 5.7 | −0.07 | −2.0 | −4.0 | −6.3 | −16.7 |
| (6,5) HiPco | 0.0 | 0.0 | 0.3 | −0.3 | −0.2 | 0.0 | 0.0 | 0.0 | −0.5 | 0.0 | −0.5 | 0.0 | 0.0 | 2.7 | - |
| (8,7) HiPco | −1.5 | −2.7 | −2.7 | −4.8 | −4.7 | −4.5 | −0.8 | −3.7 | −5.7 | - | −6.2 | - | - | - | - |
| (9,4) HiPco | 0.0 | 0.0 | 1.0 | 0.0 | −0.2 | −0.8 | 0.0 | 0.0 | −2.0 | - | −0.5 | −1.5 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsukawa, Y.; Umemura, K. Optical Response Characteristics of Single-Walled Carbon Nanotube Chirality Exposed to Oxidants with Different Oxidizing Power. Molecules 2021, 26, 1091. https://doi.org/10.3390/molecules26041091
Matsukawa Y, Umemura K. Optical Response Characteristics of Single-Walled Carbon Nanotube Chirality Exposed to Oxidants with Different Oxidizing Power. Molecules. 2021; 26(4):1091. https://doi.org/10.3390/molecules26041091
Chicago/Turabian StyleMatsukawa, Yuji, and Kazuo Umemura. 2021. "Optical Response Characteristics of Single-Walled Carbon Nanotube Chirality Exposed to Oxidants with Different Oxidizing Power" Molecules 26, no. 4: 1091. https://doi.org/10.3390/molecules26041091
APA StyleMatsukawa, Y., & Umemura, K. (2021). Optical Response Characteristics of Single-Walled Carbon Nanotube Chirality Exposed to Oxidants with Different Oxidizing Power. Molecules, 26(4), 1091. https://doi.org/10.3390/molecules26041091





