Response Surface Methodology: Photocatalytic Degradation Kinetics of Basic Blue 41 Dye Using Activated Carbon with TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regeants and Materials
2.1.1. Chemicals
2.1.2. Synthetized Textile Effluent
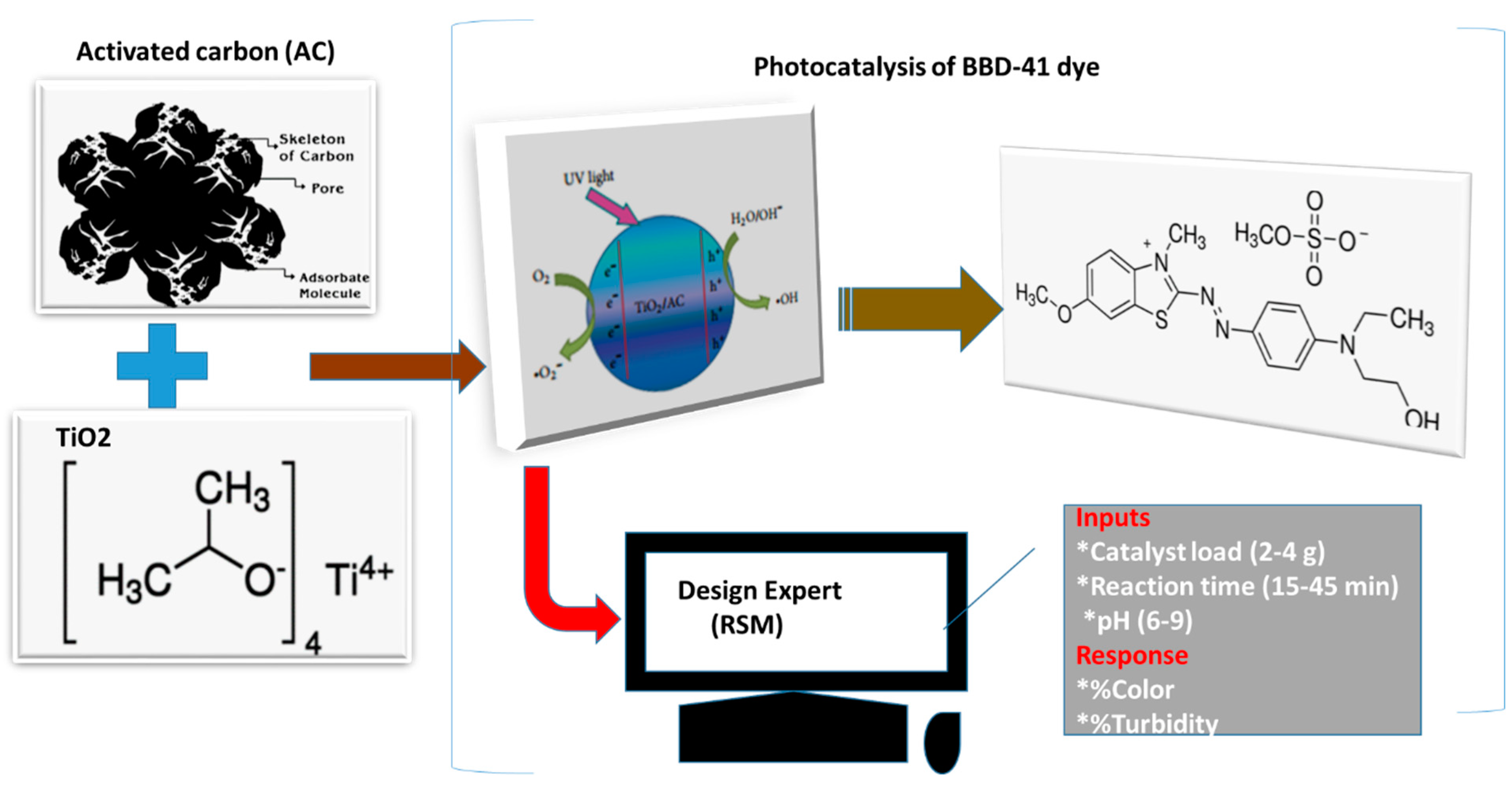
2.1.3. AC-TiO2 Composite Preparation and Characterization
2.2. Experimental Procedure
2.2.1. Photocatalytic Degradation
2.2.2. Design of Experiments
3. Results and Discussion
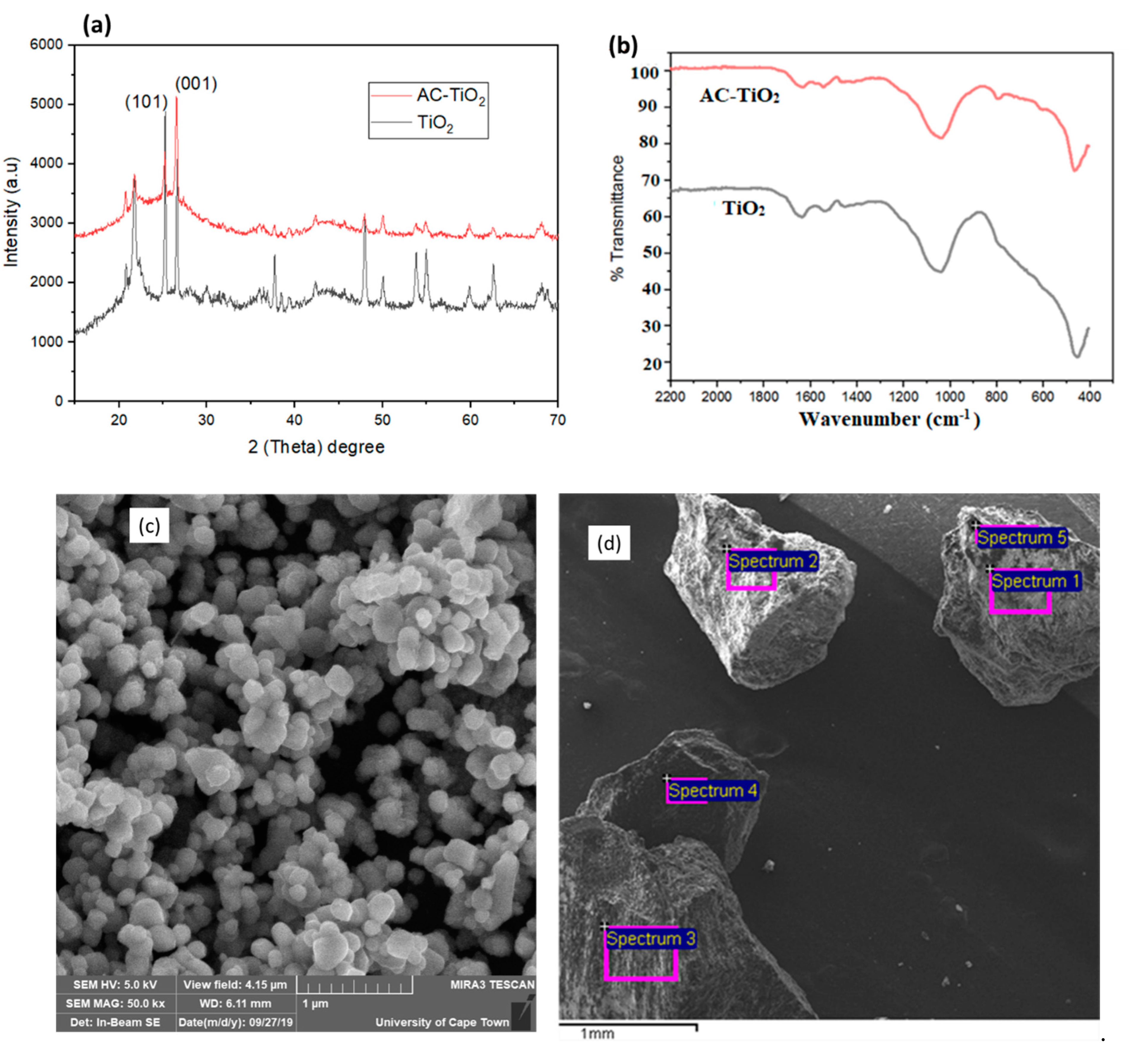
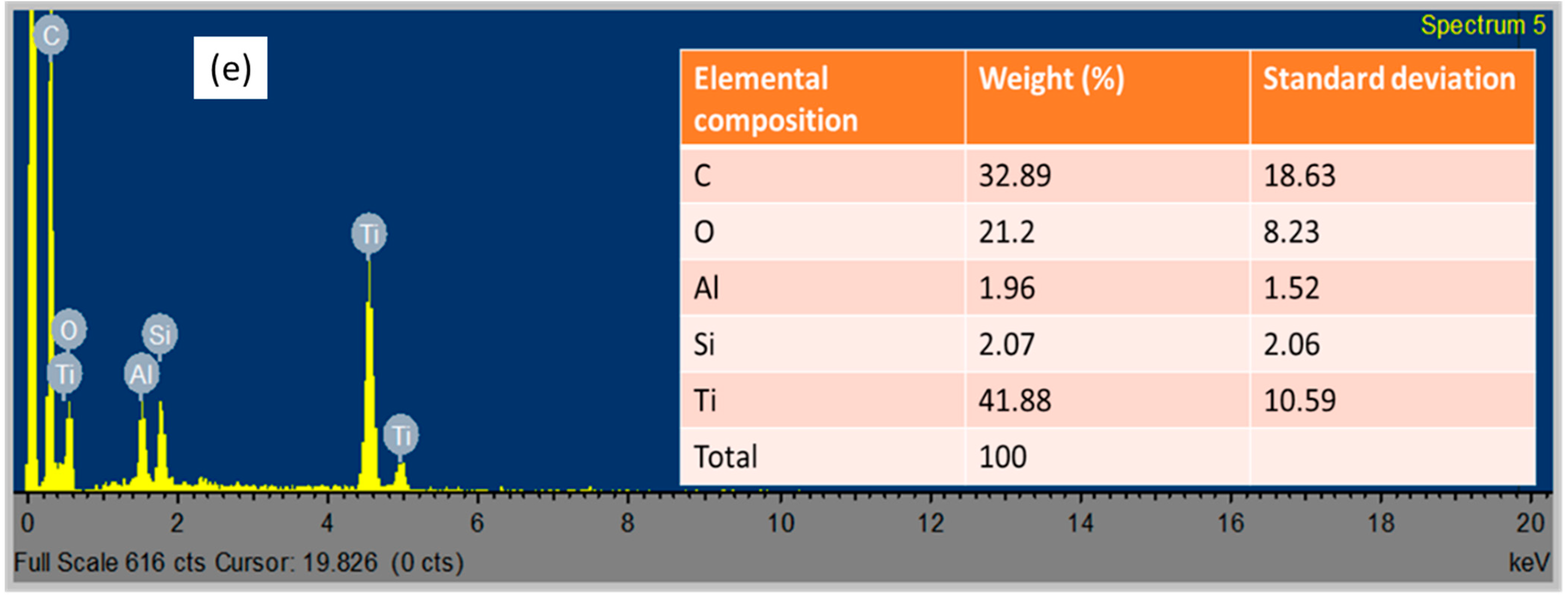
3.1. Physiochemical Properties of the Photocatalyst
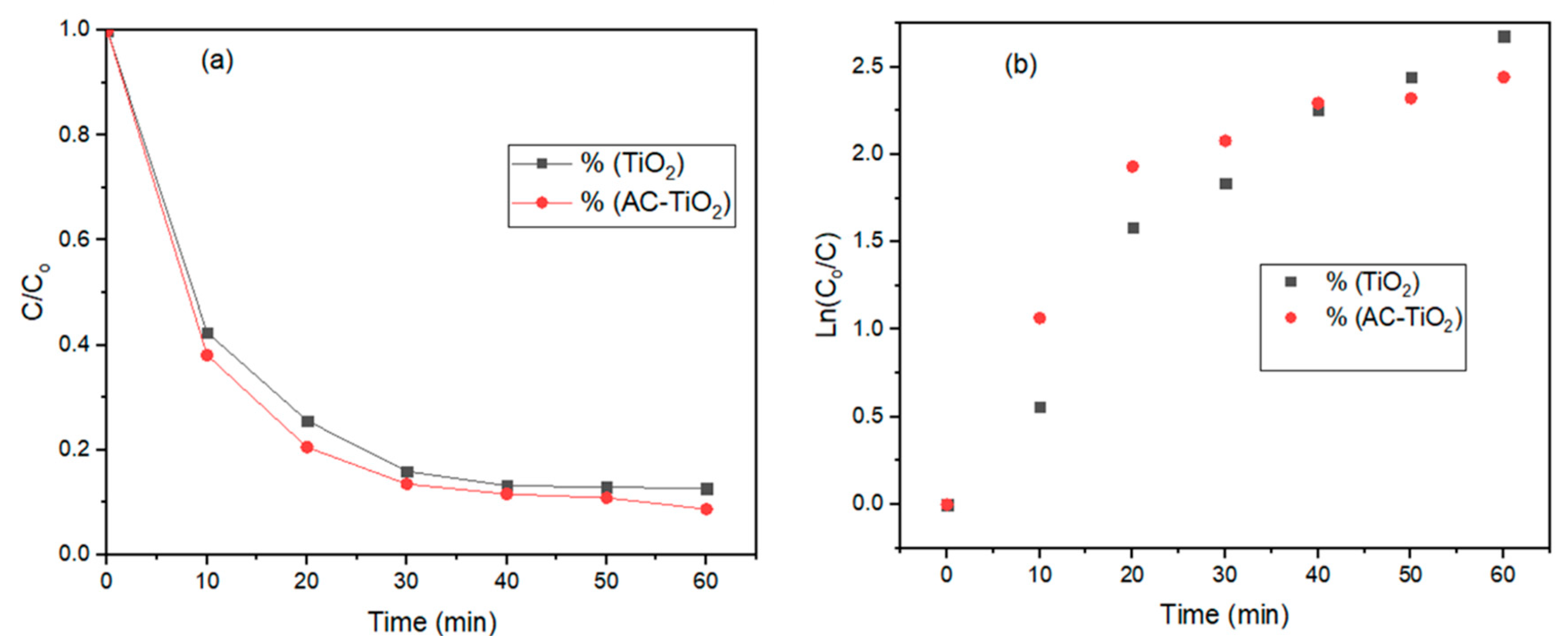
3.2. Photocatalytic Degradation Kinetics
3.3. Response Surface Methodology (RSM)
3.3.1. Analysis of Variance (ANOVA)
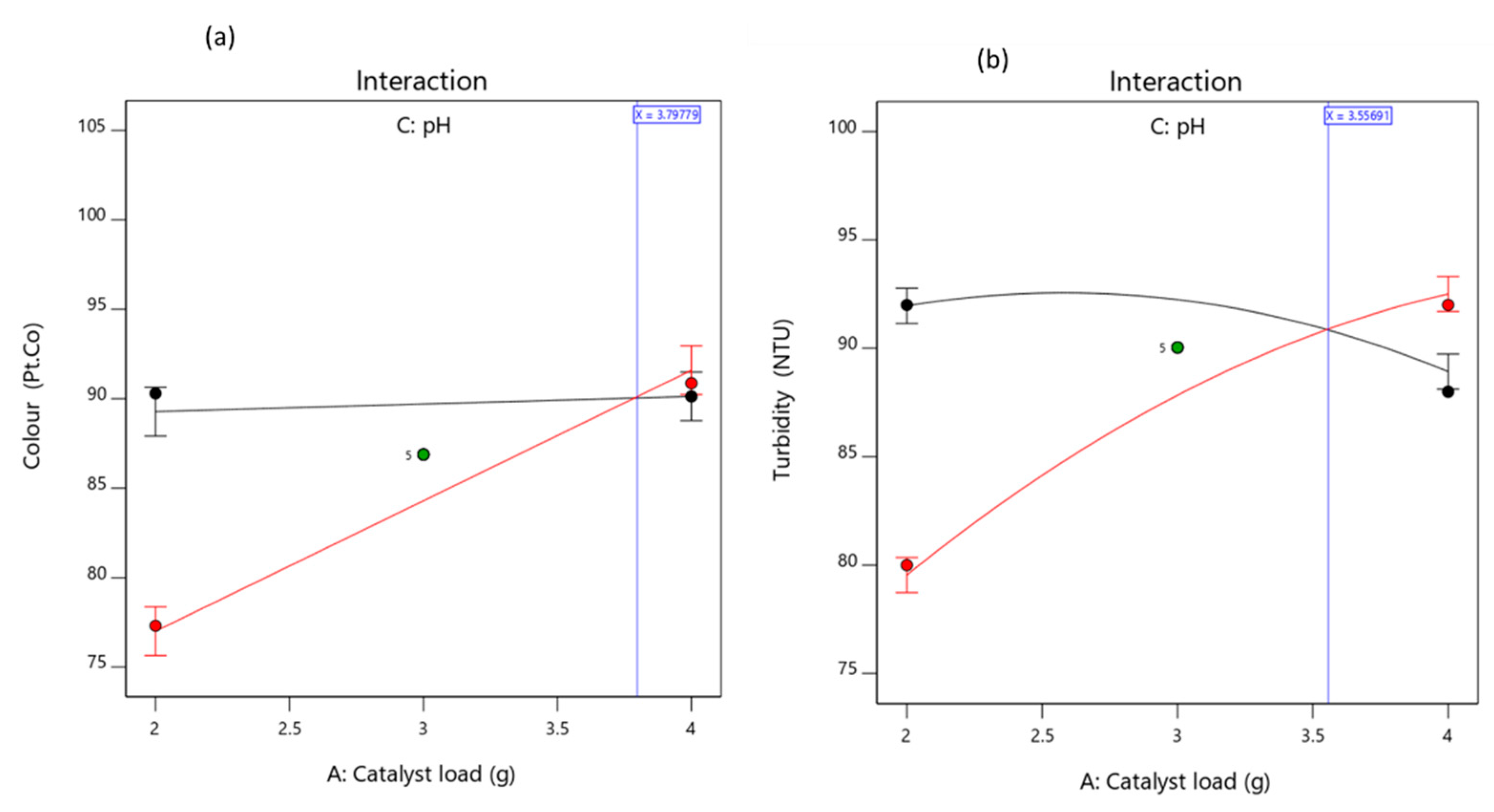
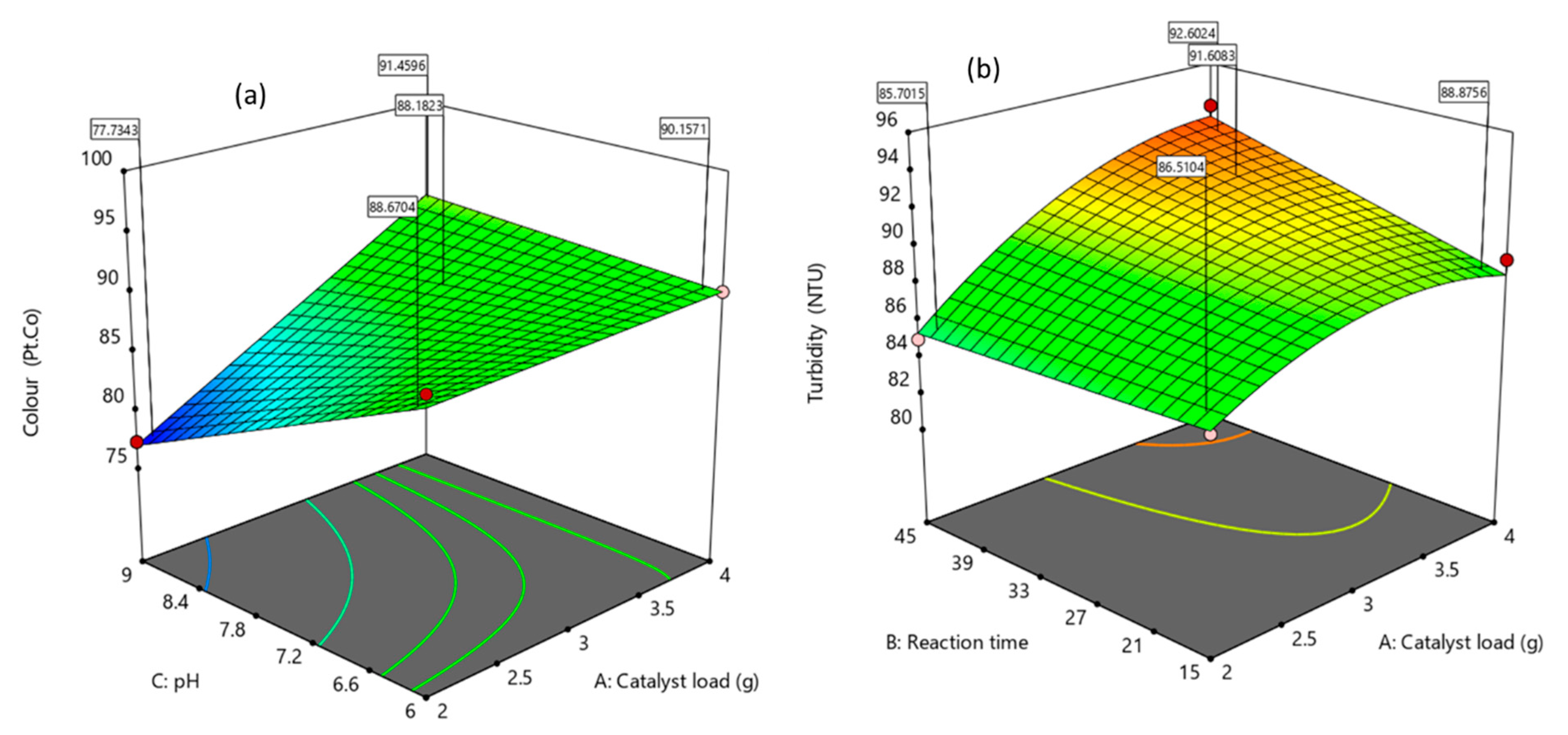
3.3.2. Effects of the Factors and Their Interaction on the Responses
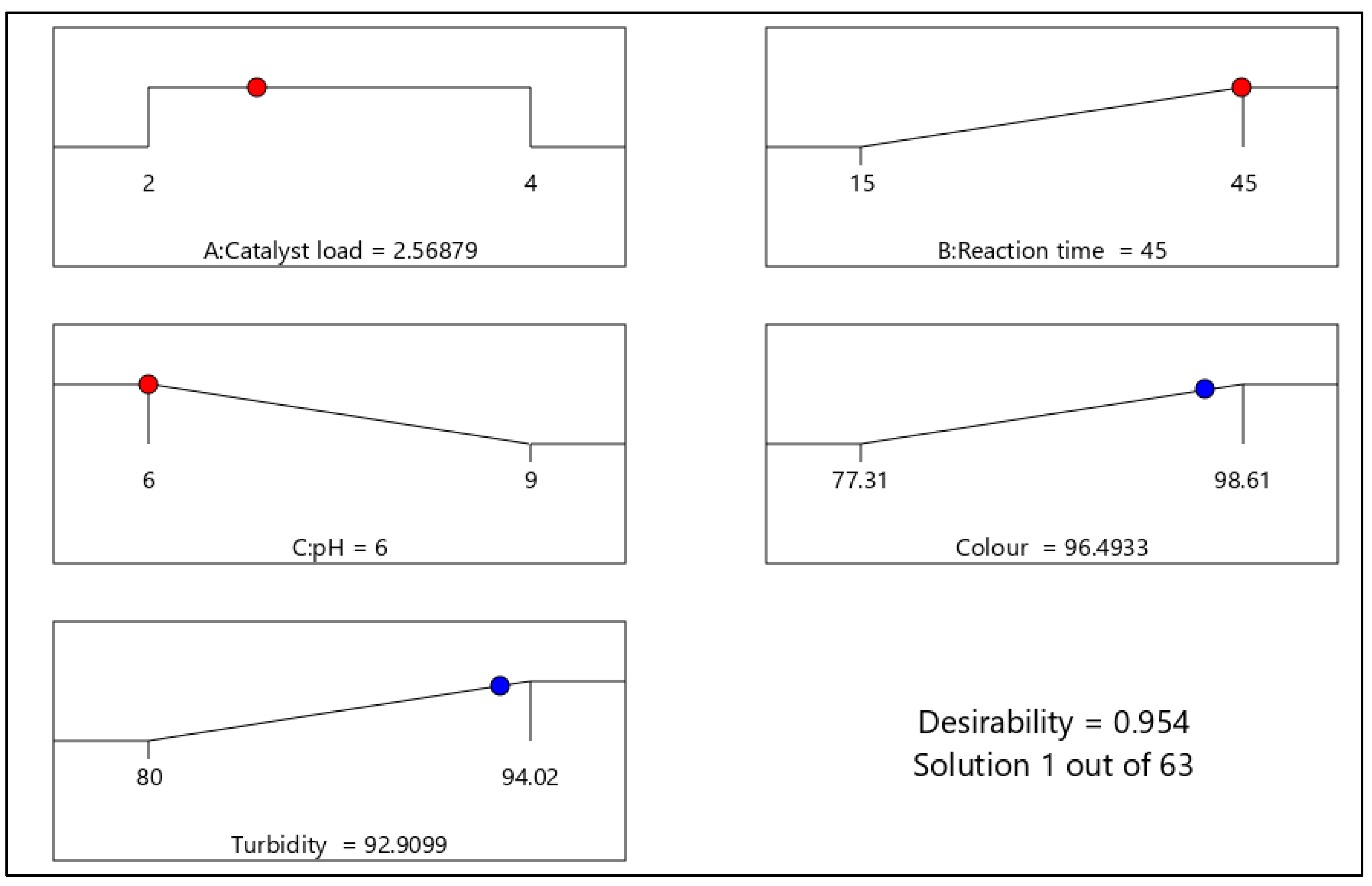
3.3.3. Numerical Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumara, N.T.R.N.; Lim, A.; Lim, C.M.; Petra, M.I.; Ekanayake, P. Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 78, 301–317. [Google Scholar]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dyes Pigments 2017, 145, 505–513. [Google Scholar]
- Singh, H.B.; Bharati, K.A. Handbook of Natural Dyes and Pigments; Woodhead Publishing India: New Delhi, India, 2014. [Google Scholar]
- Ahmad, W.A.; Ahmad, W.Y.W.; Zakaria, Z.A.; Yusof, N.Z. Application of Bacterial Pigments as Colorant; Springer: Cham, Switzerland, 2012. [Google Scholar]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments: Their Structure and Properties. In Dyes and Pigments; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Gao, Y.; Yang, B.; Wang, Q. Biodegradation and Decolorization of Dye Wastewater: A Review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Arora, S. Textile Dyes: It’s Impact on Environment and its Treatment. J. Bioremediation Biodegrad. 2014. [Google Scholar] [CrossRef]
- Haq, I.U.; Ahmad, W.; Ahmad, I.; Yaseen, M. Photocatalytic oxidative degradation of hydrocarbon pollutants in refinery wastewater using TiO2 as catalyst. Water Environ. Res. 2020. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar]
- Cheremisinoff, P.N. Handbook of Water and Wastewater Treatment Technology; Butterworth–Heinemann: Oxford, UK, 2019. [Google Scholar]
- Tetteh, E.K.; Rathilal, S. Kinetics and nanoparticle catalytic enhancement of biogas production from wastewater using a magnetized biochemical methane potential (Mbmp) system. Catalysts 2020, 10, 1200. [Google Scholar]
- Durgalakshmi, D.; Rakkesh, R.A.; Rajendran, S.; Naushad, M. Principles and Mechanisms of Green Photocatalysis; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Raja, S.; Mattoso, L.H.C. Functionalized Polymer-Based Composite Photocatalysts; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Huang, C.W.; Wu, M.C. Photocatalytic degradation of methylene blue by UV-assistant TiO2 and natural sericite composites. J. Chem. Technol. Biotechnol. 2020, 95, 2715–2722. [Google Scholar]
- Zhong, J.; Chen, F.; Zhang, J. Carbon-deposited TiO2: Synthesis, characterization, and visible photocatalytic performance. J. Phys. Chem. C. 2010, 114, 933–939. [Google Scholar]
- Sacco, O.; Vaiano, V.; Sannino, D. Main parameters influencing the design of photocatalytic reactors for wastewater treatment: A mini review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar]
- Fibers, T.N.; Alahiane, S.; Qourzal, S.; el Ouardi, M.; Abaamrane, A.; Assabbane, A. Factors Influencing the Photocatalytic Degradation of Reactive Yellow 145 by TiO2-Coated Non-Woven Fibers. Am. J. Anal. Chem. 2014, 5, 445–454. [Google Scholar]
- Chanderia, K.; Kumar, S.; Sharma, J.; Ameta, R.; Punjabi, P.B. Degradation of Sunset Yellow FCF using copper loaded bentonite and H2O2 as photo-Fenton like reagent. Arab. J. Chem. 2017, 10, S205–S211. [Google Scholar]
- Narayanasamy, L.; Murugesan, T. Degradation of Alizarin Yellow R using UV/H2O2 advanced oxidation process. Environ. Prog. Sustain. Energy 2014, 33, 482–489. [Google Scholar]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar]
- Usman, M.; Ahmed, A.; Yu, B.; Peng, Q.; Shen, Y.; Cong, H. Photocatalytic potential of bio-engineered copper nanoparticles synthesized from Ficus carica extract for the degradation of toxic organic dye from waste water: Growth mechanism and study of parameter affecting the degradation performance. Mater. Res. Bull. 2019, 120, 110583. [Google Scholar]
- Rosu, M.C.; Coros, M.; Pogacean, F.; Magerusan, L.; Socaci, C.; Turza, A.; Pruneanu, S. Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 2017, 70, 13–20. [Google Scholar]
- Liu, N.; Wang, H.; Weng, C.H.; Hwang, C.C. Adsorption characteristics of Direct Red 23 azo dye onto powdered tourmaline. Arab. J. Chem. 2018, 11, 1281–1291. [Google Scholar]
- Tetteh, E.K.; Rathilal, S.; Naidoo, D.B. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar]
- Rodrigues, C.S.D.; Madeira, L.M.; Boaventura, R.A.R. Optimization of the azo dye Procion Red H-EXL degradation by Fenton’s reagent using experimental design. J. Hazard. Mater. 2009, 164, 987–994. [Google Scholar]
- Chaker, H.; Ameur, N.; Saidi-Bendahou, K.; Djennas, M.; Fourmentin, S. Modeling and Box-Behnken design optimization of photocatalytic parameters for efficient removal of dye by lanthanum-doped mesoporous TiO2. J. Environ. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Garg, A.; Kaur, G.; Sangal, V.K.; Bajpai, P.K.; Upadhyay, S. Optimization methodology based on neural networks and box-behnken design applied to photocatalysis of acid red 114 dye. Environ. Eng. Res. 2020, 25, 753–762. [Google Scholar]
- Lee, Y.F.; Chang, K.H.; Hu, C.C.; Lin, K.M. Synthesis of activated carbon-surrounded and carbon-doped anatase TiO2 nanocomposites. J. Mater. Chem. 2010, 20, 5682–5688. [Google Scholar]
- Shen, M.; Wu, Z.; Huang, H.; Du, Y.; Zou, Z.; Yang, P. Carbon-doped anatase TiO2 obtained from TiC for photocatalysis under visible light irradiation. Mater. Lett. 2006, 60, 693–697. [Google Scholar]
- Ross, J.R.H. The Kinetics and Mechanisms of Catalytic Reactions. In Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Vannice, M.A. Kinetics of Catalytic Reactions; Springer: New York, NY, USA, 2005. [Google Scholar]
- Zakaria, W.F.W.; Jalil, A.A.; Hassan, N.S.; Ibrahim, M.; Azami, M.S. Visible-light driven photodegradation of phenol over niobium oxide-loaded fibrous silica titania composite catalyst. J. Chem. Technol. Biotechnol. 2020, 95, 2638–2647. [Google Scholar]
- Tetteh, E.K.; Ezugbe, E.O.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO42-from oil refinery wastewater using a photo-catalytic system-comparing TiO2 and zeolite efficiencies. Water 2020, 12, 214. [Google Scholar]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar]
- Zhao, J.; Chen, C.; Ma, W. Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Top. Catal. 2005, 35, 269–278. [Google Scholar]

| Factor | Low Level (−1) | Medium (0) | High (+1) | Ref |
|---|---|---|---|---|
| A: Catalyst load (g) | 2 | 3 | 4 | [24] |
| B: Reaction time (min) | 15 | 30 | 45 | [26] |
| C: pH | 6 | 7.5 | 9 | [28] |
| Factor 1 | Factor 2 | Factor 3 | Y1: Colour (%) | Y2: Turbidity (%) | |||
|---|---|---|---|---|---|---|---|
| Run | A: Catalyst Load (g) | B: Reaction Time (min) | C:pH | Exp | Pred | Exp | Pred |
| 1 | 2 | 15 | 7.5 | 85 | 86.32 | 86 | 86.16 |
| 2 | 3 | 30 | 7.5 | 86.88 | 87.00 | 90.04 | 90.04 |
| 3 | 4 | 30 | 9 | 90.87 | 91.59 | 92 | 92.51 |
| 4 | 2 | 30 | 9 | 77.31 | 77.00 | 80 | 79.54 |
| 5 | 3 | 45 | 9 | 88.98 | 90.22 | 88.08 | 88.74 |
| 6 | 3 | 30 | 7.5 | 86.88 | 87.00 | 90.04 | 90.04 |
| 7 | 2 | 30 | 6 | 90.3 | 89.28 | 92 | 91.96 |
| 8 | 3 | 30 | 7.5 | 86.88 | 87.00 | 90.04 | 90.04 |
| 9 | 4 | 30 | 6 | 90.13 | 90.13 | 88 | 88.93 |
| 10 | 4 | 15 | 7.5 | 98.61 | 98.90 | 89.31 | 88.50 |
| 11 | 2 | 45 | 7.5 | 91.92 | 91.50 | 85 | 85.35 |
| 12 | 3 | 30 | 7.5 | 86.88 | 87.00 | 90.04 | 90.04 |
| 13 | 3 | 45 | 6 | 95 | 95.63 | 94.02 | 93.16 |
| 14 | 4 | 45 | 7.5 | 95.8 | 94.35 | 93.57 | 92.95 |
| 15 | 3 | 30 | 7.5 | 86.88 | 87.00 | 90.04 | 90.04 |
| 16 | 3 | 15 | 6 | 95.09 | 95.31 | 90.9 | 91.34 |
| 17 | 3 | 15 | 9 | 91.73 | 89.90 | 87.18 | 86.92 |
| Parameter | Colour | Turbidity |
|---|---|---|
| Standard deviation | 1.06 | 0.63 |
| Mean | 89.71 | 89.19 |
| Coefficient of variance (CV, %) | 1.18 | 0.71 |
| Coefficient of determination (R2) | 0.972 | 0.988 |
| Adjusted R2 | 0.966 | 0.975 |
| Predicted R2 | 0.886 | 0.893 |
| Adequate precision | 32.33 | 33.67 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 389.44 | 6 | 64.91 | 58.28 | <0.0001 | significant |
| A-Catalyst load | 119.20 | 1 | 119.20 | 107.02 | <0.0001 | |
| B-Reaction time | 0.2016 | 1 | 0.2016 | 0.1810 | 0.0495 | |
| C-pH | 58.48 | 1 | 58.48 | 52.51 | <0.0001 | |
| AB | 23.67 | 1 | 23.67 | 21.25 | 0.0010 | |
| AC | 47.13 | 1 | 47.13 | 42.31 | <0.0001 | |
| B2 | 140.77 | 1 | 140.77 | 126.39 | <0.0001 | |
| Residual | 11.14 | 10 | 1.11 | |||
| Lack of Fit | 11.14 | 6 | 1.86 | 12.32 | 0.132 | Non-significant |
| Pure Error | 0.0000 | 4 | 0.0000 | |||
| Cor Total | 400.58 | 16 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 179.76 | 6 | 29.96 | 75.42 | <0.0001 | significant |
| A-Catalyst load | 49.40 | 1 | 49.40 | 124.36 | <0.0001 | |
| B-Reaction time | 6.62 | 1 | 6.62 | 16.68 | 0.0022 | |
| C-pH | 38.98 | 1 | 38.98 | 98.14 | <0.0001 | |
| AB | 6.92 | 1 | 6.92 | 17.41 | 0.0019 | |
| AC | 64.00 | 1 | 64.00 | 161.11 | <0.0001 | |
| A2 | 13.83 | 1 | 13.83 | 34.82 | 0.0002 | |
| Residual | 3.97 | 10 | 0.3972 | |||
| Lack of Fit | 3.97 | 6 | 0.6621 | 10. 52 | 0.154 | Non-significant |
| Pure Error | 0.0000 | 4 | 0.0000 | |||
| Cor Total | 183.73 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kweinor Tetteh, E.; Obotey Ezugbe, E.; Asante-Sackey, D.; Armah, E.K.; Rathilal, S. Response Surface Methodology: Photocatalytic Degradation Kinetics of Basic Blue 41 Dye Using Activated Carbon with TiO2. Molecules 2021, 26, 1068. https://doi.org/10.3390/molecules26041068
Kweinor Tetteh E, Obotey Ezugbe E, Asante-Sackey D, Armah EK, Rathilal S. Response Surface Methodology: Photocatalytic Degradation Kinetics of Basic Blue 41 Dye Using Activated Carbon with TiO2. Molecules. 2021; 26(4):1068. https://doi.org/10.3390/molecules26041068
Chicago/Turabian StyleKweinor Tetteh, Emmanuel, Elorm Obotey Ezugbe, Dennis Asante-Sackey, Edward Kwaku Armah, and Sudesh Rathilal. 2021. "Response Surface Methodology: Photocatalytic Degradation Kinetics of Basic Blue 41 Dye Using Activated Carbon with TiO2" Molecules 26, no. 4: 1068. https://doi.org/10.3390/molecules26041068
APA StyleKweinor Tetteh, E., Obotey Ezugbe, E., Asante-Sackey, D., Armah, E. K., & Rathilal, S. (2021). Response Surface Methodology: Photocatalytic Degradation Kinetics of Basic Blue 41 Dye Using Activated Carbon with TiO2. Molecules, 26(4), 1068. https://doi.org/10.3390/molecules26041068









