Treatment with Uncaria tomentosa Promotes Apoptosis in B16-BL6 Mouse Melanoma Cells and Inhibits the Growth of B16-BL6 Tumours
Abstract
1. Introduction
2. Results
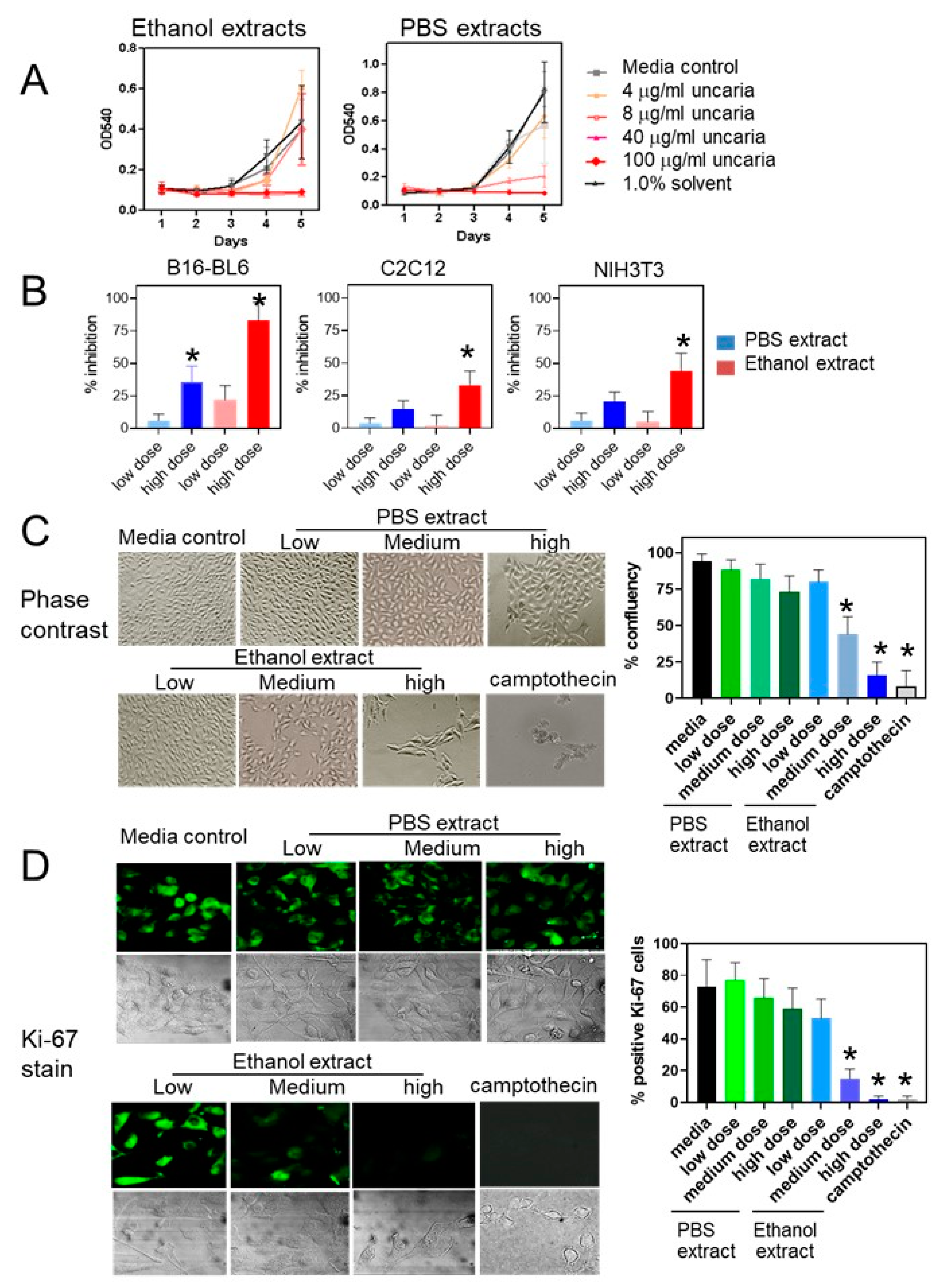
2.1. Uncaria tomentosa Extracts Inhibit Proliferation of B16-BL6 Cells
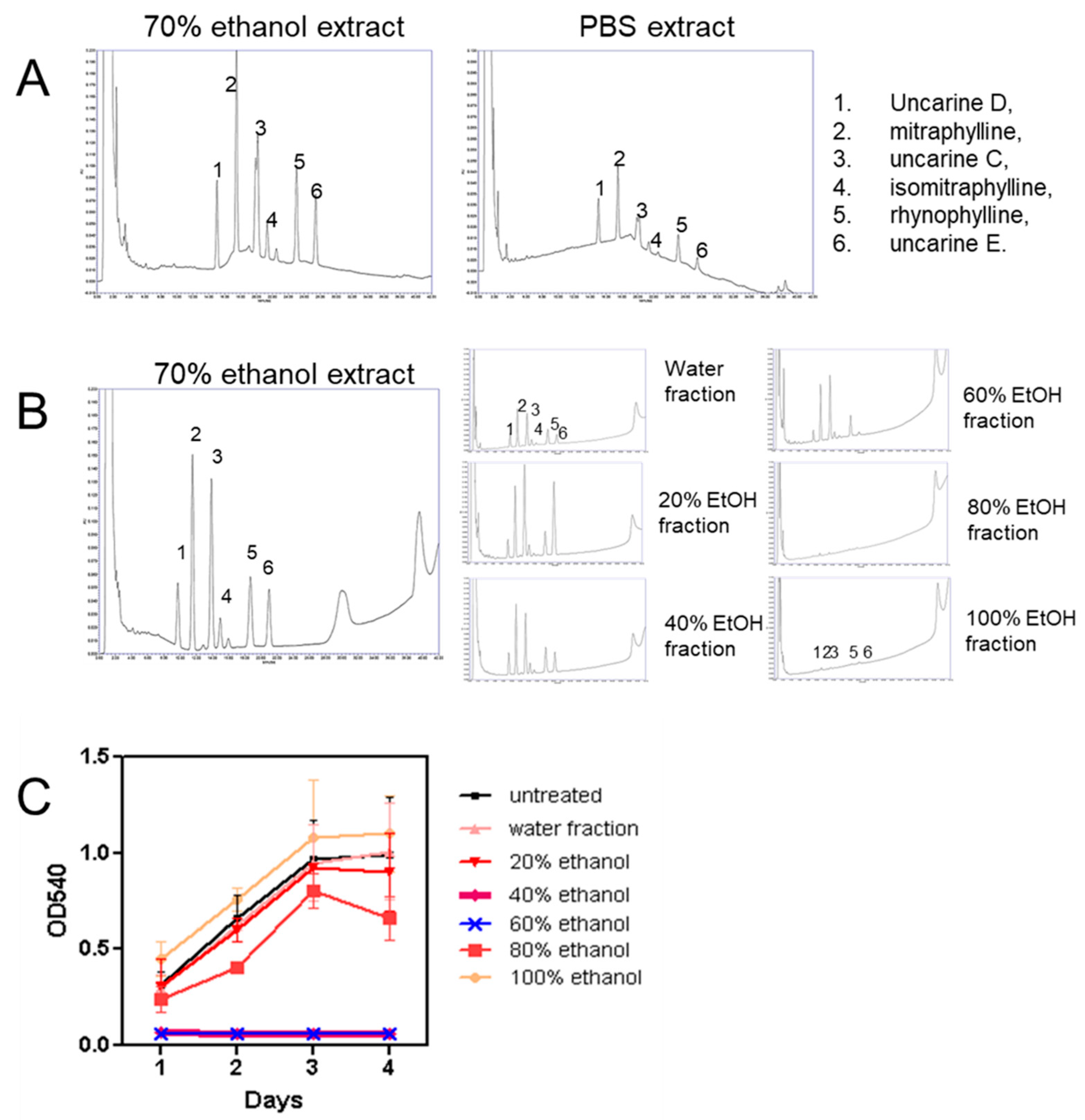
2.2. HPLC Characterization of Uncaria tomentosa Extracts
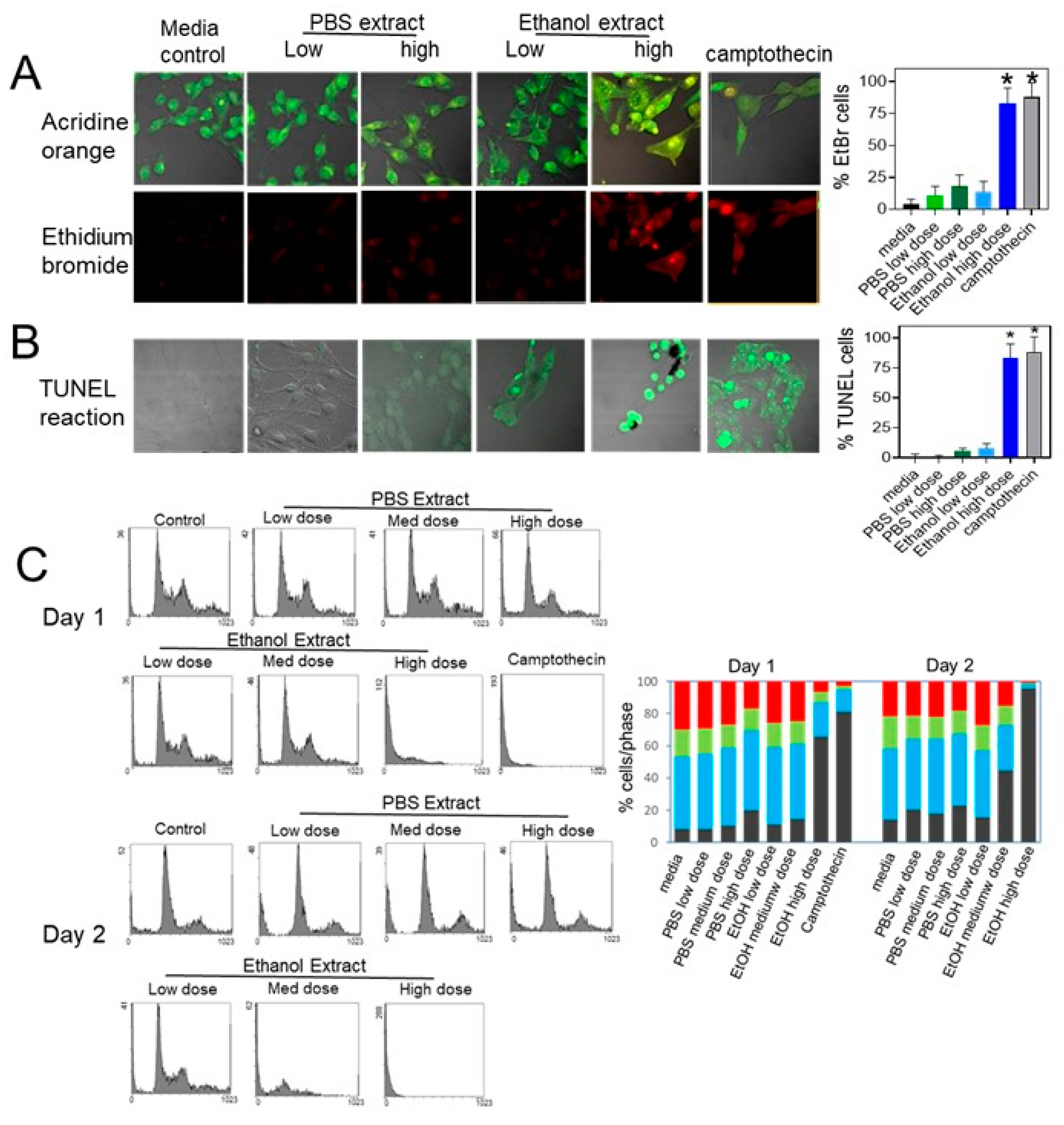
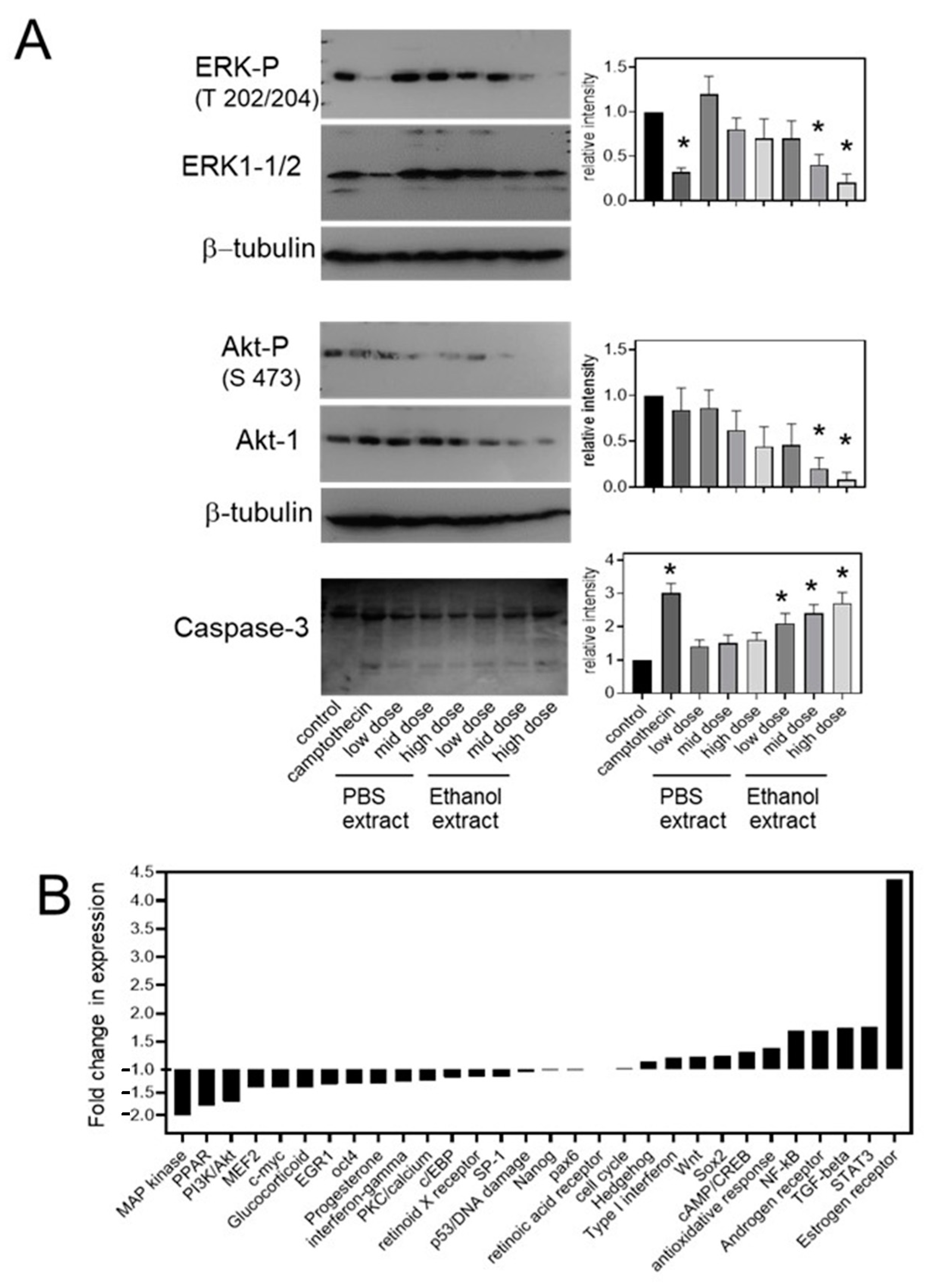
2.3. Treatment with Uncaria tomentosa Extracts Induces Apoptosis in B16-BL6 Cells
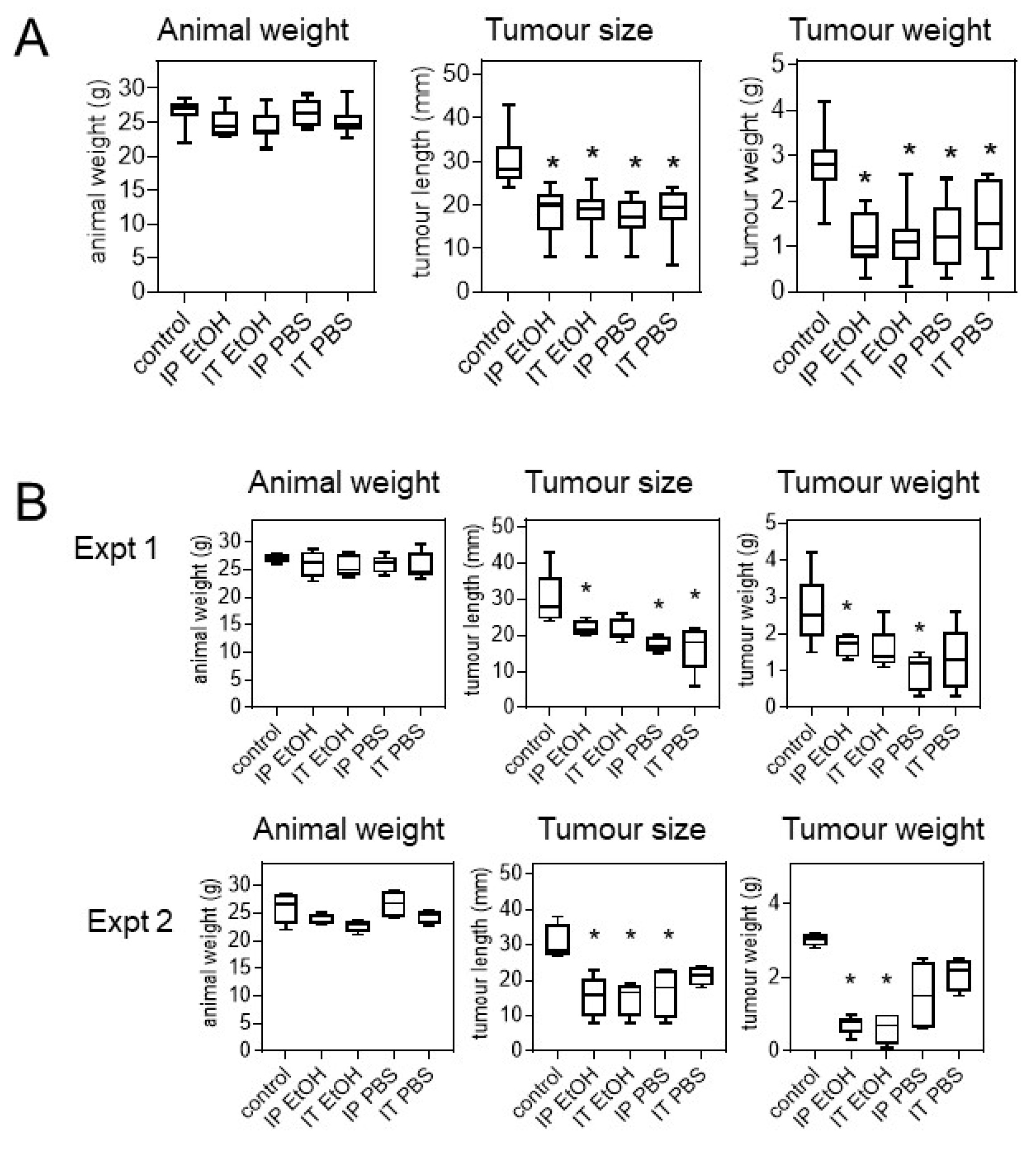
2.4. Uncaria tomentosa Extracts Inhibit B16-BL6 Tumour Growth in C57BL/6 Mice
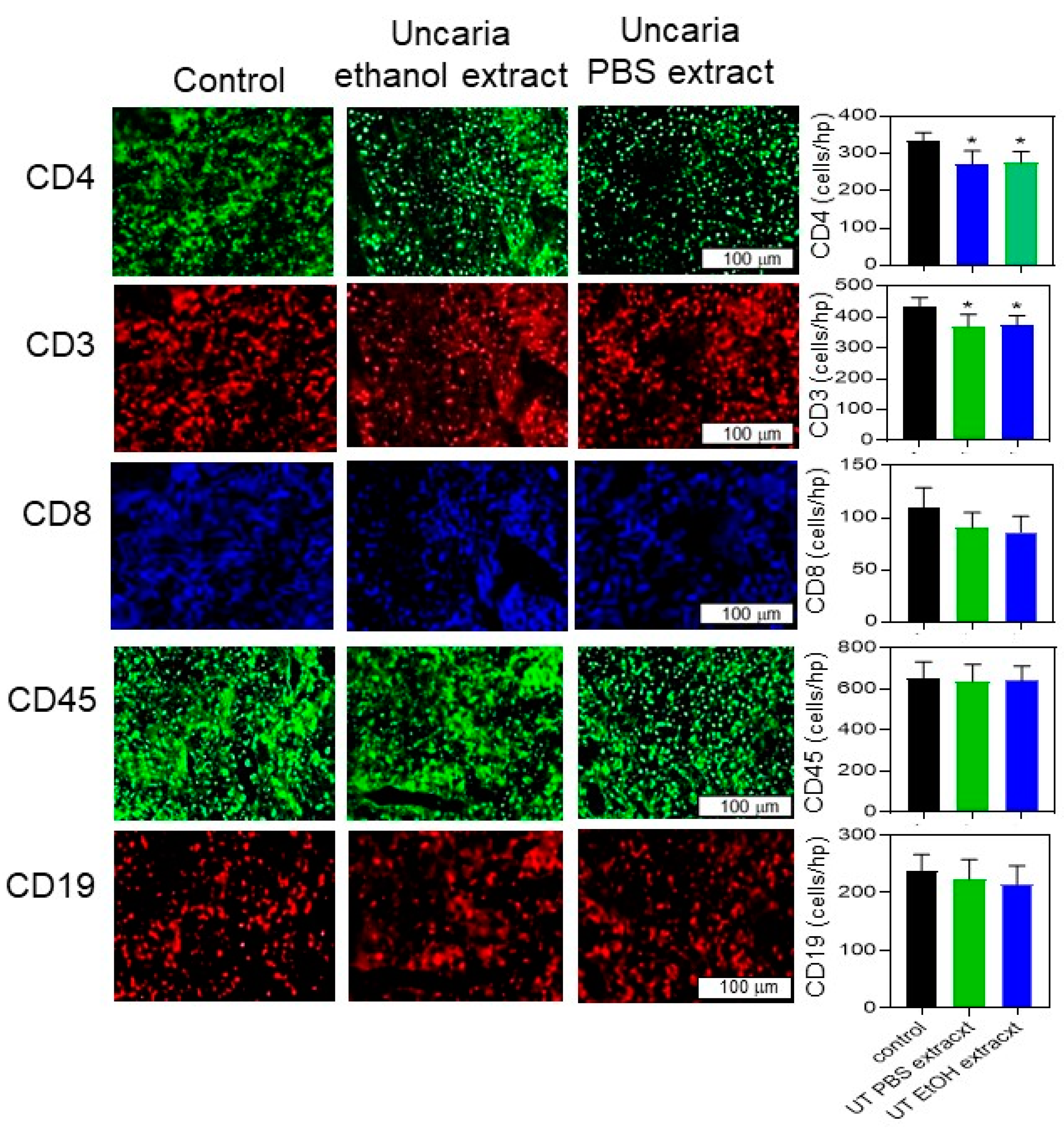
2.5. Histochemical Analysis Was Performed for B16-BL6 Tumours
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Tissue Culture
4.2. Uncaria tomentosa Extracts and Characterization
4.3. High-Performance Liquid Chromatography (HPLC)
4.4. Methyl Tetrazolium Blue Viability Assay
4.5. Flow Cytometry
4.6. Fluorescence Microscopy—Vital Staining
4.7. Fluorescence Microscopy—TUNEL
4.8. Immunoblot Analysis
4.9. Profiling Signaling Pathways Using Promoter–Reporter Assays
4.10. Animal Experiments
4.11. Histochemical Analysis of Tissue Sections
4.12. TUNEL Staining of Histochemical Sections
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Laird, S.A.; Laird, K.K. Biodiversity prospecting: The commercial use of genetic resources and best practice in benefit-sharing. In Biodiversity and Traditional Knowledge: Equitable Partnerships in Practise; Laird, S.A., Ed.; EarthScan Publications Ltd.: London, UK, 2002; pp. 241–260. [Google Scholar]
- Reinhard, K.H. Uncaria tomentosa (Willd.) D.C.: Cat’s claw, una de gato or saventaro. J. Altern. Complement. Med. 1999, 5, 143–151. [Google Scholar] [CrossRef]
- Keplinger, K.; Laus, G.; Wurm, M.; Dierich, M.P.; Teppner, H. Uncaria tomentosa (Willd.) DC-Ethnomedicinal use and new pharmacological, toxicological, and botanical results. J. Ethnopharmacol. 1999, 64, 23–34. [Google Scholar] [CrossRef]
- Heitzmann, M.E.; Neto, C.C.; Winiarz, E.; Valsberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiacease). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef]
- Allen-Hall, L.; Cano, P.; Arnason, J.T.; Rojas, R.; Lock, O.; Lafrenie, R.M. Treatment of THP-1 cells with Uncaria tomentosa extracts differentially regulates the expression of IL-1β and TNFα. J. Ethnolpharmacol. 2007, 109, 312–317. [Google Scholar] [CrossRef]
- Allen-Hall, L.; Cano, P.; Arnason, J.T.; Lafrenie, R.M. Uncaria tomentosa acts as a potent TNF-alpha inhibitor through NF-kB. J. Ethnopharmacol. 2011, 127, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Duran, R.; Gonzalez-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.H.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of mitraphylline isolated from Uncaria tomentosa bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef]
- Fazio, A.L.; Ballen, D.; Cesari, I.M.; Abad, M.J.; Arsenak, M.; Taylor, P. An ethanolic extract of Uncaria tomentosa reduces inflammation and B16-BL6 melanoma growth in C57BL/6 mice. Biol. Latinoam. Caribe Plant Med. Araomaticas 2008, 7, 217–224. [Google Scholar]
- Aquino, R.; De Feo, V.; De Simone, F.; Pizza, C.; Cirino, G. Plant metabolites. New compounds and anti-inflammatory activity of Uncaria tomentosa. J. Nat. Prod. 1991, 54, 453–459. [Google Scholar] [CrossRef]
- Mur, E.; Hartig, F.; Eibl, G.; Schirmer, M. Randomized double blind trial of an extract from the pentacyclic alkaloid-chemotype of uncaria tomentosa for the treatment of rheumatoid arthritis. J. Rheumatol. 2002, 29, 678–681. [Google Scholar]
- Rosenbaum, C.C.; O’Mathuna, D.P.; Chavez, M.; Shields, K. Antioxidants and anti-inflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern. Ther. Health Med. 2010, 16, 32–40. [Google Scholar]
- Bacher, N.; Tiefenthaler, M.; Sturm, S.; Stuppner, H.; Ausserlechner, M.J.; Kofler, R.; Konwalinka, G. Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, Go/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemica cells. Br. J. Haematol. 2006, 132, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R.; Poczekaj-Kostrzewska, M.; Ciesiolka, D.; Szyfter, K.; Gulewicz, K. Antiproliferative activity of various Uncaria tomentosa preparations on HL-60 promyelocytic leukemia cells. Pharmacol. Rep. 2007, 59, 565–572. [Google Scholar] [PubMed]
- Cheng, A.C.; Jian, C.B.; Huang, Y.T.; Lai, C.S.; Hsu, P.C.; Pan, M.H. Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrome c release, and caspases activation in human leukemia cells. Food Chem. Toxicol. 2007, 45, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Garcia Prado, E.; Garcia Gimenez, M.D.; De la Puerta Vazquez, R.; Espartero Sanchez, J.L.; Saenz Rodriguez, M.T. Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine 2007, 14, 280–284. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.Z.; Farias, I.L.; Rigo, M.L.; Glanzner, W.G.; Goncalves, P.B.; Cadona, F.C.; Cruz, I.B.; Farias, J.G.; Duarte, M.M.; Franco, L.; et al. Effect of Uncaria tomentosa extract on apoptosis triggered by oxaliplatin exposure on HT-29 cells. Evid. Based Complementary Altern. Med. 2014, 2014, 274786. [Google Scholar] [CrossRef]
- Dietrich, F.; Kaiser, S.; Rockenbach, L.; Figueiro, F.; Bergamin, L.S.; da Cunha, F.M.; Morrone, F.B.; Ortega, G.G.; Battastini, A.M. Quinovic acid glycosides purified fraction from Uncaria tomentosa induces cell death by apoptosis in the T24 human bladder cancer cell line. Food Chem. Toxicol. 2014, 67, 222–229. [Google Scholar] [CrossRef]
- Kaiser, S.; Dietrich, F.; De Resende, P.E.; Verza, S.G.; Moreaes, R.C.; Morrone, F.B.; Batistini, A.M.; Ortega, G.G. Cat’s claw oxindole alkaloid isomerization induced by cell incubation and cytotoxic activity against T24 and RT4 human bladder cancer cell lines. Planta Med. 2013, 79, 1413–1420. [Google Scholar]
- Rinner, B.; Li, Z.X.; Haas, H.; Siegl, V.; Sturm, S.; Stuppner, H.; Pfragner, R. Antiproliferative and pro-apoptotic effects of Uncaria tomentosa in human medullary thyroid carcinoma cells. Anticancer Res. 2009, 29, 4519–4528. [Google Scholar]
- Pilarski, R.; Filip, B.; Wietzyk, J.; Kuras, M.; Gulewicz, K. Anticancer activity of the Uncaria tomentosa (Willd.) DC preparations with different oxindole alkaloid composition. Phytomedicine 2010, 17, 1133–1139. [Google Scholar] [CrossRef]
- Riva, L.; Coradini, D.; Di Fronzo, G.; De Feo, V.; De Tommasi, N.; De Simone, F.; Pizza, C. The antiproliferative effects of Uncaria tomentosa extracts and fractions on the growth of breast cancer cell line. Anticancer Res. 2001, 21, 2457–2461. [Google Scholar]
- Garcia Gimenez, D.; Garcia Prado, E.; Saenz Rodriguez, T.; Fernandez Arche, A.; de la Puerta, R. Cytotoxic effect of the pentacyclic oxindole alkaloid mitraphylline isolated from Uncaria tomentosa bark on human Ewing’s sarcoma and breast cancer cell lines. Planta Med. 2010, 76, 133–136. [Google Scholar] [CrossRef]
- Dreifuss, A.A.; Bastos-Pereira, A.L.; Avila, T.V.; Soley Bda, S.; Rivero, A.J.; Aguilar, J.L.; Acco, A. Antitumoral and antioxidant effects of a hydroalcoholic extract of cat’s claw (Uncaria tomentosa (Willd. Ex Roem. & Schult) in an in vivo carcinosarcoma model. J. Ethnopharmacol. 2010, 130, 127–133. [Google Scholar] [CrossRef]
- Dreifuss, A.A.; Bastos-Pereira, A.L.; Fabossi, I.A.; Livero, F.A.; Stolf, A.M.; Alves de Souza, C.E.; Gomes Lde, O.; Constantin, R.P.; Furman, A.E.; Strapasson, R.L.; et al. Uncaria tomentosa exerts extensive anti-neoplastic effects against the Walker-256 tumour by modulating oxidative stress and not by alkaloid activity. PLoS ONE 2013, 8, e54616. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.; Arsenak, M.; Abad, M.J.; Cesari, I.M.; Taylor, P.G. Effect of 3 plant extracts on B16-BL6 melanoma cell growth and metastasis in C57bl/6 mice. Acta Cient. Venez. 2005, 56, 32–36. [Google Scholar]
- Farias, I.L.; Araujo, M.C.; Farias, J.G.; Rossato, L.V.; Elsenback, L.I.; Dalmora, S.L.; Flores, N.M.; Durigon, M.; Cruz, I.B.; Morsch, V.M.; et al. Uncaria tomentosa for reducing side effects caused by chemotherapy in CRC patients: Clinical trial. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- do Carmo Santos Araujo, M.; Farias, I.L.; Gutierres, J.; Dalmora, S.L.; Flores, N.; Farias, J.; de Cruz, I.; Chiesa, J.; Morsch, V.M.; Chitolina Schetinger, M.R. Uncaria tomentosa-adjuvant treatment for breast cancer: Clinical trial. Evid. Based Complement. Altern. Med. 2012, 2012, 676984. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, L.; Holmgren, K.; Pero, R.W. DNA repair enhancement of aqueous extracts of Uncaria tomentosa in a human volunteer study. Phytomedicine 2001, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, F.; Pietrobon Martins, J.; Kaiser, S.; Madeira Silva, R.B.; Rockenbach, L.; Albano Edelweiss, M.I.; Ortega, G.G.; Morrone, F.B.; Campos, M.M.; Battastini, A.M. The quiovic acid glycosides purified fraction from Uncaria tomentosa protects against hemorrhagic cystitis induced by cyclophosphamide in mice. PLoS ONE 2015, 10, e0131882. [Google Scholar] [CrossRef]
- De Paula, L.C.; Fonseca, F.; Perazzo, F.; Cruz, F.M.; Cubero, D.; Trufelli, D.C.; Martins, S.P.; Santi, P.X.; da Silva, E.A.; Del Giglio, A. Uncaria tomentosa (cat’s claw) improves quality of life in patients with advanced solid tumors. J. Altern. Complement. Med. 2015, 21, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Andre, N.; Wang, X.; He, Y.; Pan, G.; Kojo, A.; Liu, Y. A review of the occurrence of non-alkaloid constituents in Uncaria species and their structure-activity relationships. Am. J. Biomed. Life Sci. 2013, 1, 79–98. [Google Scholar] [CrossRef]
- Pereira, R.C.; Valente, L.M.; Pinto, J.E.; Berolucci, S.K.; Bezerra, G.M.; Alves, F.F.; dos Santos, P.F.; Benevides, P.J.; Siani, A.C.; Rosario, S.L.; et al. In vitro cultivated Uncaria tomentosa and Uncaria guianensis with determination of the pentacyclic oxindole alkaloid contents and profiles. J. Braz. Chem. Soc. 2008, 19, 1193–1200. [Google Scholar] [CrossRef]
- Sandoval, M.; Okuhama, N.N.; Zhang, X.J.; Condezo, L.; Lao, J.; Angeles, F.M.; Musah, R.A.; Borowski, P.; Miller, M.J. Anti-inflammatory and antioxidant activities of cat’s claw (Uncaria tomentosa and Uncaria guinanensis) are independent of their alkaloid content. Phytomedicine 2002, 9, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Akesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. An extract of Uncaria tomentosa inhibiting cell division and NF-kappa B activity without inducing cell death. Int. Immunopharmacol. 2003, 3, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Akesson, C.; Pero, R.W.; Ivars, F. C-Med 100, a hot water extract of Uncaria tomentosa, prolongs lymphocyte survival in vivo. Phytomedicine 2003, 10, 23–33. [Google Scholar] [CrossRef][Green Version]
- Sheng, Y.; Pero, R.W.; Wagner, H. Treatment of chemotherapy-induced leukopenia in a rat model with aqueous extract from Uncaria tomentosa. Phytomedicine 2000, 7, 137–143. [Google Scholar] [CrossRef]
- Elrod, H.A.; Sun, S.Y. PPARgamma and apoptosis in cancer. PPAR Res. 2008, 2008. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signaling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef]
- Navarro-Hoyos, M.; Alvarado-Corella, D.; Moreira-Gonzalez, I.; Arnaez-Serrano, E.; Monagas-Juan, M. Polyphenolic Composition and Antioxidant Activity of Aqueous and Ethanolic Extracts from Uncaria tomentosa Bark and Leaves. Antioxidants 2018, 7, 65. [Google Scholar] [CrossRef]
- Paniagua-Perez, R.; Madrigal-Bujaidar, E.; Molina-Jasso, D.; Reyes-Cadena, S.; Alvarez-Gonzalez, I.; Sanchez-Chapul, L.; Perez-Gallaga, J. Antigenotoxic, antioxidant and lymphocyte induction effects produced by pteropodine. Basic. Clin. Pharacol. Toxicol. 2009, 104, 222–227. [Google Scholar] [CrossRef]
- Akesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100. Int. Immunopharacol. 2005, 5, 219–229. [Google Scholar] [CrossRef]
- Mammone, T.; Akesson, C.; Gan, D.; Giampapa, V.; Pero, R.W. A water soluble extract from Uncaria tomentosa (Cat’s claw) is a potent enhancer of DNA repair in primary organ cultures of human skin. Phytother. Res. 2006, 20, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Pavei, C.; Kaiser, S.; Verza, S.G.; Borre, G.L.; Ortega, G.G. HPLC-PDA method for qunovic acid glycoseds assay in Cat’s claw (Uncaria tomentosa) associated with UPLC/Q-TOF-MS analysis. J. Pharm. Biomed. Anal. 2012, 62, 250–257. [Google Scholar] [CrossRef]
- Ranatunge, I.; Adikary, S.; Dasanayake, P.; Fernando, C.D.; Soysa, P. Development of a rapid and simple method to remove polyphenols from plant extracts. Int. J. Anal. Chem. 2017, 2017, 7230145. [Google Scholar] [CrossRef]
- Pabst, A.M.; Ackermann, M.; Wagner, W.; Haberthür, D.; Ziebart, T.; Konerding, M.A. Imaging angiogenesis: Perspectives and opportunities in tumour research-a method display. J. Craniomaxillofac. Surg. 2014, 42, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Vallien, G.; Langley, R.; Jennings, S.; Specian, R.; Granger, D.N. Expression of endothelial cell adhesion molecules in neovascularized tissue. Microcirculation 2000, 7, 249–258. [Google Scholar] [CrossRef]
- Allard, T.; Wenner, T.; Greten, H.J.; Efferth, T. Mechanisms of herb-induced nephrotoxicity. Curr. Med. Chem. 2013, 20, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Torres, L. Reversible worsening of Parkinson disease motor symptoms after oral intake of Uncaria tomentosa (cat’s claw). Clin. Neuropharmacol. 2008, 31, 293–294. [Google Scholar] [CrossRef]
- Fidler, I.J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975, 35, 218–224. [Google Scholar]
- Ganzera, M.; Muhammad, I.; Khan, R.A.; Khan, I.A. Improved method for the determination of oxindole alkaloids in Uncaria tomentosa by high performance liquid chromatography. Planta Med. 2001, 67, 447–450. [Google Scholar] [CrossRef]
- Guo, B.; Romero, J.; Kim, B.J.; Lee, H. High levels of Cdc7 and Dbf4 proteins can arrest cell-cycle progression. Eur. J. Cell Biol. 2005, 84, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Qiagen Cignal Array Kit. Available online: https://www.qiagen.com/us/resources/resourcedetail?id=e5fa0bb9-2cc5-47f9-ac7a-3f698e6d9f3e&lang=en (accessed on 4 January 2021).
- Uckner, C.A.; Buckner, A.; Koren, S.; Persinger, M.A.; Lafrenie, R.M. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE 2015, 10, e0124136. [Google Scholar] [CrossRef]
- Santa Cruz Biotech. Protocol. Immunoperoxidase staining. Available online: https://datasheets.scbt.com/protocols/protocol_06.pdf (accessed on 4 February 2021).
- Santa Cruz Biotech. Protocol. Immunofluorescence cell staining. Available online: https://datasheets.scbt.com/protocols/protocol_07.pdf (accessed on 4 February 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zari, A.; Alfarteesh, H.; Buckner, C.; Lafrenie, R. Treatment with Uncaria tomentosa Promotes Apoptosis in B16-BL6 Mouse Melanoma Cells and Inhibits the Growth of B16-BL6 Tumours. Molecules 2021, 26, 1066. https://doi.org/10.3390/molecules26041066
Zari A, Alfarteesh H, Buckner C, Lafrenie R. Treatment with Uncaria tomentosa Promotes Apoptosis in B16-BL6 Mouse Melanoma Cells and Inhibits the Growth of B16-BL6 Tumours. Molecules. 2021; 26(4):1066. https://doi.org/10.3390/molecules26041066
Chicago/Turabian StyleZari, Ali, Hajer Alfarteesh, Carly Buckner, and Robert Lafrenie. 2021. "Treatment with Uncaria tomentosa Promotes Apoptosis in B16-BL6 Mouse Melanoma Cells and Inhibits the Growth of B16-BL6 Tumours" Molecules 26, no. 4: 1066. https://doi.org/10.3390/molecules26041066
APA StyleZari, A., Alfarteesh, H., Buckner, C., & Lafrenie, R. (2021). Treatment with Uncaria tomentosa Promotes Apoptosis in B16-BL6 Mouse Melanoma Cells and Inhibits the Growth of B16-BL6 Tumours. Molecules, 26(4), 1066. https://doi.org/10.3390/molecules26041066






