Synthesis and Structural Characterization of a Silver(I) Pyrazolato Coordination Polymer †
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
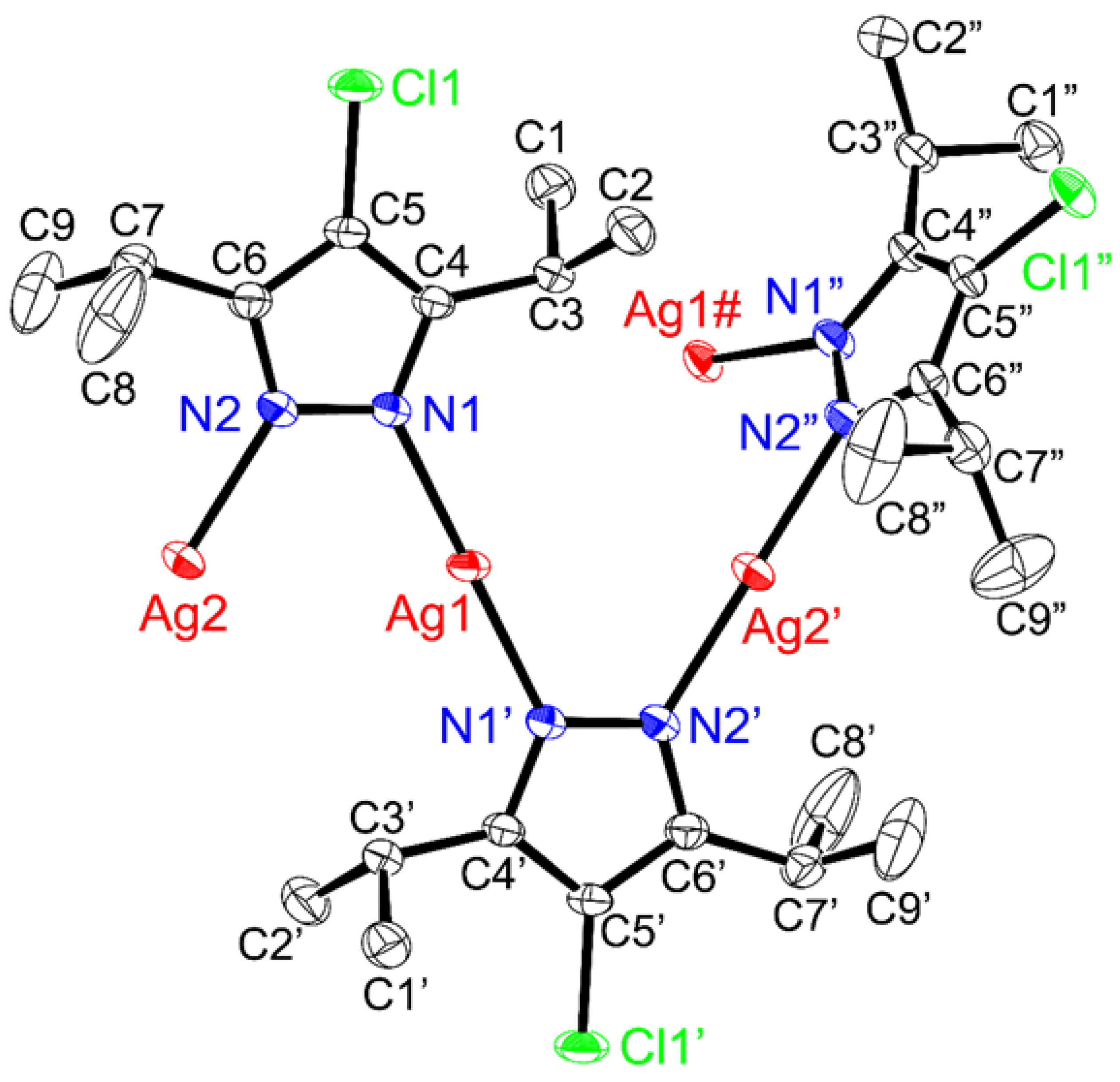
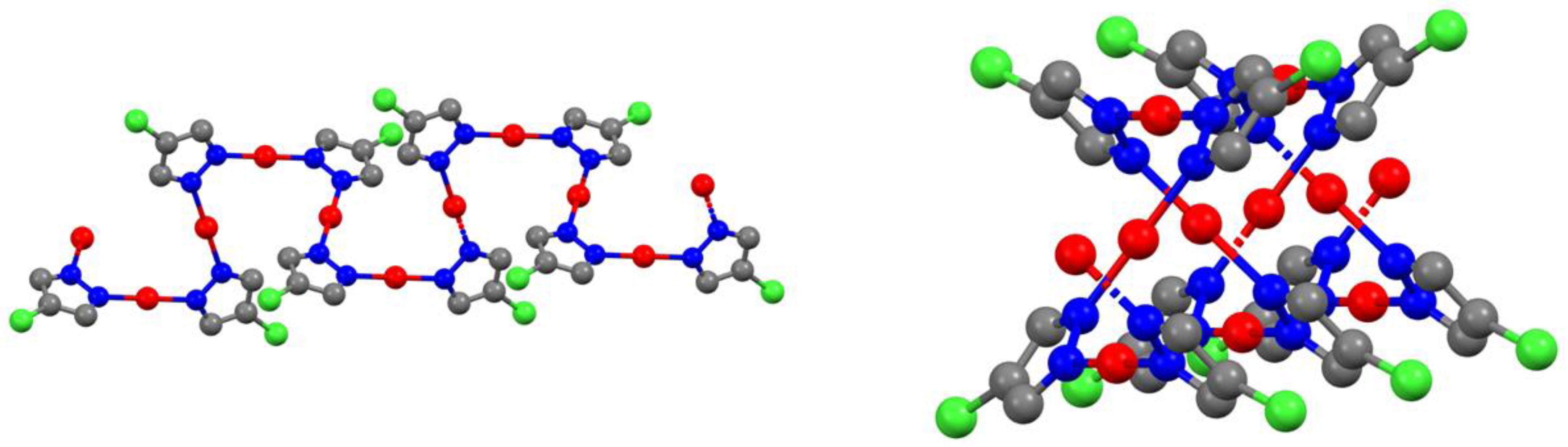
2.2. Structure
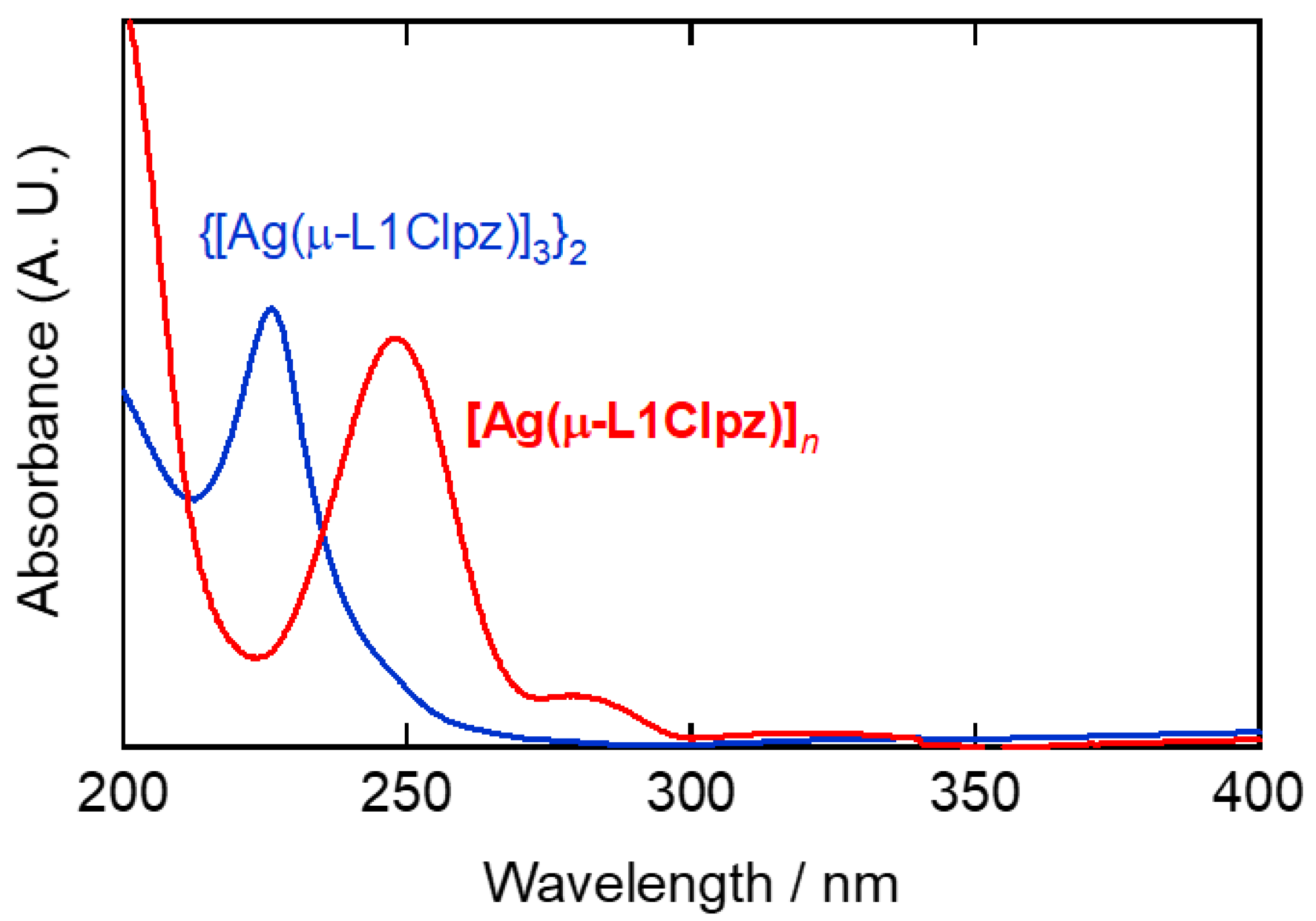
2.3. Solution-State Properties
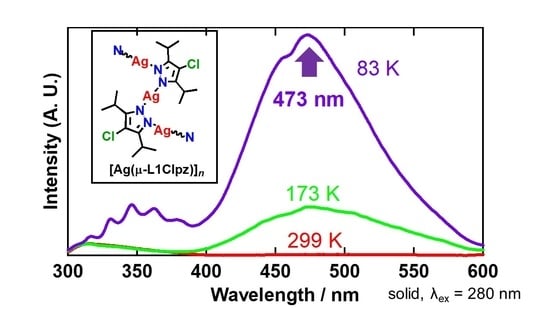
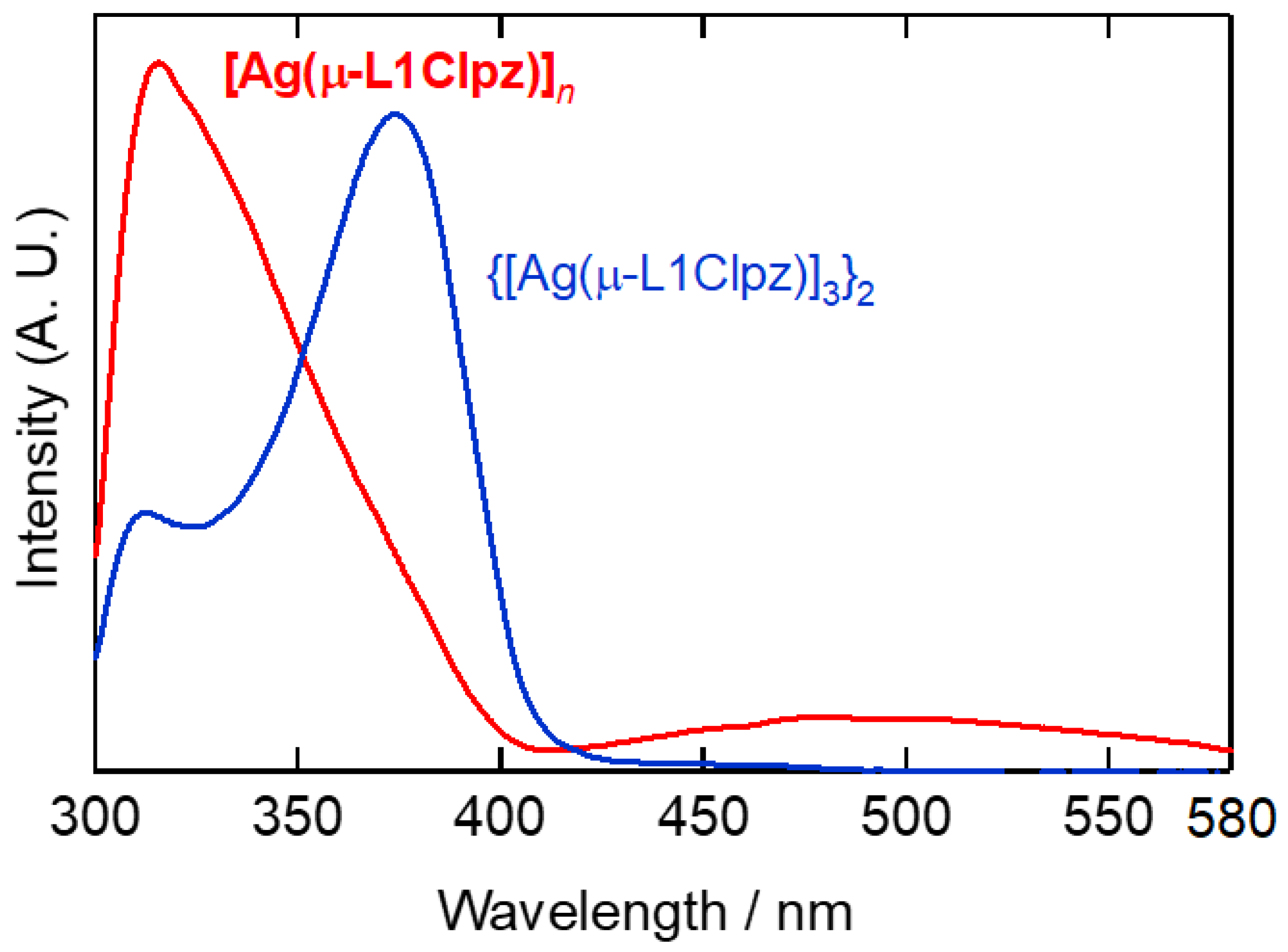
2.4. Solid-State Properties
3. Materials and Methods
3.1. Material and General Techniques
3.2. Instrumentation
3.3. Preparation of Complexes
[Ag(μ-L1Clpz)]n
3.4. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zheng, J.; Lu, Z.; Wu, K.; Ning, G.-H.; Li, D. Coinage-metal-based cyclic trinuclear complexes with metal−metal interactions: Theories to experiments and structures to functions. Chem. Rev. 2020, 120, 9675–9742. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, H.; Xie, M.; Li, D. The π-acidity/basicity of cyclic trinuclear units (CTUs): From a theoretical perspective to potential applications. Chem. Commun. 2019, 55, 7134–7146. [Google Scholar] [CrossRef] [PubMed]
- Galassi, R.; Rawashdeh-Omary, M.A.; Dias, H.V.R.; Omary, M.A. Homoleptic cyclic trinuclear d10 complexes: From self-association via metallophilic and excimeric bonding to the breakage thereof via oxidative addition, dative bonding, quadrupolar, and heterometal bonding interactions. Comments Inorg. Chem. 2019, 39, 287–348. [Google Scholar] [CrossRef]
- Elguero, J.; Alkorta, I. A computational study of metallacycles formed by pyrazolate ligands and the coinage metals M = Cu(I), Ag(I) and Au(I): (pzM)n for n = 2, 3, 4, 5 and 6. comparison with structures reported in the Cambridge crystallographic data center (CCDC). Molecules 2020, 25, 5108. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.A.; Mohamed, A.A.; Rawashdeh-Omary, M.A.; Fackler, J.P., Jr. Photophysics of supramolecular binary stacks consisting of electron-rich trinuclear Au(I) complexes and organicelectrophiles. Coord. Chem. Rev. 2005, 249, 1372–1381. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef]
- Halcrow, M.A. Pyrazoles and pyrazolides—Flexible synthons in self-assembly. Dalton Trans. 2009, 2059–2073. [Google Scholar] [CrossRef]
- Trofimenko, S. The coordination chemistry of pyrazole-derived ligands. Chem. Rev. 1972, 72, 497–509. [Google Scholar] [CrossRef]
- Trofimenko, S. Recent advances in poly(pyrazolyl)borate (scorpionate) chemistry. Chem. Rev. 1993, 93, 943–982. [Google Scholar] [CrossRef]
- Reimlinger, H.; Noels, A.; Jabot, J.; van Overstraeten, A. Synthesis with silver or sodium pyrazoles, I preparation of mono- and polypyrazoles. Chem. Ber. 1970, 103, 1942–1948. [Google Scholar] [CrossRef]
- Okkersen, H.; Groeneveld, W.L.; Reedijk, J. Pyrazoles and imidazoles as ligands. Part XVIII: Neutral and anionic pyrazole coordinated to Cu(I) and Ag(I). Recl. Trav. Chim. Pays-Bas 1973, 92, 945–953. [Google Scholar] [CrossRef]
- Murray, H.H.; Raptis, R.G.; Fackler, J.P., Jr. Syntheses and X-ray structures of group 11 pyrazole and pyrazolate complexes. X-ray crystal structures of bis(3,5-diphenylpyrazole)copper(II) dibromide, tris(μ-3,5-diphenylpyrazolato-N,N’)trisilver(I)-2-tetrahydrofuran, tris(μ-3,5-diphenylpyrazolato-N,N’)trigold(I), and hexakis(μ-3,5-diphenylpyrazolato- N,N’)hexagold(I). Inorg. Chem. 1988, 27, 26–33. [Google Scholar]
- Mohamed, A.A.; Pérez, L.M.; Fackler, J.P. Jr. Unsupported intermolecular argentophilic interaction in the dimer of trinuclear silver(I) 3,5-diphenylpyrazolates. Inorg. Chim. Acta 2005, 358, 1657–1662. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef] [PubMed]
- Masciocchi, N.; Moret, M.; Cairati, P.; Sironi, A.; Ardizzoia, G.A.; Monica, G.L. The multiphase nature of the Cu(pz) and Ag(pz) (Hpz = pyrazole) systems: Selective syntheses and ab-initio X-ray powder diffraction structural characterization of copper(I) and silver(I) pyrazolates. J. Am. Chem. Soc. 1994, 116, 7668–7676. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Feng, J.-B.; Gao, Q.; Xie, Y.-B. catena-Poly[silver(I)-μ-pyrazolato-κ2N:N’]. Acta Cryst. 2008, 64, m352. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Okano, M.; Martín-Pastor, M.; López-Sánchez, R.; Elguero, J.; Alkorta, I. Multinuclear magnetic resonance studies of five silver(I) trinuclear pyrazolate complexes. Struct. Chem. 2021, 32, 215–224. [Google Scholar] [CrossRef]
- Fujisawa, K.; Saotome, M.; Takeda, S.; Young, D.J. Structures and photoluminescence of coinage metal(I) phenylpyrazolato trinuclear complexes [M(3,5-Et2-4-Ph-pz)]3 and arene sandwich complexes {[Ag(3,5-Et2-4-Ph-pz)]3}2(Ar) (Ar = mesitylene and toluene). Chem. Lett. 2020, 49, 670–673. [Google Scholar] [CrossRef]
- Saotome, M.; Shimizu, D.; Itagaki, A.; Young, D.J.; Fujisawa, K. Structures and photoluminescence of silver(I) and gold(I) cyclic trinuclear complexes with aryl substituted pyrazolates. Chem. Lett. 2019, 48, 533–536. [Google Scholar] [CrossRef]
- Morishima, Y.; Young, D.J.; Fujisawa, K. Structure and photoluminescence of silver(I) trinuclear halopyrazolato complexes. Dalton Trans. 2014, 43, 15915–15928. [Google Scholar] [PubMed]
- Fujisawa, K.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K. Pyrazolate-bridged group 11 metal(I) complexes: Substituent effects on the supramolecular structures and physicochemical properties. Inorg. Chim. Acta 2010, 363, 2977–2989. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K. Crystal structure of pyrazolato-bridged copper(I) polynuclear complexes. Chem. Lett. 2004, 33, 66–67. [Google Scholar] [CrossRef]
- Burini, A.; Bravi, R.; Fackler, J.P., Jr.; Galassi, R.; Grant, T.A.; Omary, M.A.; Pietroni, B.R.; Staples, R.J. Luminescent chains formed from neutral, triangular gold complexes sandwiching TlI and AgI. structures of {Ag([Au(μ-C2,N3-bzim)]3)2}BF4·CH2Cl2, {Tl([Au(μ-C2,N3-bzim)]3)2}PF6·0.5THF (bzim = 1-Benzylimidazolate), and {Tl([Au(μ-C(OEt)=NC6H4CH3)]3)2}PF6·THF, with MAu6 (M = Ag+, Tl+) cluster cores. Inorg. Chem. 2000, 39, 3158–3165. [Google Scholar]
- Baril-Robert, F.; Li, X.; Katz, M.J.; Geisheimer, A.R.; Leznoff, D.B.; Patterson, H. Changes in electronic properties of polymeric one-dimensional {[M(CN) 2]−}n (M = Au, Ag) chains due to neighboring closed-shell Zn(II) or open-shell Cu(II) ions. Inorg. Chem. 2011, 50, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Shorrock, C.J.; Xue, B.-Y.; Kim, P.B.; Batchelor, R.J.; Patrick, B.O.; Leznoff, D.B. Heterobimetallic coordination polymers incorporating [M(CN)2]− (M = Cu, Ag) and [Ag2(CN)3]− units: Increasing structural dimensionality via M–M’ and M···NC interactions. Inorg. Chem. 2002, 41, 6743–6753. [Google Scholar] [CrossRef]
- Rawashdeh-Omary, M.A.; Omary, M.A.; Patterson, H.H.; Fackler, J.P., Jr. Excited-state interactions for [Au(CN)2−]n and [Ag(CN)2−]n oligomers in solution. Formation of luminescent gold–gold bonded excimers and exciplexes. J. Am. Chem. Soc. 2001, 123, 11237–11247. [Google Scholar] [CrossRef]
- Omary, M.A.; Webb, T.R.; Assefa, Z.; Shankle, G.E.; Patterson, H.H. Crystal structure, electronic structure, and temperature-dependent Raman spectra of Tl[Ag(CN)2]: Evidence for ligand-unsupported argentophilic interactions. Inorg. Chem. 1998, 37, 1380–1386. [Google Scholar] [CrossRef]
- Omary, M.A.; Patterson, H.H. Temperature-dependent photoluminescence properties of Tl[Ag(CN)2]: Formation of luminescent metal-metal-bonded inorganic exciplexes in the solid state. Inorg. Chem. 1998, 37, 1060–1066. [Google Scholar] [CrossRef]
- Dragulescu-Andrasi, A.; Hietsoi, O.; Üngör, Ö.; Dunk, P.W.; Stubbs, V.; Arroyave, A.; Kovnir, K.; Shatruk, M. Dicyanometalates as building blocks for multinuclear iron(II) spin-crossover complexes. Inorg. Chem. 2019, 58, 11920–11926. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volume and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman spectra of inorganic and coordination compounds, 6th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Omary, M.A.; Rawashdeh-Omary, M.A.; Gonser, M.W.A.; Elbjeirami, O.; Grimes, T.; Cundari, T.R.; Diyabalanage, H.V.K.; Gamage, C.S.P.; Dias, H.V.R. Metal Effect on the supramolecular structure, photophysics, and acid-base character of trinuclear pyrazolato coinage metal complexes. Inorg. Chem. 2005, 44, 8200–8210. [Google Scholar] [CrossRef] [PubMed]
- Grimes, T.; Omary, M.A.; Dias, H.V.R.; Cundari, T.R. Intertrimer and intratrimer metallophilic and excimeric bonding in the ground and phosphorescent states of trinuclear coinage metal pyrazolates: A computational study. J. Phys. Chem. A 2006, 110, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, C.V.; Rawashdeh-Omary, M.A.; Korir, D.; Kohistani, J.; Yousufuddin, M.; Dias, H.V.R. Trinuclear copper(I) and silver(I) adducts of 4-chloro-3,5-bis(trifluoromethyl)pyrazolate and 4-bromo-3,5-bis(trifluoromethyl)pyrazolate. Inorg. Chem. 2013, 52, 13576–13583. [Google Scholar] [CrossRef]
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Moro-oka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, T.; Tatsumi, K.; Nakamura, A. A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = i-Pr and Ph). J. Am. Chem. Soc. 1992, 114, 1277–1291. [Google Scholar] [CrossRef]
- CrystalClear: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Akishima, Japan, 1999.
- CrysAlisPro: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Akishima, Japan, 2015.
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Crystal Structure 4.3: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Akishima, Japan, 2019.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Jaros, S.W.; da Silva, M.F.C.G.; Florek, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I) 1,3,5-triaza-7-phosphaadamantane coordination polymers driven by substituted glutarate and malonate building blocks: Self-assembly synthesis, structural features, and antimicrobial properties. Inorg. Chem. 2016, 55, 5886–5894. [Google Scholar] [CrossRef]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal–organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Smoleński, P.; da Silva, M.F.C.G.; Florek, M.; Król, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M. New silver BioMOFs driven by 1,3,5-triaza-7-phosphaadamantane-7-sulfide (PTA=S): Synthesis, topological analysis and antimicrobial activity. CrystEngComm 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Domb, A.J.; Kunduru, K.R.; Farah, S. Antimicrobial Materials for Biomedical Applications; The Royal Society of Chemistry: Croydon, UK, 2019. [Google Scholar]


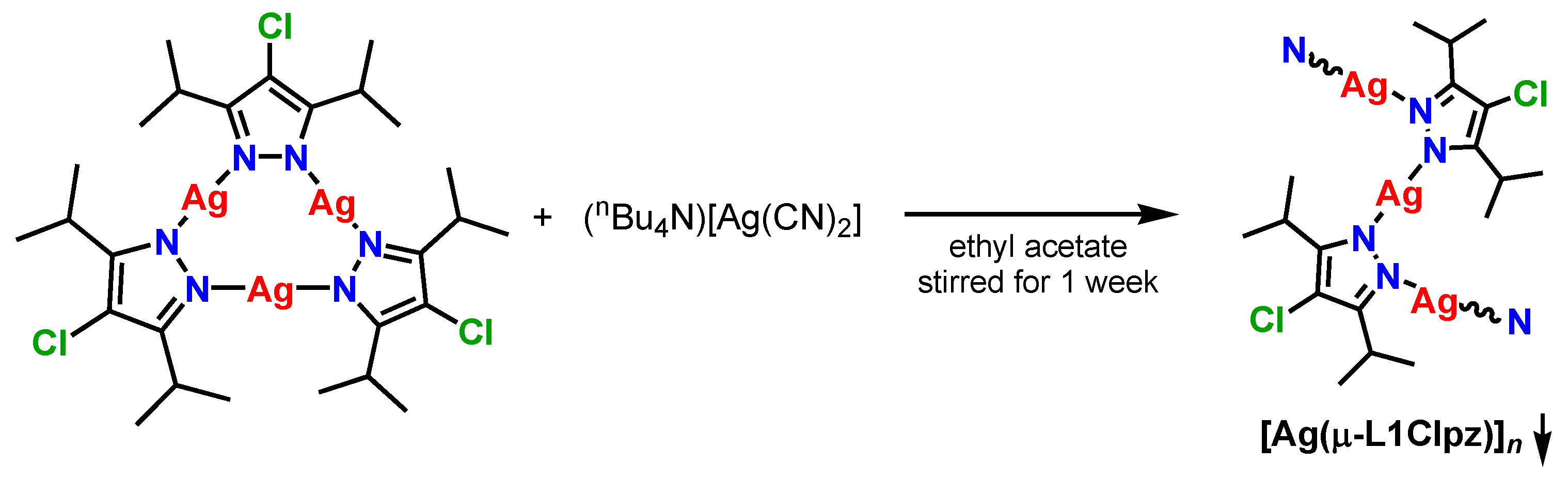

| Complex | [Ag(μ-L1Clpz)]n |
|---|---|
| CCDC number | 2053891 |
| Empirical Formula | C9H14AgClN2 |
| Formula Weight | 293.54 |
| Crystal System | Monoclinic |
| Space Group | I2/a (#15) |
| a/Å | 9.4667(11) |
| b/Å | 11.0962(3) |
| c/Å | 21.5508(7) |
| β/° | 94.859(2) |
| V/Å3 | 2255.66(11) |
| Z | 8 |
| Dcalc/g cm−3 | 1.729 |
| μ(MoKα)/cm−1 | 19.771 |
| Temperature/°C | −80 |
| 2θ range, ° | 6–55 |
| Reflections collected | 8801 |
| Unique reflections | 2589 |
| Rint | 0.0111 |
| Number of Variables | 120 |
| Refls./Para ratio | 21.57 |
| Residuals: R1 (I > 2 σ (I)) | 0.0155 |
| Residuals: R (All reflections) | 0.0167 |
| Residuals: wR2 (All reflections) | 0.0406 |
| Goodness of fit indicator | 1.046 |
| Max/min peak,/e Å−3 | 0.43/−0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Nemoto, T.; Morishima, Y.; Leznoff, D.B. Synthesis and Structural Characterization of a Silver(I) Pyrazolato Coordination Polymer. Molecules 2021, 26, 1015. https://doi.org/10.3390/molecules26041015
Fujisawa K, Nemoto T, Morishima Y, Leznoff DB. Synthesis and Structural Characterization of a Silver(I) Pyrazolato Coordination Polymer. Molecules. 2021; 26(4):1015. https://doi.org/10.3390/molecules26041015
Chicago/Turabian StyleFujisawa, Kiyoshi, Takuya Nemoto, Yui Morishima, and Daniel B. Leznoff. 2021. "Synthesis and Structural Characterization of a Silver(I) Pyrazolato Coordination Polymer" Molecules 26, no. 4: 1015. https://doi.org/10.3390/molecules26041015
APA StyleFujisawa, K., Nemoto, T., Morishima, Y., & Leznoff, D. B. (2021). Synthesis and Structural Characterization of a Silver(I) Pyrazolato Coordination Polymer. Molecules, 26(4), 1015. https://doi.org/10.3390/molecules26041015