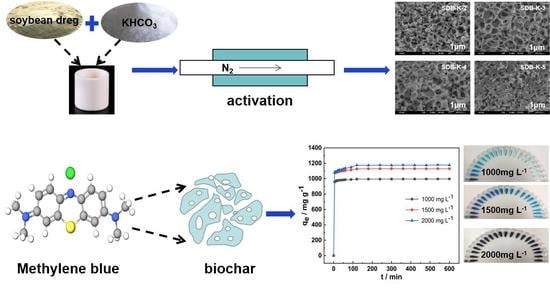
Efficient Adsorption of Methylene Blue by Porous Biochar Derived from Soybean Dreg Using a One-Pot Synthesis Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
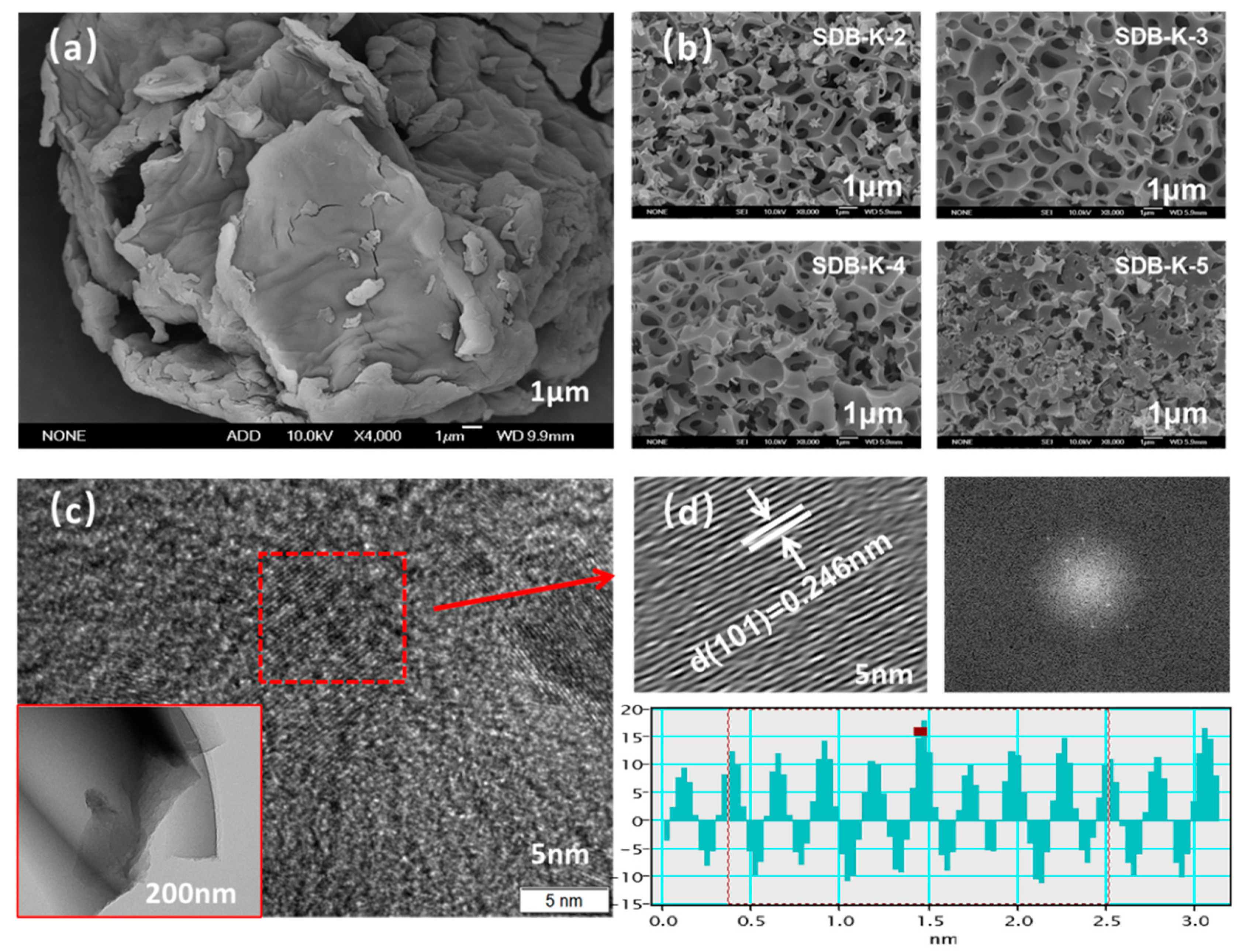
2.1.1. SEM and TEM
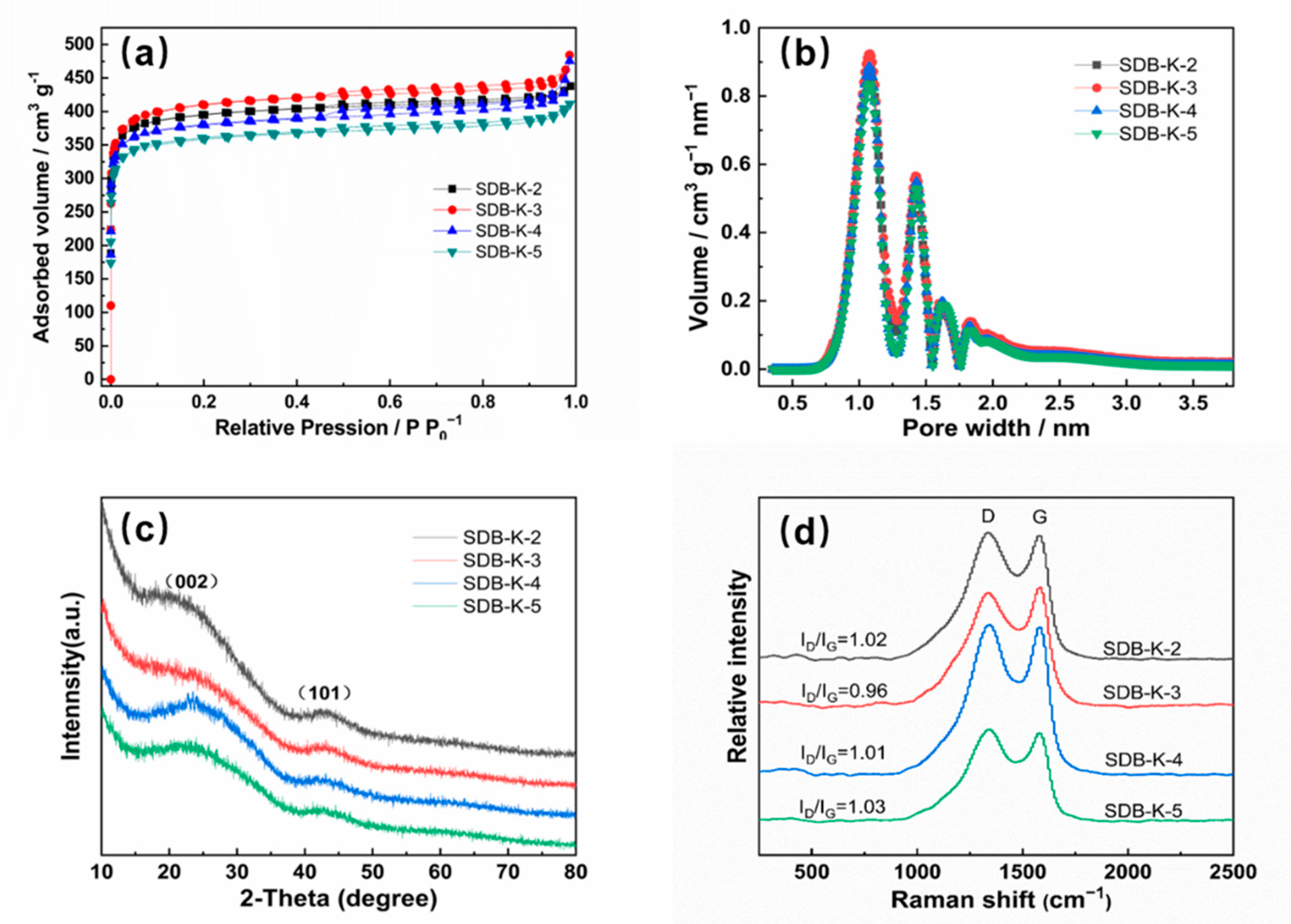
2.1.2. EA and BET
2.1.3. XRD and Raman
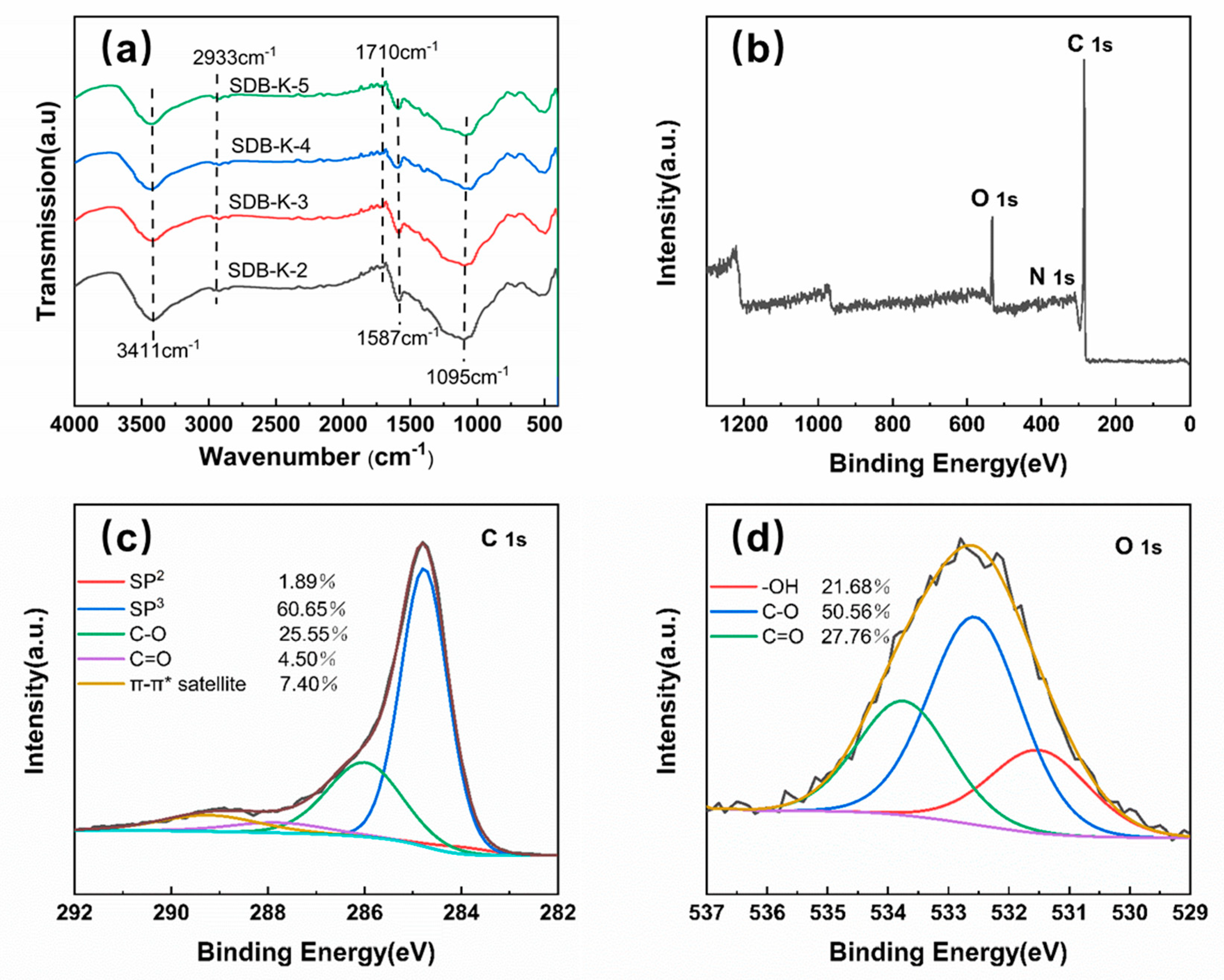
2.1.4. FTIR and XPS
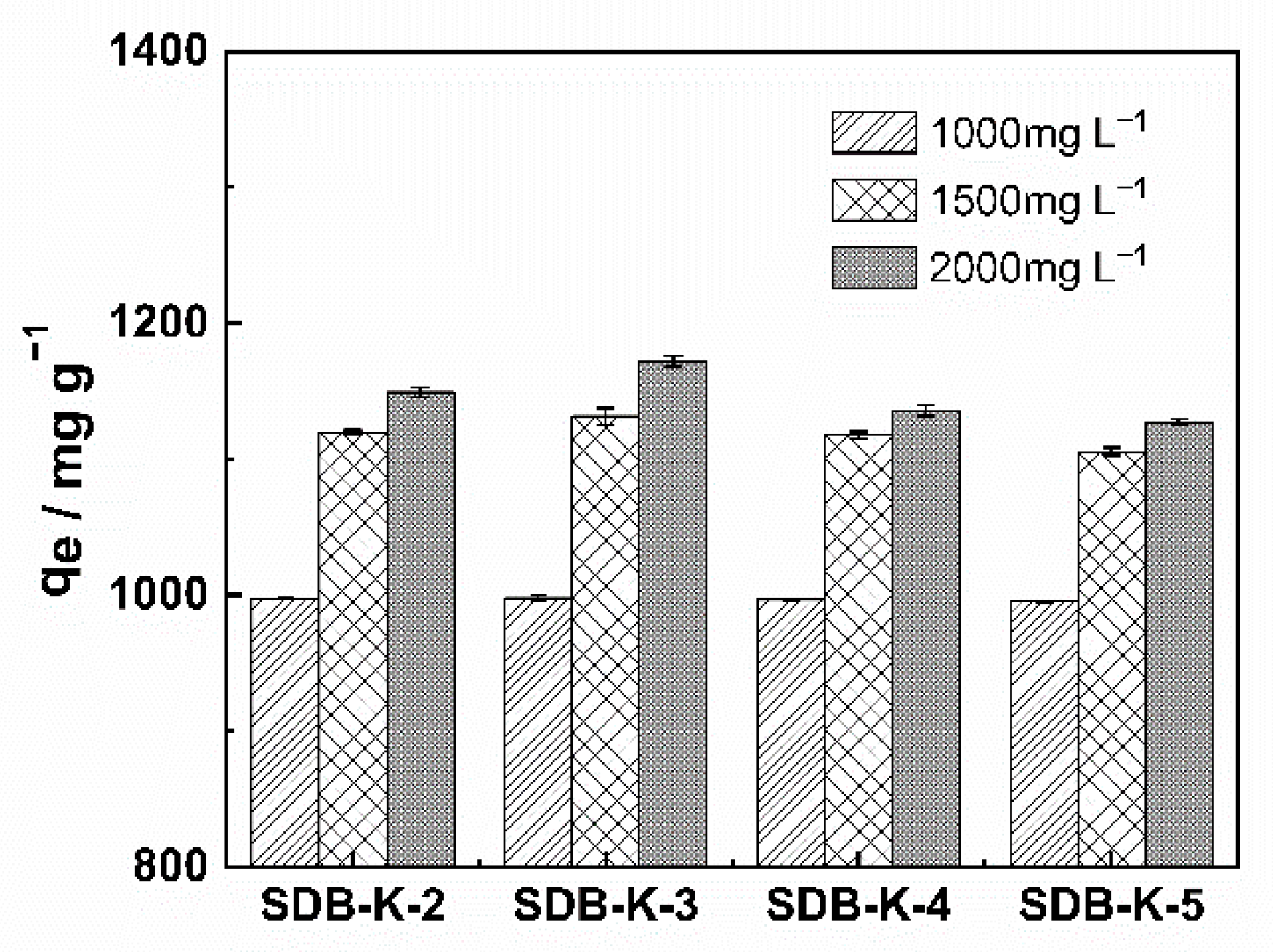
2.2. The Adsorption Process
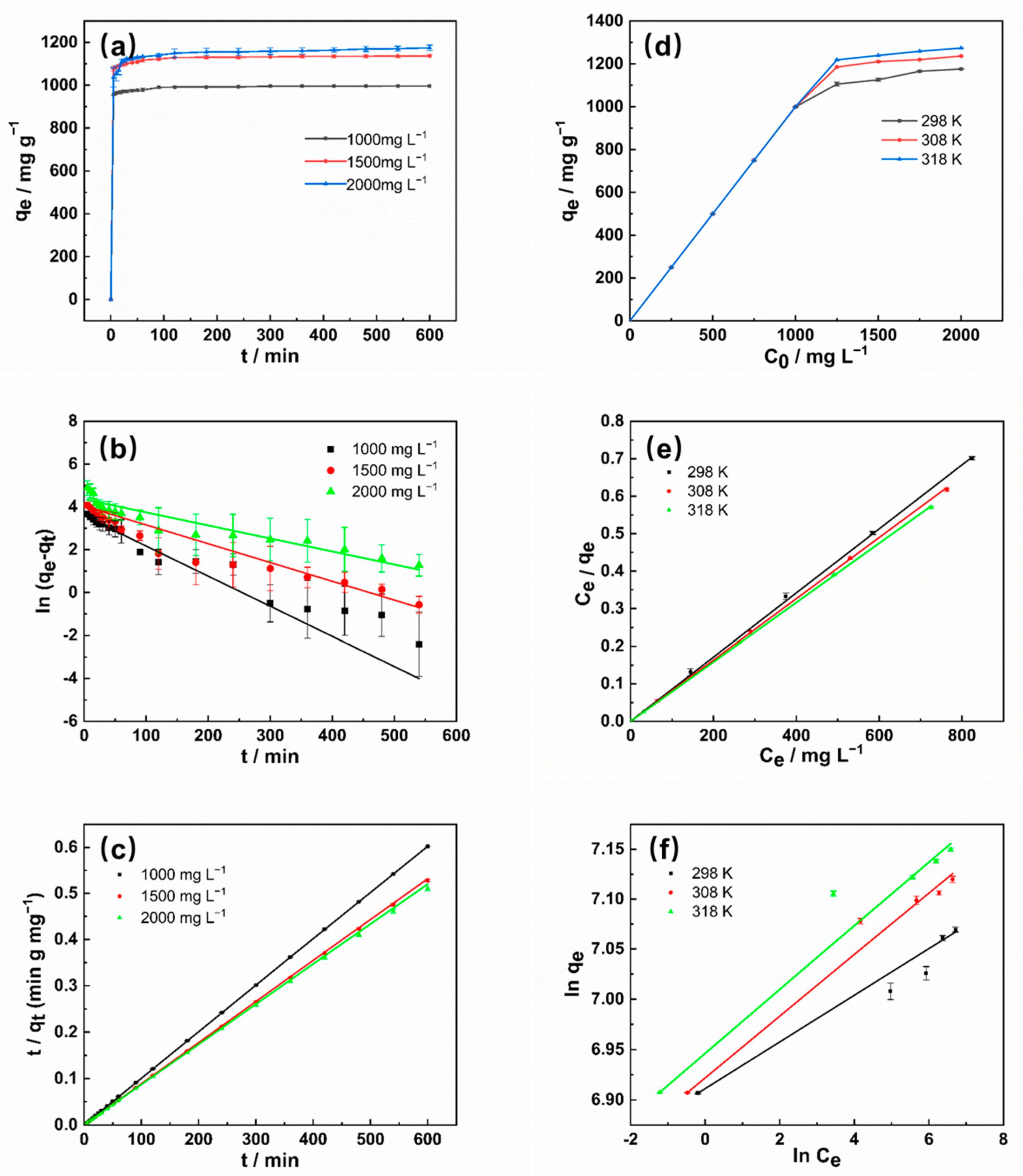
2.2.1. Adsorption Kinetics
2.2.2. Adsorption Equilibrium
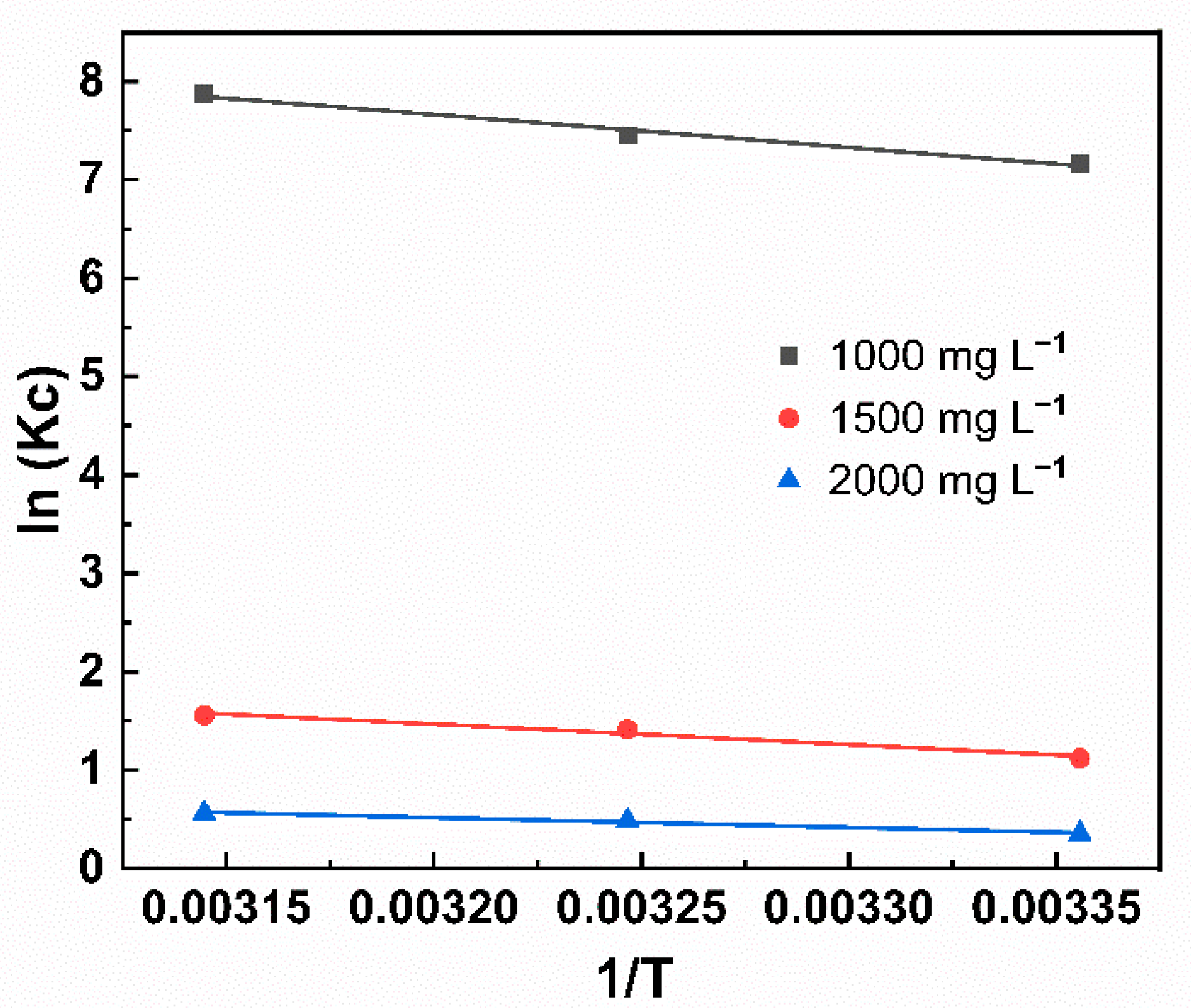
2.2.3. Thermodynamic Analysis
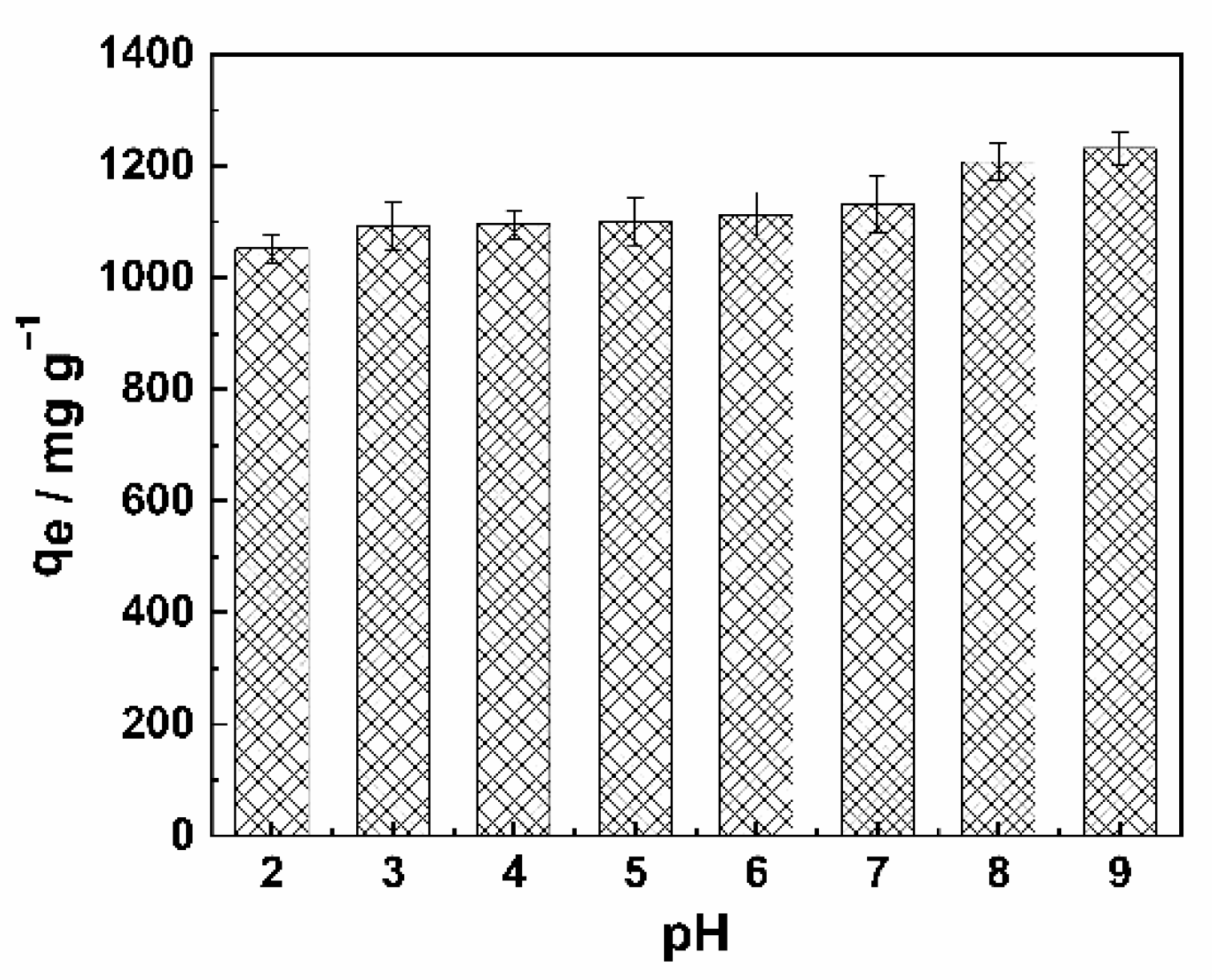
2.2.4. Effect of pH on MB Adsorption
2.3. Adsorption Performance Comparison with Other Adsorbents
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of SDB-K-X
3.3. Characterization of SDB-K-X
3.4. Adsorption Experiments
3.4.1. Adsorption Kinetics
3.4.2. Adsorption Isotherm
3.4.3. Effect of Solution pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Corso, C.R.; Almeida, E.J.; Santos, G.C.; Morao, L.G.; Fabris, G.S.; Mitter, E.K. Bioremediation of direct dyes in simulated textile effluents by a paramorphogenic form of Aspergillus oryzae. Wat. Sci. Tech. 2012, 65, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Nethaji, S.; Sivasamy, A.; Thennarasu, G.; Saravanan, S. Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. J. Hazard. Mater. 2010, 181, 271–280. [Google Scholar] [CrossRef]
- Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Methylene blue adsorption by algal biomass based materials: Biosorbents characterization and process behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriy, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Dincer, A.R.; Gunes, Y.; Karakaya, N.; Gunes, E. Comparison of activated carbon and bottom ash for removal of reactive dye from aqueous solution. Bioresour. Technol. 2007, 98, 834–839. [Google Scholar] [CrossRef]
- Shen, D.; Fan, J.; Zhou, W.; Gao, B.; Yue, Q.; Kang, Q. Adsorption kinetics and isotherm of anionic dyes onto organo-bentonite from single and multisolute systems. J. Hazard. Mater. 2009, 172, 99–107. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Varadarajan, P.R.; Porkodi, K.; Subbhuraam, C.V. Adsorption of methylene blue onto jute fiber carbon: Kinetics and equilibrium studies. J. Colloid Interf. Sci. 2005, 284, 78–82. [Google Scholar] [CrossRef]
- Tan, I.A.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye using activated carbon prepared from oil palm shell: Batch and fixed bed studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Ciardelli, G.; Corsi, L.; Marcucci, M. Membrane separation for wastewater reuse in the textile industry. Resour. Conserv. Recy. 2000, 31, 189–197. [Google Scholar] [CrossRef]
- Thongchai, P.; Somkid, W. Mechanisms of dye wastewater colour removal by magnesium carbonate-hydrated basic. Wat. Sci. Tech. 1986, 18, 139–144. [Google Scholar]
- Dong, Y.; Han, Z.; Liu, C.; Du, F. Preparation and photocatalytic performance of Fe (III)-amidoximated PAN fiber complex for oxidative degradation of azo dye under visible light irradiation. Sci. Total Environ. 2010, 408, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile Dyeing Wastewater Treatment. Adv. Treat. Text. Effl. 2011, 5, 91–116. [Google Scholar]
- Astuti, W.; Sulistyaningsih, T.; Kusumastuti, E.; Thomas, G.; Kusnadi, R.Y. Thermal conversion of pineapple crown leaf waste to magnetized activated carbon for dye removal. Bioresour. Technol. 2019, 287, 121426. [Google Scholar] [CrossRef] [PubMed]
- Khattri, S.D.; Singh, M.K. Removal of malachite green from dye wastewater using neem sawdust by adsorption. J. Hazard. Mater. 2009, 167, 1089–1094. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. One-pot synthesis and characterization of engineered hydrochar by hydrothermal carbonization of biomass with ZnCl2. Chemosphere 2020, 254, 126866. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. Comparison of characteristics of twenty-one types of biochar and their ability to remove multi-heavy metals and methylene blue in solution. Fuel Process. Technol. 2017, 160, 55–63. [Google Scholar] [CrossRef]
- Juan, R.; Wu, F.; Tseng, R. Characterization and use of activated carbons prepared from bagasses for liquid-phase adsorption. Colloids Surf. A 2002, 201, 191–199. [Google Scholar] [CrossRef]
- Arami, M.; Limaee, N.Y.; Mahmoodi, N.M.; Tabrizi, N.S. Removal of dyes from colored textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies. J. Colloid Interface Sci. 2005, 288, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Production of reducing sugars from bean dregs waste by hydrolysis in subcritical water. J. Anal. Appl. Pyrol. 2011, 90, 182–186. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Xiao, Z.; Zhou, R.; Yi, F. Pyrolysis characteristics of bean dregs and in situ visualization of pyrolysis transformation. Waste Manage. 2012, 32, 2287–2293. [Google Scholar] [CrossRef]
- Li, B.; Qiao, M.; Lu, F. Composition, Nutrition, and Utilization of Okara (Soybean Residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Kumar, G.; Shobana, S.; Chen, W.; Bach, Q.; Kim, S.-H.; Atabani, A.E.; Chang, J.-S. A review of thermochemical conversion of microalgal biomass for biofuels: Chemistry and processes. Green Chem. 2017, 19, 44–67. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sust. Energ. Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Xu, F.; Li, M.; Han, C.; Gong, Y.; Wang, H.; Wang, Y. Inspired by Bread Leavening: One-pot Synthesisof Hierarchically Porous Carbon for Supercapacitors. Green Chem. 2015, 17, 4053–4060. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Wang, Z.; Yuan, Z. Bean dregs-derived hierarchical porous carbons as metal-free catalysts for efficient dehydrogenation of propane to propylene. J. Chem. Technol. Biot. 2018, 93, 3410–3417. [Google Scholar] [CrossRef]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-Moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Hydrothermal conversion of urban food waste to chars for removal of textile dyes from contaminated waters. Bioresour. Technol. 2014, 161, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Zhang, P.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.; Deng, S. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem. Eng. J. 2019, 355, 309–319. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Yu, Y.; Qiao, N.; Wang, D.; Zhu, Q.; Fu, F.; Cao, R.; Wang, R.; Liu, W.; Xu, B. Fluffy honeycomb-like activated carbon from popcorn with high surface area and well-developed porosity for ultra-high efficiency adsorption of organic dyes. Bioresour. Technol. 2019, 285, 121340. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Hydrothermal deoxygenation of graphene oxide: Chemical and structural evolution. Chem. Asian J. 2013, 8, 2070–2078. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasisb, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–844. [Google Scholar] [CrossRef]
- Luo, C.; Tian, Z.; Yang, B.; Zhang, L.; Yan, S. Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal. Chem. Eng. J. 2013, 234, 256–265. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, C.; Sun, J.; Li, H.; Sun, Z.; Yan, S. Enhanced adsorption removal of methyl orange from aqueous solution by nanostructured proton-containing δ-MnO2. J. Mater. Chem. A 2015, 3, 5674–5682. [Google Scholar] [CrossRef]
- Wang, D.; Shen, H.; Guo, L.; Wang, C.; Fu, F. Porous BiOBr/Bi2MoO6 Heterostructures for Highly Selective Adsorption of Methylene Blue. ACS Omega 2016, 1, 566–577. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Ueber die adsorption in loesungen. Zeitschrift für physikalische Chemie 1907, 57, 385–470. [Google Scholar]
- Rajesh, V.; Rajesh, N. Adsorption isotherms, kinetics and thermodynamic studies towards understanding the interaction between a microbe immobilized polysaccharide matrix and lead. Chem. Eng. J. 2014, 248, 342–351. [Google Scholar]
- Zhang, P.; O’Connora, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; He, B.; Xu, F.; Chen, X. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 2011, 170, 82–89. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhou, X.; Zhang, Z.; Zhang, J.; Dang, F. Biomass-derived highly porous functional carbon fabricated by using a free-standing template for efficient removal of methylene blue. Bioresour. Technol. 2014, 154, 138–147. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour. Technol. 2019, 278, 159–164. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J. Environ. Manage. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T.; Hashim, R.; Said, N.; Akhtar, M.N.; Mohamad-Saleh, J.; Sulaiman, O. Comparison of surface properties of wood biomass activated carbons and their application against rhodamine B and methylene blue dye. Surf. Interfaces 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotox. Environ. Safe. 2017, 138, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, W.; Zhang, C.; Zhang, S.; Liu, L.; Zhu, L.; Zhao, W. Effect of a magnetic field on the adsorptive removal of methylene blue onto wheat straw biochar. Bioresour. Technol. 2016, 206, 16–22. [Google Scholar] [CrossRef] [PubMed]
| Sample | Yield (%) | C (%) | O (%) | H (%) | N (%) | S (%) |
|---|---|---|---|---|---|---|
| SD | / | 41.14 | 46.63 | 7.895 | 3.370 | 0.476 |
| SDB-K-2 | 15.34% | 66.31 | 28.27 | 2.136 | 0.530 | 0.348 |
| SDB-K-3 | 15.61% | 66.28 | 28.11 | 1.669 | 0.542 | 0.383 |
| SDB-K-4 | 15.11% | 69.71 | 27.81 | 1.487 | 0.795 | 0.528 |
| SDB-K-5 | 15.00% | 69.87 | 26.84 | 1.434 | 0.551 | 0.445 |
| Sample | BET (m2 g−1) | Vtot (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| SDB-K-2 | 1572 | 0.6786 | 1.727 |
| SDB-K-3 | 1620 | 0.7509 | 1.859 |
| SDB-K-4 | 1510 | 0.7375 | 1.953 |
| SDB-K-5 | 1425 | 0.6376 | 1.790 |
| Sample | C0 (mg L−1) | qe, exp (mg g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| qe,cal (mg·g−1) | K1 (min−1) | R2 | qe,cal (mg·g−1) | K2 (g·mg−1·min−1) | R2 | |||
| SBD-K-3 | 1000 | 996.37 | 35.74 | 0.01404 | 0.87406 | 1000.00 | 0.00356 | 1 |
| 1500 | 1136.83 | 56.61 | 0.00877 | 0.94358 | 1129.14 | 0.00221 | 0.9999 | |
| 2000 | 1174.73 | 77.62 | 0.00608 | 0.85346 | 1155.79 | 0.00086 | 0.99986 | |
| Sample | T/K | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qm (mg·g−1) | KL (L·mg−1) | R2 | n | KF | R2 | ||
| SBD-K-3 | 298 | 1170.72 | 3.0296 | 0.99989 | 43.10 | 1003.44 | 0.96722 |
| 308 | 1225.55 | 6.65189 | 0.99992 | 32.58 | 1014.14 | 0.99206 | |
| 318 | 1264.97 | 11.2932 | 0.99989 | 31.45 | 1039.18 | 0.99186 | |
| Concentration (mg L−1) | T (K) | ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (J·K−1·mol−1) | R2 |
|---|---|---|---|---|---|
| 1000 | 298 | −17.70 | 27.77 | 152.56 | 0.96523 |
| 308 | −19.22 | ||||
| 318 | −20.75 | ||||
| 1500 | 298 | −2.82 | 17.41 | 67.89 | 0.9409 |
| 308 | −3.50 | ||||
| 318 | −4.18 | ||||
| 2000 | 298 | −0.898 | 8.14 | 30.33 | 0.96866 |
| 308 | −1.201 | ||||
| 318 | −1.505 |
| Adsorbents | Max Adsorption Capacity qmax (mg g−1) | Reference |
|---|---|---|
| Banana peel | 862 mg g−1 | [46] |
| Garlic peel | 142.86 mg g−1 | [47] |
| Seaweed | 512.67 mg g−1 | [48] |
| Coconut shells | 200 mg g−1 | [49] |
| Wood | 159.89 mg g−1 | [50] |
| Rattan | 359 mg g−1 | [51] |
| Wheat straw | 62.5 mg g−1 | [52] |
| SD | 1273.51 mg g−1 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, Z.; Chen, X.; Li, H.; Liu, X.; Zhang, C.; Zhang, J.; Yi, G. Efficient Adsorption of Methylene Blue by Porous Biochar Derived from Soybean Dreg Using a One-Pot Synthesis Method. Molecules 2021, 26, 661. https://doi.org/10.3390/molecules26030661
Ying Z, Chen X, Li H, Liu X, Zhang C, Zhang J, Yi G. Efficient Adsorption of Methylene Blue by Porous Biochar Derived from Soybean Dreg Using a One-Pot Synthesis Method. Molecules. 2021; 26(3):661. https://doi.org/10.3390/molecules26030661
Chicago/Turabian StyleYing, Zhiwei, Xinwei Chen, He Li, Xinqi Liu, Chi Zhang, Jian Zhang, and Guofu Yi. 2021. "Efficient Adsorption of Methylene Blue by Porous Biochar Derived from Soybean Dreg Using a One-Pot Synthesis Method" Molecules 26, no. 3: 661. https://doi.org/10.3390/molecules26030661
APA StyleYing, Z., Chen, X., Li, H., Liu, X., Zhang, C., Zhang, J., & Yi, G. (2021). Efficient Adsorption of Methylene Blue by Porous Biochar Derived from Soybean Dreg Using a One-Pot Synthesis Method. Molecules, 26(3), 661. https://doi.org/10.3390/molecules26030661