Comparison of Metal Nanoparticles (Au, Ag, Eu, Cd) Used for Immunoanalysis Using LA-ICP-MS Detection
Abstract
1. Introduction
2. Results and Discussion
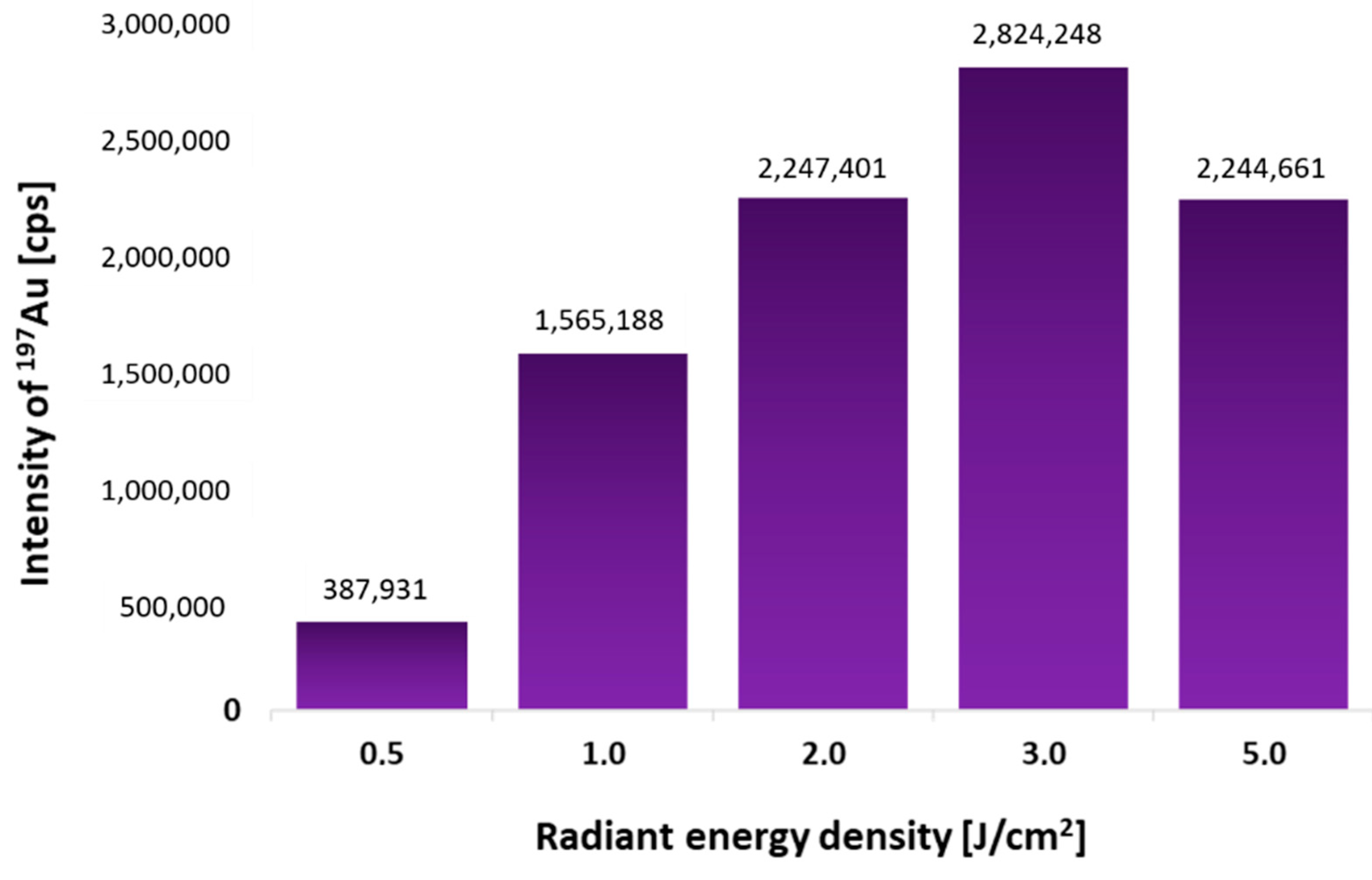
2.1. Laser Beam Fluence Optimization
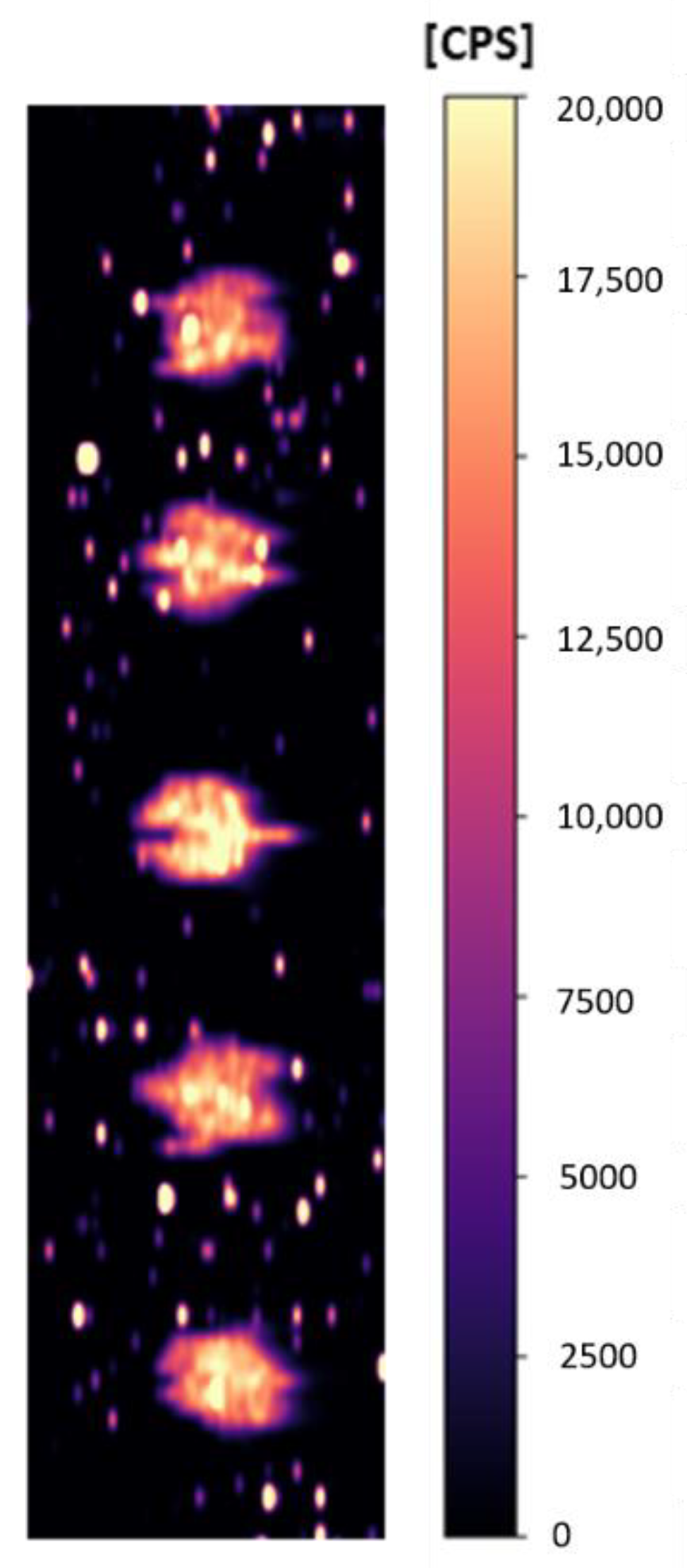
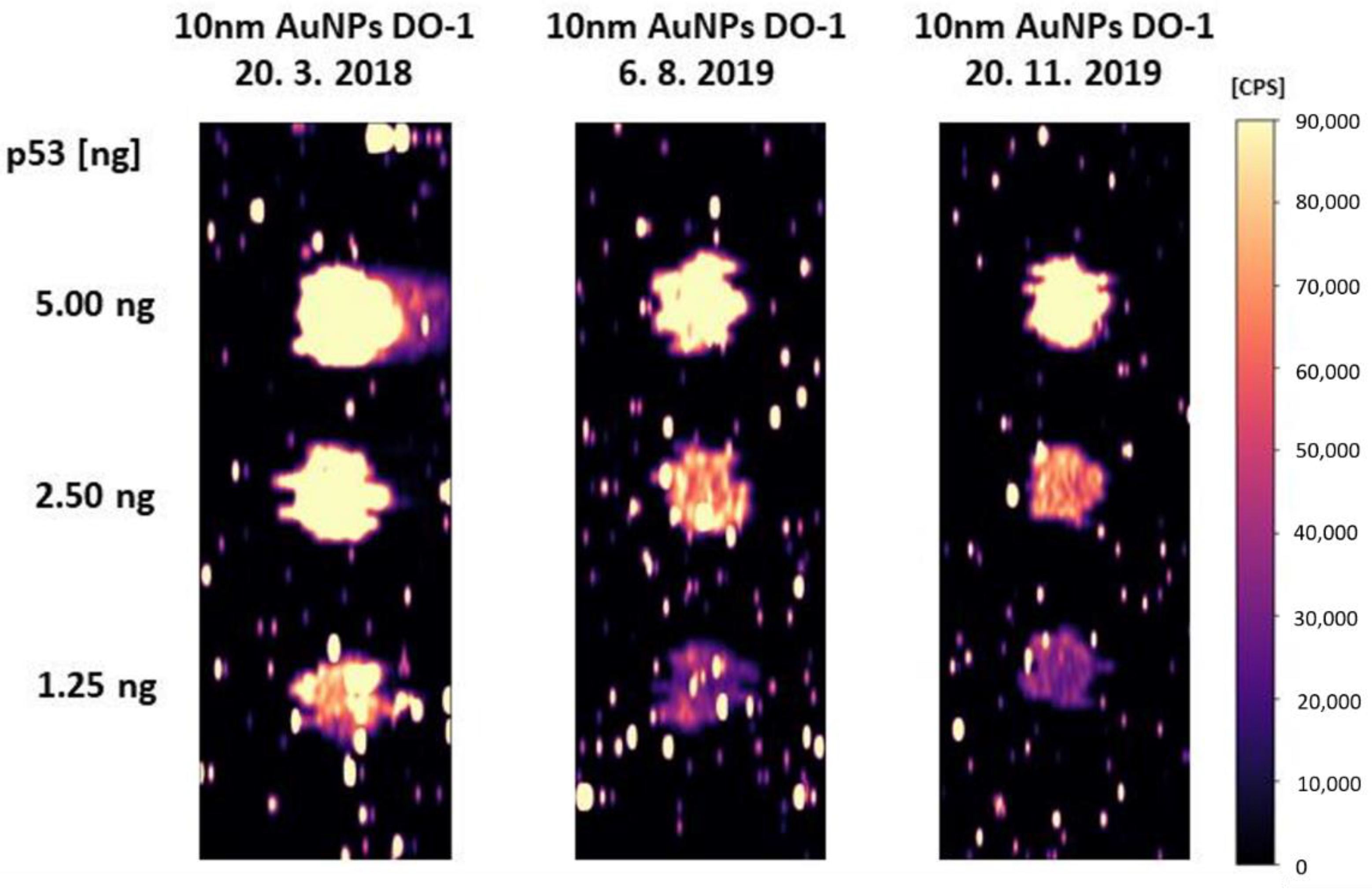
2.2. Repeatability of the Analysis
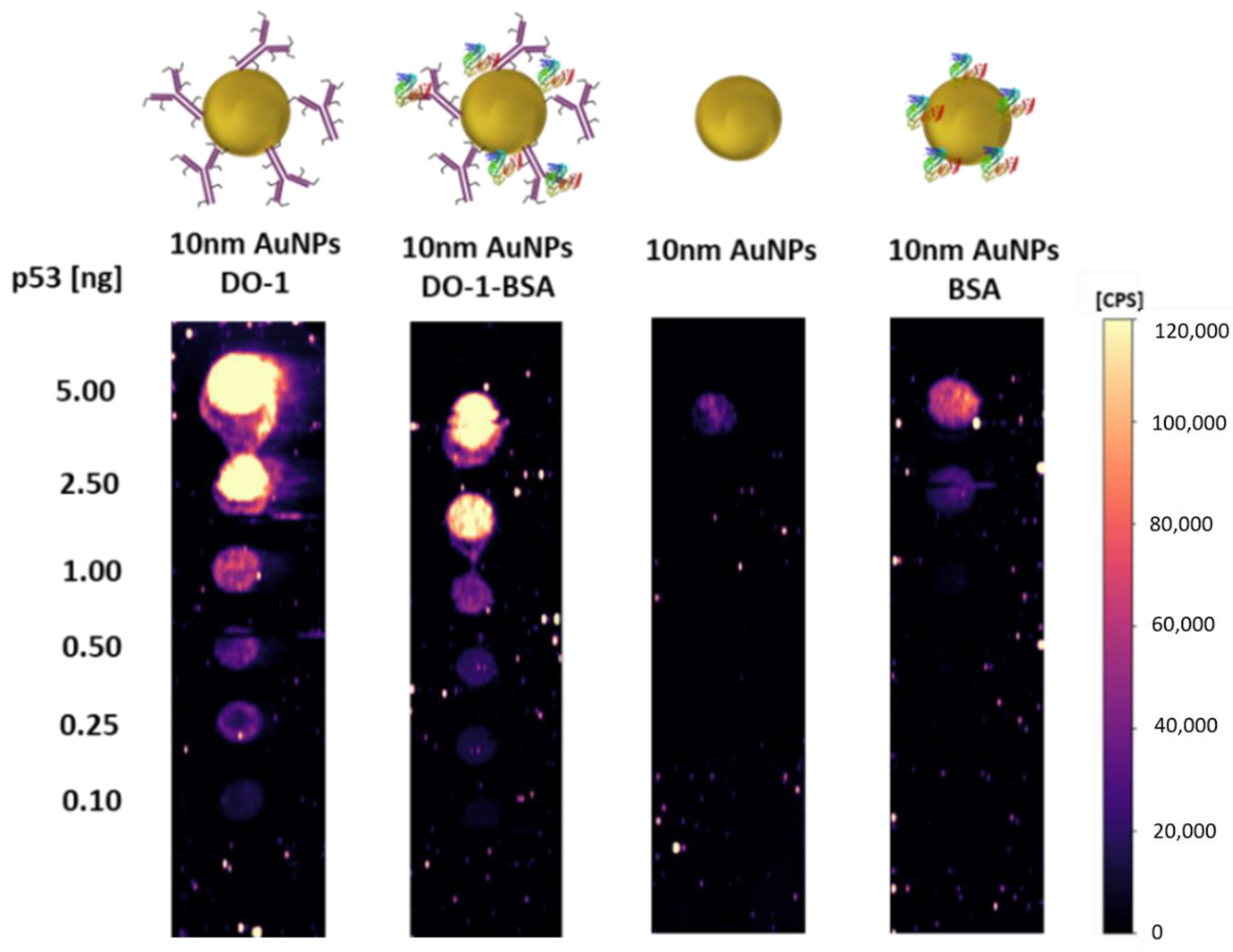
2.3. Verification of Antibody Activity after Binding to Various Nanoparticles
2.4. Contribution of Bare (Incompletely Covered) Nanoparticles to Non-Specific Sorption
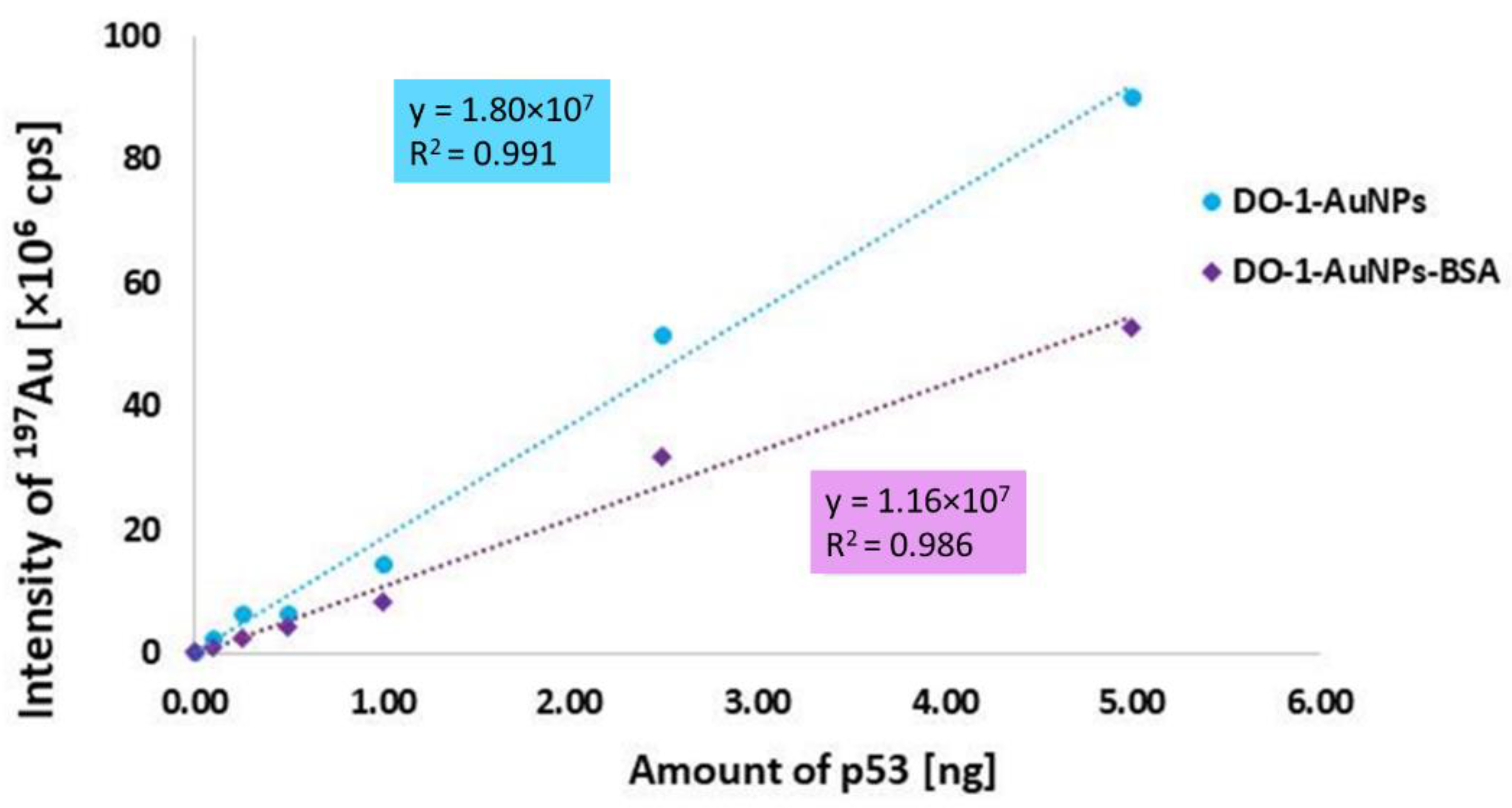
2.5. Linearity of Calibration Dependence
2.6. Dot-Blot Analysis of p53 Protein in a Protein Mixture
3. Materials and Methods
3.1. Chemicals
3.2. Nanoparticles
3.3. The Composition of the Solutions
3.4. Preparation of Antibody-Nanoparticle Conjugates with AuNPs, AgNPs and EuNPs
3.5. Preparation of Antibody-Nanoparticle Conjugates with QDs
3.6. Dot-Blot
3.7. LA-ICP-MS Analysis
3.8. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanner, S.D.; Baranov, V.I.; Ornatsky, O.I.; Bandura, D.R.; George, T.C. An introduction to mass cytometry: Fundamentals and applications. Cancer Immunol. Immun. 2013, 62, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.D.; Zhang, G.H.; Herrera, I.; Kinach, R.; Ornatsky, O.; Baranov, V.; Nitz, M.; Winnik, M.A. Polymer-based elemental tags for sensitive bioassays. Angew. Chem. Int. Edit. 2007, 46, 6111–6114. [Google Scholar] [CrossRef] [PubMed]
- Giesen, C.; Mairinger, T.; Khoury, L.; Waentig, L.; Jakubowski, N.; Panne, U. Multiplexed immunohistochemical detection of tumor markers in breast cancer tissue using laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 8177–8183. [Google Scholar] [CrossRef] [PubMed]
- Waentig, L.; Techritz, S.; Jakubowski, N.; Roos, P.H. A multi-parametric microarray for protein profiling: Simultaneous analysis of 8 different cytochromes via differentially element tagged antibodies and laser ablation icp-ms. Analyst 2013, 138, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, B.B.; He, M.; Hu, B. Simultaneous determination of two phosphorylated p53 proteins in scc-7 cells by an icp-ms immunoassay using apoferritin-templated europium(iii) and lutetium(iii) phosphate nanoparticles as labels. Microchim. Acta 2019, 186, 424. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Alonso, M.; Fernandez, B.; Navarro, A.; Junceda, S.; Astudillo, A.; Pereiro, R. Laser ablation icp-ms for simultaneous quantitative imaging of iron and ferroportin in hippocampus of human brain tissues with alzheimer’s disease. Talanta 2019, 197, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Vega, R.; Clases, D.; Luisa Fernandez-Sanchez, M.; Eiro, N.; Gonzalez, L.O.; Vizoso, F.J.; Doble, P.A.; Sanz-Medel, A. Mmp-11 as a biomarker for metastatic breast cancer by immunohistochemical-assisted imaging mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Tvrdonova, M.; Vlcnovska, M.; Vanickova, L.P.; Kanicky, V.; Adam, V.; Ascher, L.; Jakubowski, N.; Vaculovicova, M.; Vaculovic, T. Gold nanoparticles as labels for immunochemical analysis using laser ablation inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.pipety.cz/data/machines/flyer_101-7301-a1_hyperion_imaging_system.pdf (accessed on 20 December 2020).
- Available online: https://www.fluidigm.com/applications/imaging-mass-cytometry#Products (accessed on 20 December 2020).
- Available online: https://www.abcam.com/human-p53-elisa-kit-pser15-ab156027.html (accessed on 20 December 2020).
- Baldacchini, C.; Montanarella, A.F.; Francioso, L.; Signore, M.A.; Cannistraro, S.; Bizzarri, A.R. A reliable biofet immunosensor for detection of p53 tumour suppressor in physiological-like environment. Sensors 2020, 20, 6364. [Google Scholar] [CrossRef]
- Attallah, A.M.; Abdel-Aziz, M.M.; El-Sayed, A.M.; Tabll, A.A. Detection of serum p53 protein in patients with different gastrointestinal cancers. Cancer Detect. Prev. 2003, 27, 127–131. [Google Scholar] [CrossRef]
- Thomas, M.D.; McIntosh, G.G.; Anderson, J.J.; McKenna, D.M.; Parr, A.H.; Johnstone, R.; Lennard, T.W.J.; Horne, C.H.W.; Angus, B. A novel quantitative immunoassay system for p53 using antibodies selected for optimum designation of p53 status. J. Clin. Pathol. 1997, 50, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Shwetha, N.; Selvakumar, L.S.; Thakur, M.S. Aptamer-nanoparticle-based chemiluminescence for p53 protein. Anal. Biochem. 2013, 441, 73–79. [Google Scholar] [CrossRef]
- Available online: https://www.abcam.com/human-p53-elisa-kit-ab171571.html (accessed on 20 December 2020).
- Sabapathy, K.; Lane, D.P. Understanding p53 functions through p53 antibodies. J. Mol. Cell Biol. 2019, 11, 317–329. [Google Scholar] [CrossRef]
- Hwang, L.A.; Phang, B.H.; Liew, O.W.; Iqbal, J.; Koh, X.H.; Koh, X.Y.; Othman, R.; Xue, Y.Z.; Richards, A.M.; Lane, D.P.; et al. Monoclonal antibodies against specific p53 hotspot mutants as potential tools for precision medicine. Cell Rep. 2018, 22, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody fragments as nanoparticle targeting ligands: A step in the right direction. Chem. Sci. 2017, 8, 63–77. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Method | Sample Volume | LOD * | Note | Ref. |
|---|---|---|---|---|
| ELISA Kit | 50 μL | 8 µg/mL 183 nM | Commercial Human p53 ELISA Kit (ab156027), Abcam UK records OD at 450 nm. | [11] |
| Bio-FET | 20 μL | 4.37 ng/mL 100 pM * | Label-free field-effect transistor-based immunosensor. | [12] |
| LA-ICP-MS | 0.5 μL | 2.6 ng/mL 59.5 pM | Direct immunoassay using nanoparticle labelled antibodies. | This study |
| ELISA | 50 μL | 0.44 ng/mL 10 pM | PNPP was used as a substrate and absorbance was read at 405 nm. | [13] |
| ELISA | 50 μL | 0.1 ng/mL 2.3 pM | TMB was used as a substrate and absorbance was read at 250 nm. | [14] |
| Aptasensor | 100 μL | 10 pg/mL 229 fM | The catalytic activity of aggregated AuNPs increased chemiluminescence intensity. | [15] |
| p53 ELISA Kit | 50 μL | 65 pg/mL 1.49 pM | Commercial Human p53 ELISA Kit (ab171571), Abcam UK records the OD at 450 nm. | [16] |
| p53 [ng] | Sum Au (AuNPs-DO-1) [CPS] |
|---|---|
| 2 | 2,460,000 |
| 2 | 2,410,000 |
| 2 | 2,570,000 |
| 2 | 2,520,000 |
| 2 | 2,390,000 |
| Average | 2,470,000 |
| SD | 75,400 |
| RSD [%] | 3.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlcnovska, M.; Stossova, A.; Kuchynka, M.; Dillingerova, V.; Polanska, H.; Masarik, M.; Hrstka, R.; Adam, V.; Kanicky, V.; Vaculovic, T.; et al. Comparison of Metal Nanoparticles (Au, Ag, Eu, Cd) Used for Immunoanalysis Using LA-ICP-MS Detection. Molecules 2021, 26, 630. https://doi.org/10.3390/molecules26030630
Vlcnovska M, Stossova A, Kuchynka M, Dillingerova V, Polanska H, Masarik M, Hrstka R, Adam V, Kanicky V, Vaculovic T, et al. Comparison of Metal Nanoparticles (Au, Ag, Eu, Cd) Used for Immunoanalysis Using LA-ICP-MS Detection. Molecules. 2021; 26(3):630. https://doi.org/10.3390/molecules26030630
Chicago/Turabian StyleVlcnovska, Marcela, Aneta Stossova, Michaela Kuchynka, Veronika Dillingerova, Hana Polanska, Michal Masarik, Roman Hrstka, Vojtech Adam, Viktor Kanicky, Tomas Vaculovic, and et al. 2021. "Comparison of Metal Nanoparticles (Au, Ag, Eu, Cd) Used for Immunoanalysis Using LA-ICP-MS Detection" Molecules 26, no. 3: 630. https://doi.org/10.3390/molecules26030630
APA StyleVlcnovska, M., Stossova, A., Kuchynka, M., Dillingerova, V., Polanska, H., Masarik, M., Hrstka, R., Adam, V., Kanicky, V., Vaculovic, T., & Vaculovicova, M. (2021). Comparison of Metal Nanoparticles (Au, Ag, Eu, Cd) Used for Immunoanalysis Using LA-ICP-MS Detection. Molecules, 26(3), 630. https://doi.org/10.3390/molecules26030630









