Catechol-Based Antimicrobial Polymers
Abstract
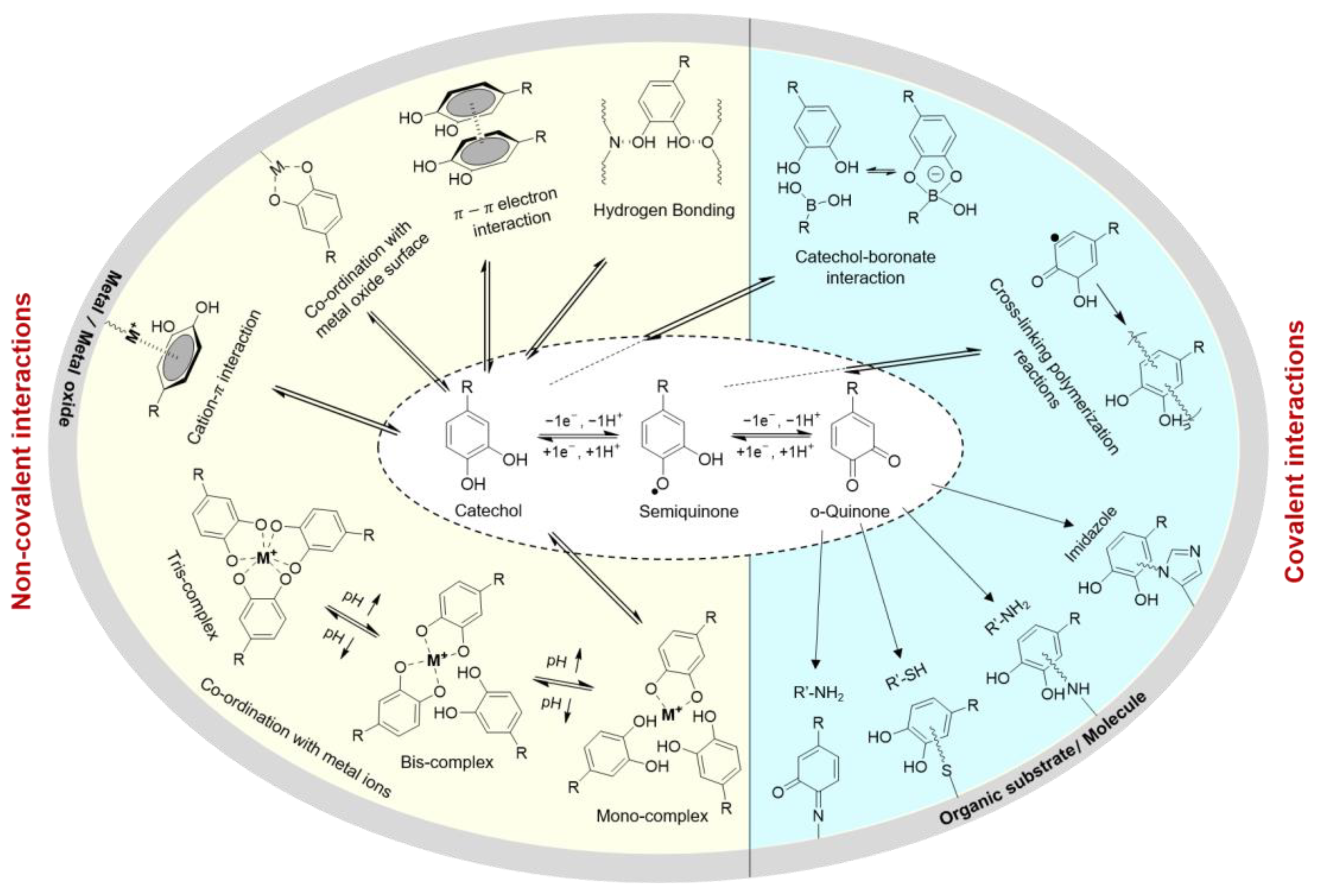
1. Introduction
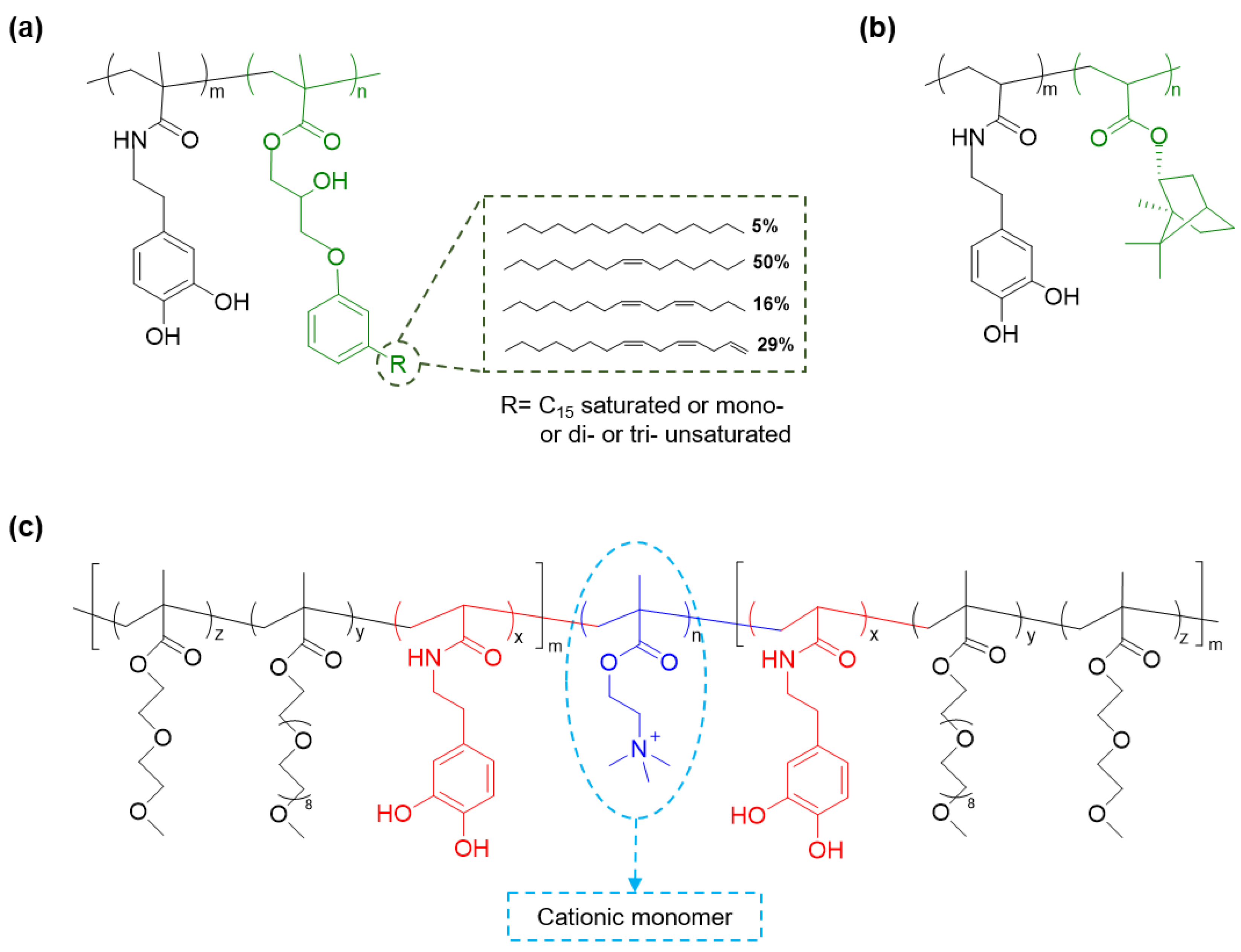
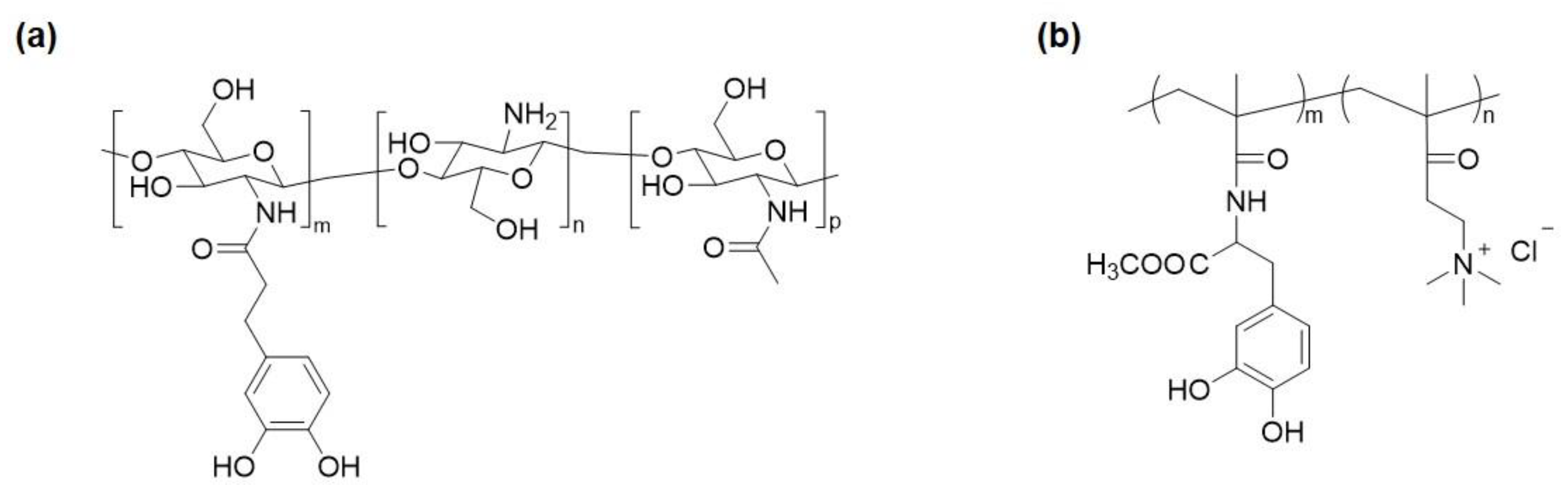
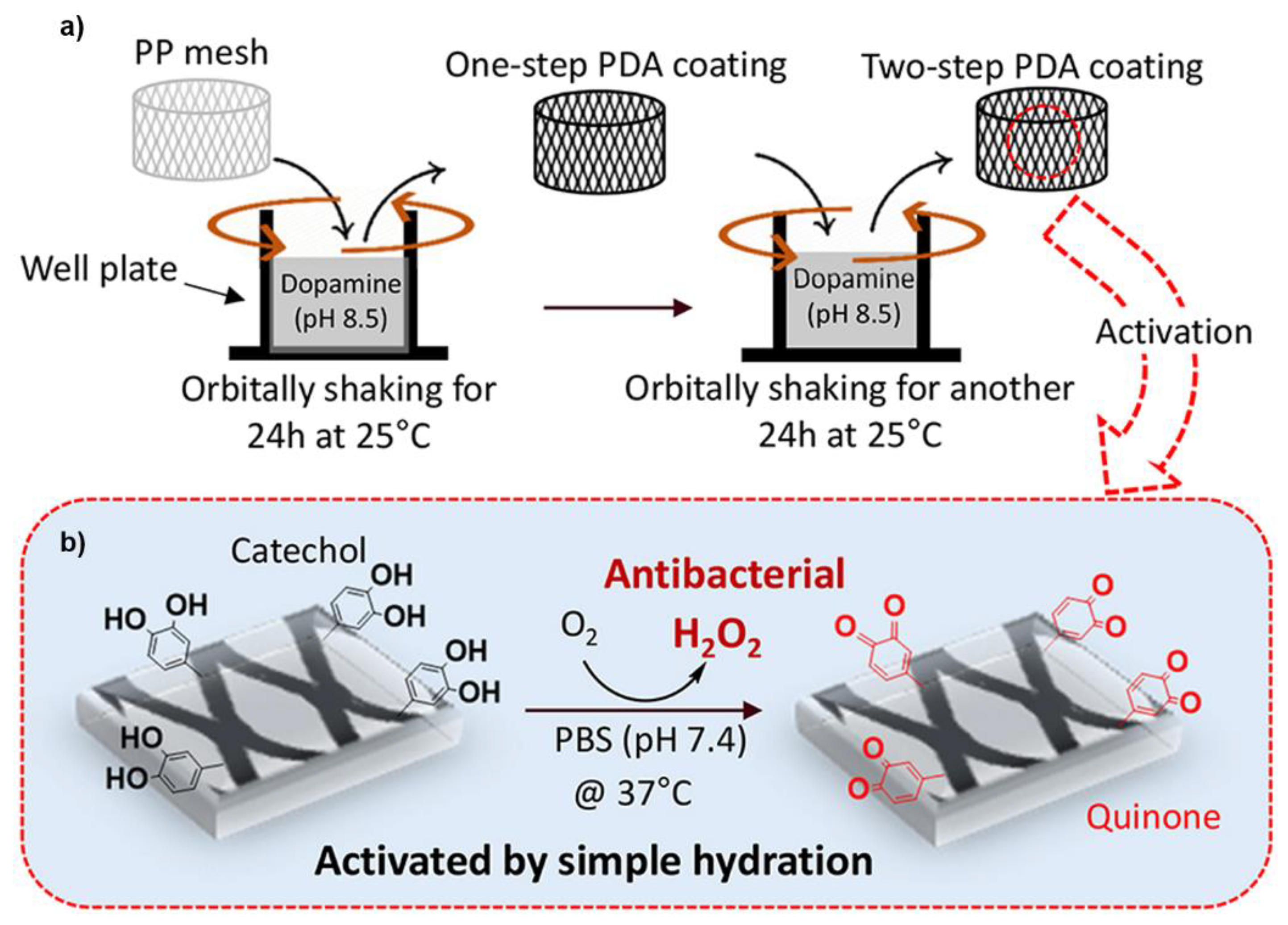
2. Catechol-Modified Polymers with Innate Antimicrobial Properties
3. Catechol-Based Polymers in Combination with Metal Ions and Nanoparticles
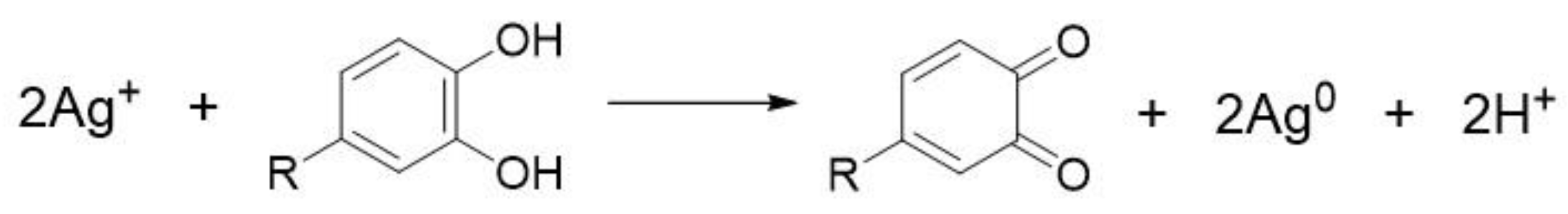
3.1. Silver Ions (Ag+) and Silver Nanoparticles (AgNPs)
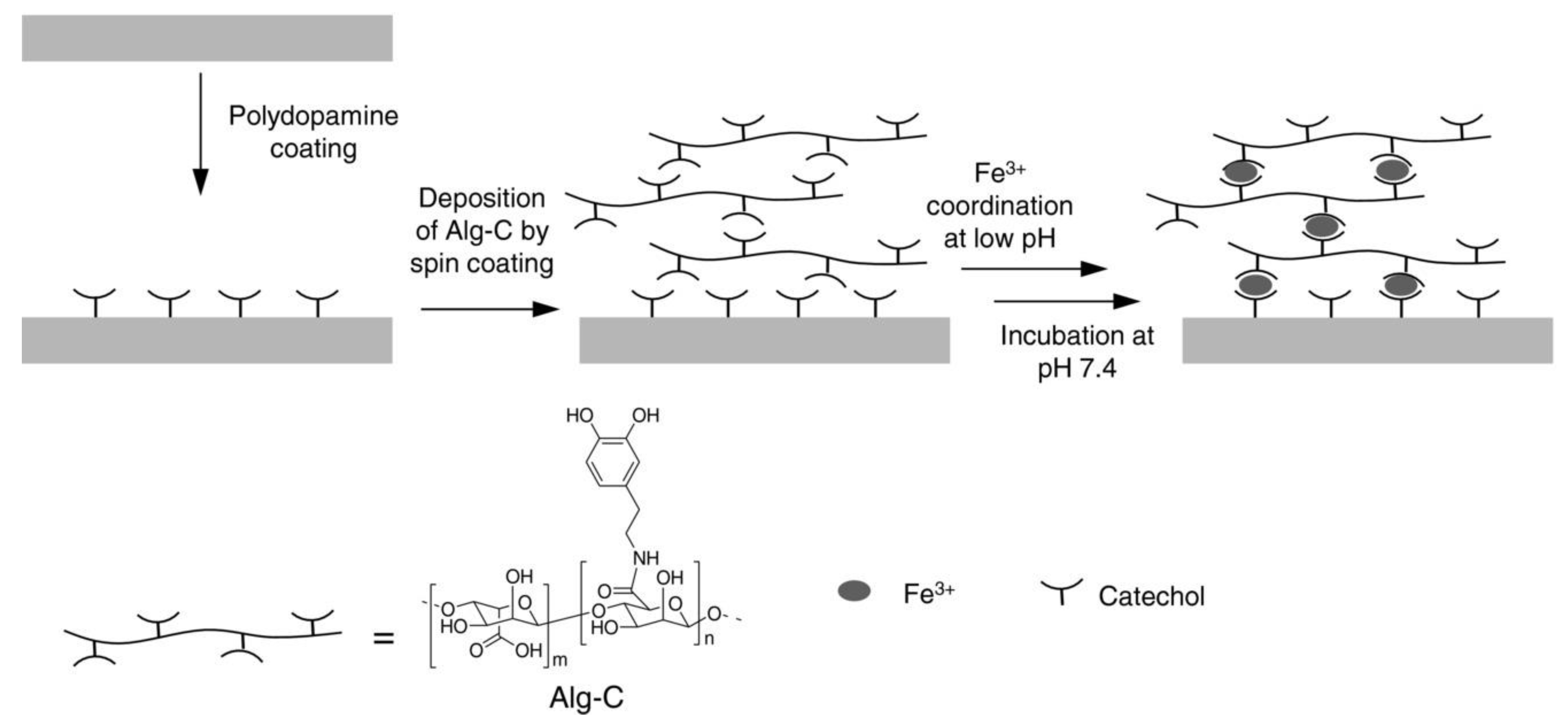
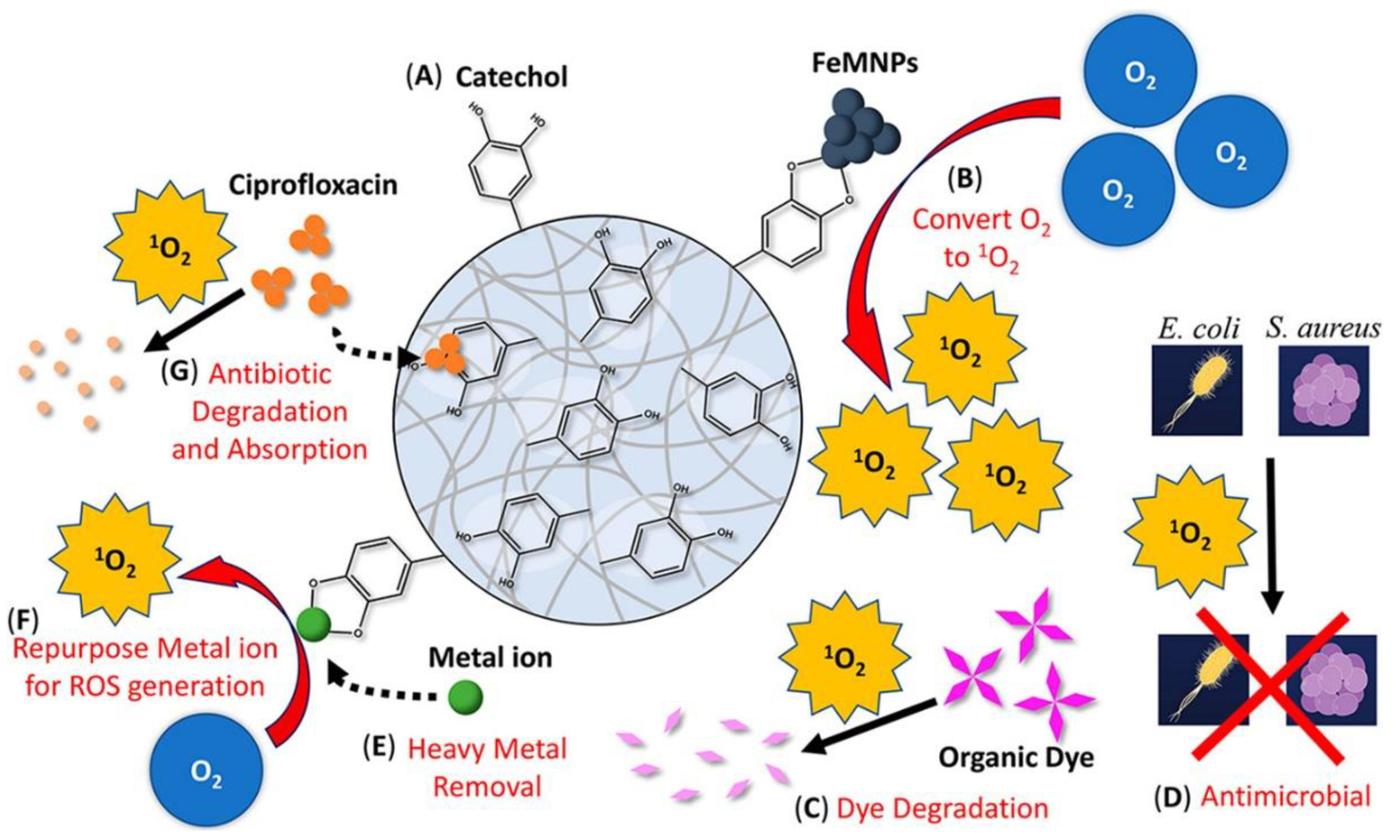
3.2. Other Metal Ions and Nanoparticles
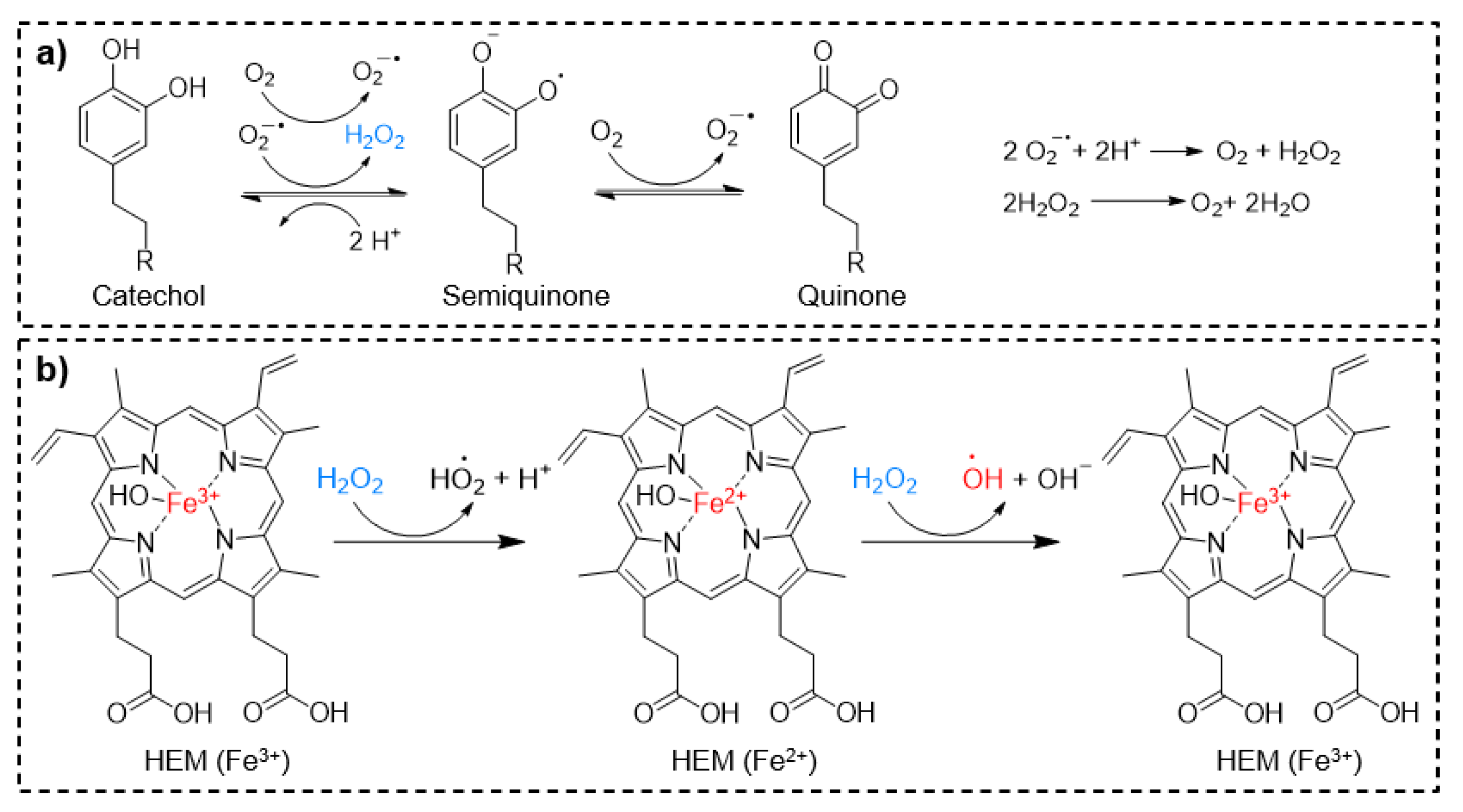
4. ROS-Releasing Catechol-Based Polymers
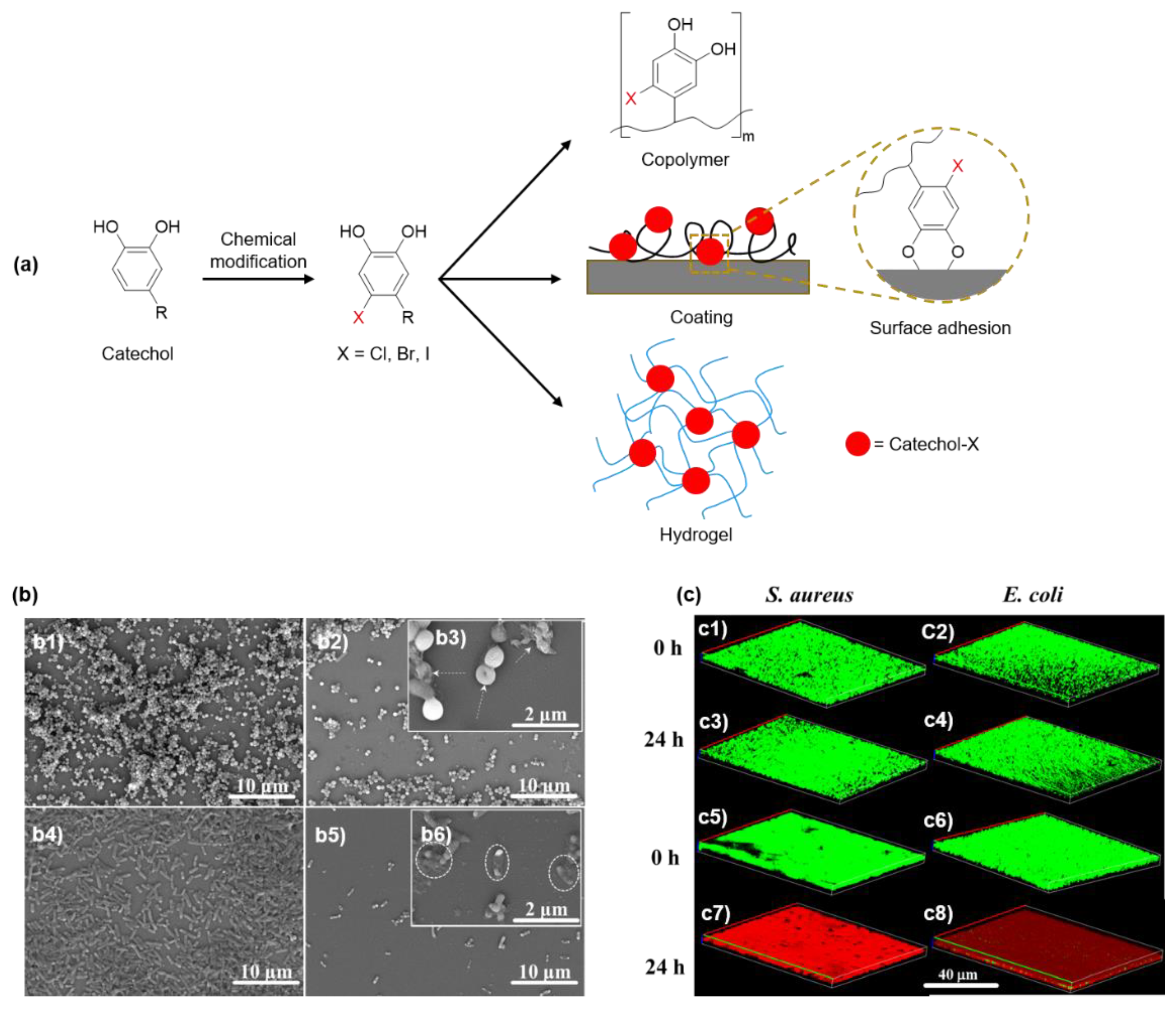
5. Innate Antimicrobial Property of Halogenated Catechol and Polyphenols
5.1. Antimicrobial Halogenated Catechol
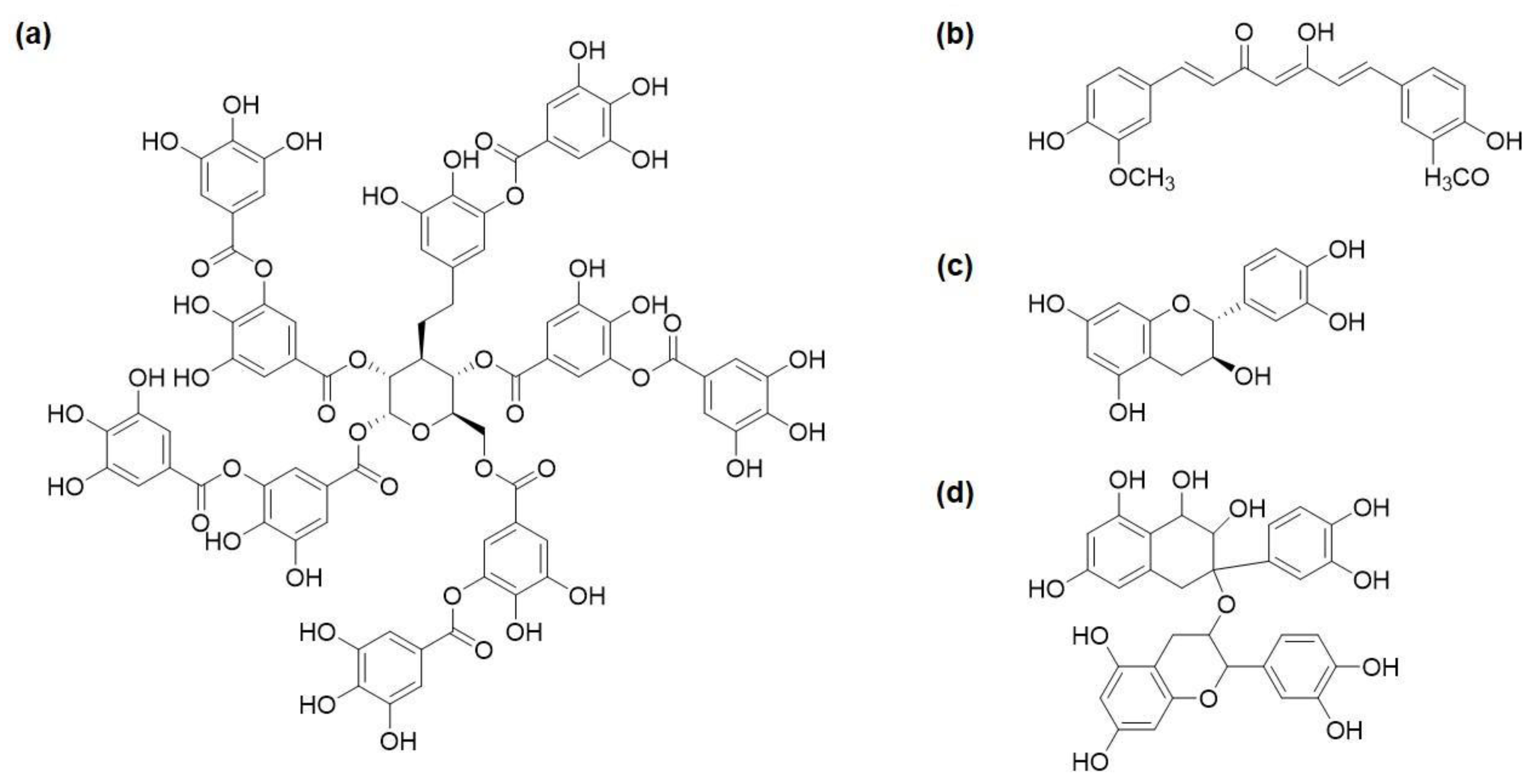
5.2. Antimicrobial Polyphenols
6. Summary and Future Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jang, J. Antimicrobial polymer nanostructures: Synthetic route, mechanism of action and perspective. Adv. Colloid Interface Sci 2014, 203, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Baym, M.; Stone, L.K.; Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 2016, 351, aad3292. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Cheng, W.; Wang, G.; Liu, Y. Developments in antimicrobial polymers. J. Polym. Sci. Part. A Polym. Chem. 2017, 55, 632–639. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Engler, A.C.; Wiradharma, N.; Ong, Z.Y.; Coady, D.J.; Hedrick, J.L.; Yang, Y.-Y. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 2012, 7, 201–222. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Kenawyel, R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef]
- Tew, G.N.; Scott, R.W.; Klein, M.L.; DeGrado, W.F. De Novo Design of Antimicrobial Polymers, Foldamers, and Small Molecules: From Discovery to Practical Applications. Acc. Chem. Res. 2010, 43, 30–39. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Saravanan, R.; Li, C.M.; Leong, S.S.J. Antimicrobial macromolecules: Synthesis methods and future applications. Rsc Adv. 2012, 2, 4031–4044. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial cationic polymers: From structural design to functional control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.d.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef]
- Rotem, S.; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1582–1592. [Google Scholar] [CrossRef]
- Sobczak, M.; Dębek, C.; Olędzka, E.; Kozłowski, R. Polymeric systems of antimicrobial peptides--strategies and potential applications. Molecules 2013, 18, 14122–14137. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Munoz-Bonilla, A.; Echeverria, C.; Sonseca, A.; Arrieta, M.P.; Fernandez-Garcia, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixeira, J.P. Self-disinfecting surfaces and infection control. Colloids Surf. B Biointerfaces 2019, 178, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef] [PubMed]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part. A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Tanzer, M.L. Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038. [Google Scholar] [CrossRef]
- Patil, N.; Jérôme, C.; Detrembleur, C. Recent advances in the synthesis of catechol-derived (bio)polymers for applications in energy storage and environment. Prog. Polym. Sci. 2018, 82, 34–91. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Meng, H.; Forooshani, P.K.; Joshi, P.U.; Osborne, J.; Mi, X.; Meingast, C.; Pinnaratip, R.; Kelley, J.; Narkar, A.; He, W.; et al. Biomimetic recyclable microgels for on-demand generation of hydrogen peroxide and antipathogenic application. Acta Biomater. 2019, 83, 109–118. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Polega, E.; Thomson, K.; Bhuiyan, M.S.A.; Pinnaratip, R.; Trought, M.; Kendrick, C.; Gao, Y.; Perrine, K.A.; Pan, L.; et al. Antibacterial Properties of Mussel-Inspired Polydopamine Coatings Prepared by a Simple Two-Step Shaking-Assisted Method. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, S.; Song, R.; Zhang, W.; Zhang, S.; Li, J. The synergy between natural polyphenol-inspired catechol moieties and plant protein-derived bio-adhesive enhances the wet bonding strength. Sci. Rep. UK 2017, 7, 9664. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shin, M.; Koh, M.-Y.; Ryu, J.H.; Lee, M.S.; Hong, S.; Lee, H. TAPE: A Medical Adhesive Inspired by a Ubiquitous Compound in Plants. Adv. Funct. Mater. 2015, 25, 2402–2410. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Faust, M.; Konst, S.; Lee, B.P. Hydrogen peroxide generation and biocompatibility of hydrogel-bound mussel adhesive moiety. Acta Biomater. 2015, 17, 160–169. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Dogan, E.M.; Sudur Zalluhoglu, F.; Orbey, N. Effect of potassium ion on the stability and release rate of hydrogen peroxide encapsulated in silica hydrogels. Aiche J. 2016, 63, 409–417. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, C.; Zhang, Z.; Roland, J.D.; Lee, B.P. Antimicrobial property of halogenated catechols. Chem. Eng. J. 2021, 403, 126340. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kang, H.; Kim, D.-G.; Cha, S.-H.; Lee, J.-C. Mussel-Inspired Dopamine- and Plant-Based Cardanol-Containing Polymer Coatings for Multifunctional Filtration Membranes. ACS Appl. Mater. Interfaces 2014, 6, 21297–21307. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Himejima, M.; Kubo, I. Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 1991, 39, 418–421. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, K.-H.; Kim, D.-G.; Kim, H.J.; Cha, S.-H.; Lee, J.-C. Synthesis and characterization of self-cross-linkable and bactericidal methacrylate polymers having renewable cardanol moieties for surface coating applications. RSC Adv. 2014, 4, 41195–41203. [Google Scholar] [CrossRef]
- Granger, R.E.; Campbell, E.L.; Johnston, G.A. (+)- And (-)-borneol: Efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABA(A) receptors. Biochem Pharm. 2005, 69, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, G.; Luan, D.; Yuan, Q.; Wei, Y.; Wang, X. Antibacterial Adhesion of Borneol-Based Polymer via Surface Chiral Stereochemistry. ACS Appl. Mater. Interfaces 2014, 6, 19371–19377. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jiang, H.; Wang, R.; Xie, Y.; Zhao, W.; Zhao, C. A versatile approach towards multi-functional surfaces via covalently attaching hydrogel thin layers. J. Colloid Interface Sci. 2016, 484, 60–69. [Google Scholar] [CrossRef]
- Wang, X.; Jing, S.; Liu, Y.; Liu, S.; Tan, Y. Diblock copolymer containing bioinspired borneol and dopamine moieties: Synthesis and antibacterial coating applications. Polymer 2017, 116, 314–323. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J.; Huang, W.; Chen, L.; Zeng, H. Injectable Self-Healing Hydrogel with Antimicrobial and Antifouling Properties. ACS Appl. Mater. Interfaces 2017, 9, 9221–9225. [Google Scholar] [CrossRef]
- Plachá, D.; Muñoz-Bonilla, A.; Škrlová, K.; Echeverria, C.; Chiloeches, A.; Petr, M.; Lafdi, K.; Fernández-García, M. Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials. Nanomaterials 2020, 10, 1218. [Google Scholar] [CrossRef]
- Han, H.; Wu, J.; Avery, C.W.; Mizutani, M.; Jiang, X.; Kamigaito, M.; Chen, Z.; Xi, C.; Kuroda, K. Immobilization of amphiphilic polycations by catechol functionality for antimicrobial coatings. Langmuir ACS J. Surf. Colloids 2011, 27, 4010–4019. [Google Scholar] [CrossRef]
- Qiu, W.-Z.; Zhao, Z.-S.; Du, Y.; Hu, M.-X.; Xu, Z.-K. Antimicrobial membrane surfaces via efficient polyethyleneimine immobilization and cationization. Appl. Surf. Sci. 2017, 426, 972–979. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Xu, Z. Mechanics of metal-catecholate complexes: The roles of coordination state and metal types. Sci. Rep. 2013, 3, 2914. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired metal–polyphenol materials: Self-healing and beyond. Biomimetics 2019, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Zollfrank, C.; Gutbrod, K.; Wechsler, P.; Guggenbichler, J.P. Antimicrobial activity of transition metal acid MoO(3) prevents microbial growth on material surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 47–54. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Nadres, E.T.; Alamani, B.G.; Rodrigues, D.F. Designing polymeric adhesives for antimicrobial materials: Poly(ethylene imine) polymer, graphene, graphene oxide and molybdenum trioxide—A biomimetic approach. J. Mater. Chem. B 2017, 5, 6616–6628. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Sadoon, A.A.; Khadka, P.; Freeland, J.; Gundampati, R.K.; Manso, R.H.; Ruiz, M.; Krishnamurthi, V.R.; Thallapuranam, S.K.; Chen, J.; Wang, Y. Silver Ions Caused Faster Diffusive Dynamics of Histone-Like Nucleoid-Structuring Proteins in Live Bacteria. Appl. Environ. Microbiol. 2020, 86, e02479-19. [Google Scholar] [CrossRef]
- Tan, M.; Choi, Y.; Kim, J.; Kim, J.-H.; Fromm, K.M. Polyaspartamide Functionalized Catechol-Based Hydrogels Embedded with Silver Nanoparticles for Antimicrobial Properties. Polymers 2018, 10, 1188. [Google Scholar] [CrossRef]
- Huang, X.; Bao, X.; Liu, Y.; Wang, Z.; Hu, Q. Catechol-Functional Chitosan/Silver Nanoparticle Composite as a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. Sci. Rep. 2017, 7, 1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, X.; Xiang, T.; Liu, Q.; Su, B.; Zhao, W.; Zhao, C. Mussel-inspired chitosan-polyurethane coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. Carbohydr. Polym. 2017, 168, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Zhang, J.Y.; Wang, Y.B.; Li, C.M.; Lu, Z.S.; Hu, X.F.; Xu, L.Q. Deposition of catechol-functionalized chitosan and silver nanoparticles on biomedical titanium surfaces for antibacterial application. Mater. Sci. Eng. C 2019, 98, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Falentin-Daudré, C.; Faure, E.; Svaldo-Lanero, T.; Farina, F.; Jérôme, C.; Van De Weerdt, C.; Martial, J.; Duwez, A.-S.; Detrembleur, C. Antibacterial Polyelectrolyte Micelles for Coating Stainless Steel. Langmuir 2012, 28, 7233–7241. [Google Scholar] [CrossRef] [PubMed]
- Charlot, A.; Sciannaméa, V.; Lenoir, S.; Faure, E.; Jérôme, R.; Jérôme, C.; Van De Weerdt, C.; Martial, J.; Archambeau, C.; Willet, N.; et al. All-in-one strategy for the fabrication of antimicrobial biomimetic films on stainless steel. J. Mater. Chem. 2009, 19, 4117–4125. [Google Scholar] [CrossRef]
- Le Thi, P.; Lee, Y.; Hoang Thi, T.T.; Park, K.M.; Park, K.D. Catechol-rich gelatin hydrogels in situ hybridizations with silver nanoparticle for enhanced antibacterial activity. Mater. Sci. Eng. C 2018, 92, 52–60. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223. [Google Scholar] [CrossRef]
- Sambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the Toxic Effect of Silver Nanoparticles on Mammalian Cell Lines. J. Nanomater. 2015, 2015, 136765. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Nie, C.; Cheng, C.; Ma, L.; Deng, J.; Zhao, C. Mussel-Inspired Antibacterial and Biocompatible Silver–Carbon Nanotube Composites: Green and Universal Nanointerfacial Functionalization. Langmuir 2016, 32, 5955–5965. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Moon, J.-M.; Choi, J.S.; Cho, W.K.; Kang, S.M. Mussel-Inspired Approach to Constructing Robust Multilayered Alginate Films for Antibacterial Applications. Adv. Funct. Mater. 2016, 26, 4099–4105. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ji, C.; Juárez-Hernández, R.E.; Miller, M.J. Exploiting bacterial iron acquisition: Siderophore conjugates. Future Med. Chem. 2012, 4, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Kinzel, O.; Tappe, R.; Gerus, I.; Budzikiewicz, H. The synthesis and antibacterial activity of two pyoverdin-ampicillin conjugates, entering Pseudomonas aeruginosa via the pyoverdin-mediated iron uptake pathway. J. Antibiot. 1998, 51, 499–507. [Google Scholar] [CrossRef]
- Ohi, N.; Aoki, B.; Kuroki, T.; Matsumoto, M.; Kojima, K.; Nehashi, T. Semisynthetic beta-lactam antibiotics. III. Effect on antibacterial activity and comt-susceptibility of chlorine-introduction into the catechol nucleus of 6-[(R)-2-[3-(3,4-dihydroxybenzoyl)-3-(3-hydroxypropyl)-1-ureido]-2-phenylacetamido]penicillanic acid. J. Antibiot. 1987, 40, 22–28. [Google Scholar] [CrossRef]
- Forooshani, P.K.; Meng, H.; Lee, B.P. Catechol Redox Reaction: Reactive Oxygen Species Generation, Regulation, and Biomedical Applications. In Advances in Bioinspired and Biomedical Materials Volume 1; American Chemical Society: Washington, DC, USA, 2017; Volume 1252, pp. 179–196. [Google Scholar]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Ye, Y.; Xiao, L.; Bin, H.; Zhang, Q.; Nie, T.; Yang, X.; Wu, D.; Cheng, H.; Li, P.; Wang, Q. Oxygen-tuned nanozyme polymerization for the preparation of hydrogels with printable and antibacterial properties. J. Mater. Chem. B 2017, 5, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. The biology of oxygen radicals. Science 1978, 201, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Chapter 4—Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial Actions of Reactive Oxygen Species. mBio 2011, 2, e00141-11. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Felix, C.C.; Sealy, R.C. Semiquinone anion radicals of catechol(amine)s, catechol estrogens, and their metal ion complexes. Environ. Health Perspect. 1985, 64, 185–198. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Zhou, C.; Reaume, M.; Wu, M.; Liu, B.; Lee, B.P. Iron Magnetic Nanoparticle-Induced ROS Generation from Catechol-Containing Microgel for Environmental and Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21210–21220. [Google Scholar] [CrossRef]
- Mustafa, H.S.I. Staphylococcus aureus Can Produce Catalase Enzyme When Adding to Human WBCs as a Source of H2O2 Productions in Human Plasma or Serum in the Laboratory. Open J. Med. Microbiol. 2014, 4, 249. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Pinnaratip, R.; Polega, E.; Tyo, A.G.; Pearson, E.; Liu, B.; Folayan, T.-O.; Pan, L.; Rajachar, R.M.; Heldt, C.L.; et al. Hydroxyl Radical Generation through the Fenton-like Reaction of Hematin- and Catechol-Functionalized Microgels. Chem. Mater. 2020. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. EJB Rev. 1994, 1993, 101–107. [Google Scholar]
- Kruk, I.; Lichszteld, K.; Michalska, T.; Wronska, J.; Bounias, M. The Formation of Singlet Oxygen during Oxidation of Catechol Amines as Detected by Infrared Chemiluminescence and Spectrophotometric Method. Z. Nat. C 1989, 44, 895–900. [Google Scholar] [CrossRef]
- Tyo, A.; Welch, S.; Hennenfent, M.; Kord Fooroshani, P.; Lee, B.P.; Rajachar, R. Development and Characterization of an Antimicrobial Polydopamine Coating for Conservation of Humpback Whales. Front. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, J.; Corre, J.; Cremieux, A. Antibacterial activity of phenolic compounds and aromatic alcohols. Res. Microbiol. 1990, 141, 499–510. [Google Scholar] [CrossRef]
- Sivaraman, S.; Sullivan, T.J.; Johnson, F.; Novichenok, P.; Cui, G.; Simmerling, C.; Tonge, P.J. Inhibition of the bacterial enoyl reductase FabI by triclosan: A structure− reactivity analysis of FabI inhibition by triclosan analogues. J. Med. Chem. 2004, 47, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Rafi, S.B.; Cui, G.; Song, K.; Cheng, X.; Tonge, P.J.; Simmerling, C. Insight through molecular mechanics Poisson− Boltzmann surface area calculations into the binding affinity of triclosan and three analogues for FabI, the E. coli enoyl reductase. J. Med. Chem. 2006, 49, 4574–4580. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.J.; Srivastava, A.; Reifert, J.R.; Waite, J.H. Halogenated DOPA in a marine adhesive protein. J. Adhes. 2009, 85, 126–138. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, L.; Cui, J.; Serrano, C.; Shafiq, Z.; Gropeanu, R.A.; Miguel, V.S.; Ramos, J.I.; Wang, M.; Auernhammer, G.K.; Ritz, S. Antibacterial Strategies from the Sea: Polymer-Bound Cl-Catechols for Prevention of Biofilm Formation. Adv. Mater. 2013, 25, 529–533. [Google Scholar] [CrossRef]
- Chien, H.-W.; Chiu, T.-H. Stable N-halamine on polydopamine coating for high antimicrobial efficiency. Eur. Polym. J. 2020, 130, 109654. [Google Scholar] [CrossRef]
- Widsten, P.; Heathcote, C.; Kandelbauer, A.; Guebitz, G.; Nyanhongo, G.S.; Prasetyo, E.N.; Kudanga, T. Enzymatic surface functionalisation of lignocellulosic materials with tannins for enhancing antibacterial properties. Process. Biochem. 2010, 45, 1072–1081. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly (Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef]
- Li, N.; Yang, X.; Liu, W.; Xi, G.; Wang, M.; Liang, B.; Ma, Z.; Feng, Y.; Chen, H.; Shi, C. Tannic acid cross-linked polysaccharide-based multifunctional hemostatic microparticles for the regulation of rapid wound healing. Macromol. Biosci. 2018, 18, 1800209. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zheng, J.; Guo, R.; Luo, J.; Yuan, Y.; Liu, X. Synthesis of New Biobased Antibacterial Methacrylates Derived from Tannic Acid and Their Application in UV-Cured Coatings. Ind. Eng. Chem. Res. 2014, 53, 10835–10840. [Google Scholar] [CrossRef]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Dai Tran, L.; Park, J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Xie, M.; Fan, D.; Zhao, Z.; Li, Z.; Li, G.; Chen, Y.; He, X.; Chen, A.; Li, J.; Lin, X. Nano-curcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int. J. Pharm. 2015, 496, 732–740. [Google Scholar] [CrossRef]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. 2002, 26, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Awan, J.A.; Rehman, S.U.; Kashif Bangash, M.; Hussain, F.; Jaubert, J.-N. Development and characterization of electrospun curcumin-loaded antimicrobial nanofibrous membranes. Text. Res. J. 2020. [Google Scholar] [CrossRef]
- Ana, T.; Nieves, B.; Raul, D.; Ana, B.; Eduardo, R.; Diego, M.; Cristina, G. Natural bioactive compounds from winery by-products as health promoters. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar]
- Sato, J.; Nakayama, M.; Tomita, A.; Sonoda, T.; Miyamoto, T. Difference in the antibacterial action of epigallocatechin gallate and theaflavin 3, 3′-di-O-gallate on Bacillus coagulans. J. Appl. Microbiol. 2020, 129, 601–611. [Google Scholar] [CrossRef]
- George, D.; Begum, K.M.S.; Maheswari, P.U. Sugarcane bagasse (SCB) based pristine cellulose hydrogel for delivery of grape pomace polyphenol drug. Waste Biomass Valorization 2020, 11, 851–860. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.-Y.; Tang, R.-C.; Yuan, H.-B. Grape Seed Proanthocyanidins: Novel Coloring, Flame-Retardant, and Antibacterial Agents for Silk. ACS Sustain. Chem. Eng. 2020, 8, 5966–5974. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Narkar, A.; Lee, B.P. Gelatin Microgel Incorporated Poly(ethylene glycol)-Based Bioadhesive with Enhanced Adhesive Property and Bioactivity. ACS Appl. Mater. Interfaces 2016, 8, 11980–11989. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, H.; Konst, S.; Sarmiento, R.; Rajachar, R.; Lee, B.P. Injectable Dopamine-Modified Poly(Ethylene Glycol) Nanocomposite Hydrogel with Enhanced Adhesive Property and Bioactivity. ACS Appl. Mater. Interfaces 2014, 6, 16982–16992. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.; Yang, J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef]
- Brubaker, C.E.; Kissler, H.; Wang, L.-J.; Kaufman, D.B.; Messersmith, P.B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials 2010, 31, 420–427. [Google Scholar] [CrossRef]
- Colinas, I.R.; Rojas-Andrade, M.D.; Chakraborty, I.; Oliver, S.R. Two structurally diverse Zn-based coordination polymers with excellent antibacterial activity. Cryst. Eng. Comm. 2018, 20, 3353–3362. [Google Scholar] [CrossRef]
- Patel, K.M.; Patel, K.; Patel, N.; Patel, M. Synthesis, characterization, and antimicrobial activities of some transition metal complexes with a tridentate dibasic Schiff base and bidentate 2, 2′-bipyridylamine. Synth. React. Inorg. Met. Org. Chem. 2001, 31, 239–246. [Google Scholar] [CrossRef]
- Huppmann, T.; Yatsenko, S.; Leonhardt, S.; Krampe, E.; Radovanovic, I.; Bastian, M.; Wintermantel, E. Antimicrobial polymers-The Antibacterial Effect of Photoactivated Nano Titanium Dioxide Polymer Composites. In AIP Conference Proceedings; American Institute of Physics: University Park City, MD, USA, 2014; pp. 440–443. [Google Scholar]
- Neilson, A.H.; Allard, A.S.; Hynning, P.Å.; Remberger, M. Distribution, fate and persistence of organochlorine compounds formed during production of bleached pulp. Toxicol. Environ. Chem. 1991, 30, 3–41. [Google Scholar] [CrossRef]
- Pizer, R.; Babcock, L. Mechanism of the complexation of boron acids with catechol and substituted catechols. Inorg. Chem. 1977, 16, 1677–1681. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Liu, S.; Wong, C.; Chu, S. Mechanical chameleon through dynamic real-time plasmonic tuning. ACS Nano 2016, 10, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.E.K.; Wong, Y.T.; Ho, R.; Wasser, M.; Du, T.; Ng, W.T.; Halliwell, B. Effects of Hydrogen Peroxide on Wound Healing in Mice in Relation to Oxidative Damage. PLoS ONE 2012, 7, e49215. [Google Scholar] [CrossRef] [PubMed]
- Brian, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive Oxygen Species (ROS)-A Family of Fate Deciding Molecules Pivotal in Constructive Inflammation and Wound Healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- Pinnaratip, R.; Forooshani, P.K.; Li, M.; Hu, Y.H.; Rajachar, R.M.; Lee, B.P. Controlling the Release of Hydrogen Peroxide from Catechol-Based Adhesives Using Silica Nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 4502–4511. [Google Scholar] [CrossRef] [PubMed]
- Statz, A.R.; Barron, A.E.; Messersmith, P.B. Protein, cell and bacterial fouling resistance of polypeptoid-modified surfaces: Effect of side-chain chemistry. Soft Matter 2008, 4, 131–139. [Google Scholar] [CrossRef]
- Pechey, A.; Elwood, C.N.; Wignall, G.R.; Dalsin, J.L.; Lee, B.P.; Vanjecek, M.; Welch, I.; Ko, R.; Razvi, H.; Cadieux, P.A. Anti-adhesive coating and clearance of device associated uropathogenic escherichia coli cystitis. J. Urol. 2009, 182, 1628–1636. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.A.; Roland, J.D.; Liu, B.; Reaume, M.; Zhang, Z.; Kelley, J.D.; Lee, B.P. In Situ Deactivation of Catechol-Containing Adhesive Using Electrochemistry. J. Am. Chem. Soc. 2020, 142, 4631–4638. [Google Scholar] [CrossRef]
- Nicklisch, S.C.; Spahn, J.E.; Zhou, H.; Gruian, C.M.; Waite, J.H. Redox Capacity of an Extracellular Matrix Protein Associated with Adhesion in Mytilus californianus. Biochemistry 2016, 55, 2022–2030. [Google Scholar] [CrossRef]
- Narkar, A.R.; Kelley, J.D.; Pinnaratip, R.; Lee, B.P. Effect of Ionic Functional Groups on the Oxidation State and Interfacial Binding Property of Catechol-Based Adhesive. Biomacromolecules 2018, 19, 1416–1424. [Google Scholar] [CrossRef]
- Narkar, A.R.; Barker, B.; Clisch, M.; Jiang, J.; Lee, B.P. pH Responsive and Oxidation Resistant Wet Adhesive based on Reversible Catechol–Boronate Complexation. Chem. Mater. 2016, 28, 5432–5439. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razaviamri, S.; Wang, K.; Liu, B.; Lee, B.P. Catechol-Based Antimicrobial Polymers. Molecules 2021, 26, 559. https://doi.org/10.3390/molecules26030559
Razaviamri S, Wang K, Liu B, Lee BP. Catechol-Based Antimicrobial Polymers. Molecules. 2021; 26(3):559. https://doi.org/10.3390/molecules26030559
Chicago/Turabian StyleRazaviamri, Seyedehfatemeh, Kan Wang, Bo Liu, and Bruce P. Lee. 2021. "Catechol-Based Antimicrobial Polymers" Molecules 26, no. 3: 559. https://doi.org/10.3390/molecules26030559
APA StyleRazaviamri, S., Wang, K., Liu, B., & Lee, B. P. (2021). Catechol-Based Antimicrobial Polymers. Molecules, 26(3), 559. https://doi.org/10.3390/molecules26030559







