Synthesis of Tetravalent Thio- and Selenogalactoside-Presenting Galactoclusters and Their Interactions with Bacterial Lectin PA-IL from Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results and Discussion
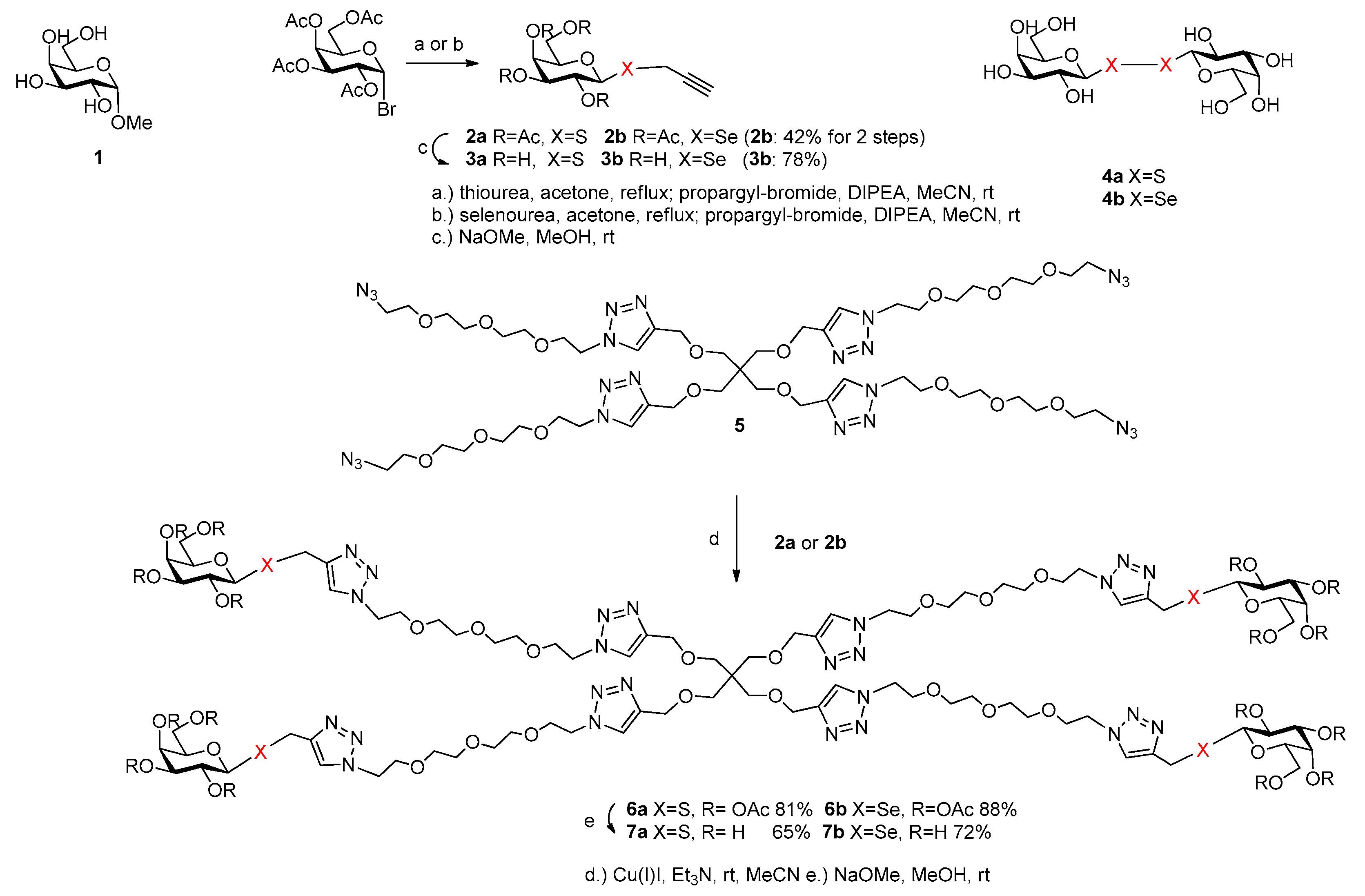
2.1. Synthesis
2.2. Inhibition of PA-IL with Thio- and Selenogalactosides
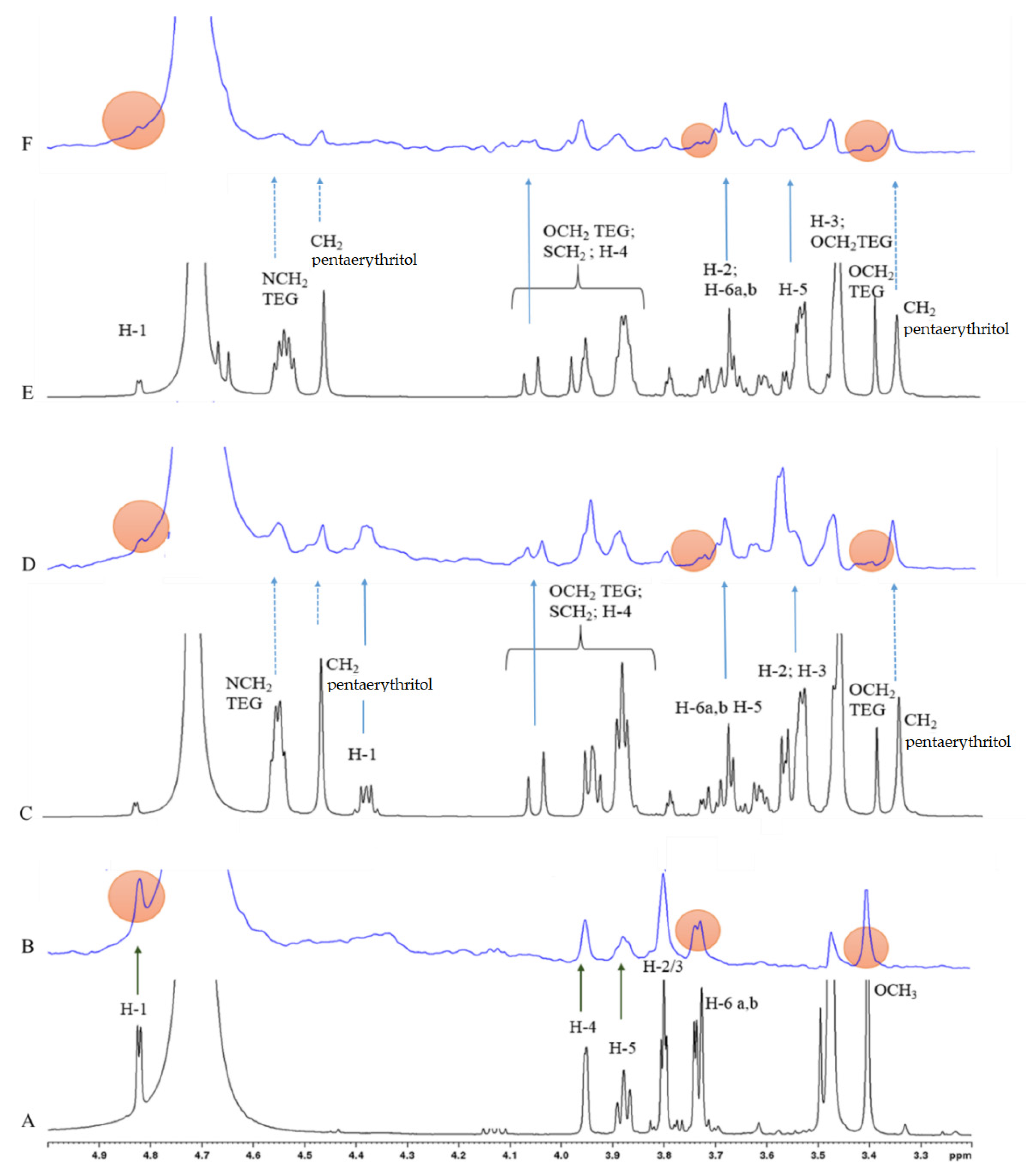
2.3. STD-NMR Studies: Binding of Galactoside-Containing Ligands to PA-IL Lectin Characterized by 1H STD and Competition NMR Experiments
3. Materials and Methods
3.1. General Methods
3.2. Synthesis
3.2.1. Compound 2b
3.2.2. Compound 3b
3.2.3. Compound 6a
3.2.4. Compound 6b
3.2.5. Compound 7a
3.2.6. Compound 7b
3.3. Hemagglutination Inhibition Assay (HIA)
3.4. 1H STD NMR Experiment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sharon, N.; Lis, H. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta 2006, 1760, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus Multivalent Glycoconjugates for the Design of High Affinity Lectin Ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012, 10, 841–851. [Google Scholar] [CrossRef]
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174–196. [Google Scholar] [CrossRef]
- Cioci, G.; Mitchell, E.P.; Gautier, C.; Wimmerová, M.; Sudakevitz, D.; Pérez, S.; Gilboa-Garber, N.; Imberty, A. Structural basis of calcium and galactose recognition by the lectin PA-IL of Pseudomonas aeruginosa. FEBS Lett. 2003, 555, 297–301. [Google Scholar] [CrossRef]
- Chemani, C.; Imberty, A.; de Bentzmann, S.; Pierre, M.; Wimmerová, M.; Guery, B.P.; Faure, K. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect. Immun. 2009, 77, 2065–2075. [Google Scholar] [CrossRef]
- Diggle, S.P.; Stacey, R.E.; Dodd, C.; Cámara, M.; Williams, P.; Winzer, K. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ. Microbiol. 2006, 8, 1095–1104. [Google Scholar] [CrossRef]
- Novoa, A.; Eierhoff, T.; Topin, J.; Varrot, A.; Barluenga, S.; Imberty, A.; Römer, W.; Winssinger, N. A LecA Ligand Identified from a Galactoside-Conjugate Array Inhibits Host Cell Invasion by Pseudomonas aeruginosa. Angew. Chem. Int. Ed. 2014, 53, 8885–8889. [Google Scholar] [CrossRef]
- Bajolet-Laudinat, O.; Bentzmann, S.G.-D.; Tournier, J.M.; Madoulet, C.; Plotkowski, M.C.; Chippaux, C.; Puchelle, E. Cytotoxicity of Pseudomonas aeruginosa internal lectin PA-I to respiratory epithelial cells in primary culture. Infect. Immun. 1994, 62, 4481–4487. [Google Scholar] [CrossRef]
- Thai, L.S.; Malinovská, L.; Vašková, M.; Mező, E.; Kelemen, V.; Borbás, A.; Hodek, P.; Wimmerová, M.; Csávás, M. Investigation of the Binding Affinity of a Broad Array of L-Fucosides with Six Fucose-Specific Lectins of Bacterial and Fungal Origin. Molecules 2019, 24, 2262. [Google Scholar] [CrossRef] [PubMed]
- Malinovská, L.; Thai, L.S.; Herczeg, M.; Vašková, M.; Houser, J.; Fujdiarová, E.; Komárek, J.; Hodek, P.; Borbás, A.; Wimmerová, M.; et al. Synthesis of β-D-galactopyranoside-presenting glycoclusters, investigation of their interactions with Pseudomonas aeruginosa lectin A (PA-IL) and evaluation of their anti-adhesion potential. Biomolecules 2019, 9, 686. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Dias, I.F.C.; Di Lorenzo, I.; Grzes, P.; Palomba, M.; Rosati, O.; Bagnoli, L.; Marini, F.; Santi, C.; Lenardao, E.J.; et al. Sweet Selenium: Synthesis and Properties of Selenium-Containing Sugars and Derivatives. Pharmaceuticals 2020, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Makyio, H.; Ando, H.; Komura, N.; Menjo, M.; Yamada, Y.; Imamura, A.; Ishida, H.; Wakatsuki, S.; Kato, R.; et al. Expanded potential of seleno-carbohydrates as a molecular tool for X-ray structural determination of a carbohydrate–protein complex with single/multi-wavelength anomalous dispersion phasing. Bioorg. Med. Chem. 2014, 22, 2090–2101. [Google Scholar] [CrossRef] [PubMed]
- Valerio, S.; Iadonisi, A.; Adinolfi, M.; Ravidá, A. Novel Approaches for the Synthesis and Activation of Thio- and Selenoglycoside Donors. J. Org. Chem. 2007, 72, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Gamblin, D.P.; Garnier, P.; Van Kasteren, S.; Oldham, N.J.; Fairbanks, A.J.; Davis, B.G. Glyco-SeS: Selenenylsulfide-Mediated Protein Glycoconjugation—A New Strategy in Post-Translational Modification. Angew. Chem. Int. Ed. 2004, 43, 828–833. [Google Scholar] [CrossRef]
- Kim, E.J.; Love, N.C.; Darout, E.; Abdo, M.; Rempel, B.; Withers, S.G.; Rablen, P.R.; Hanover, J.A.; Knapp, S. OGA inhibition by GlcNAc-selenazoline. Bioorg. Med. Chem. 2010, 18, 7058–7064. [Google Scholar] [CrossRef]
- Mehta, S.; Andrews, J.S.; Svensson, B.; Pinto, B.M. Synthesis and Enzymic Activity of Novel Glycosidase Inhibitors Containing Sulfur and Selenium. J. Am. Chem. Soc. 1995, 117, 9783–9790. [Google Scholar] [CrossRef]
- André, S.; Kövér, K.E.; Gabius, H.-J.; Szilágyi, L. Thio- and selenoglycosides as ligands for biomedically relevant lectins: Valency–activity correlations for benzene-based dithiogalactoside clusters and first assessment for (di)selenodigalactosides. Bioorg. Med. Chem. Lett. 2015, 25, 931–935. [Google Scholar] [CrossRef]
- Cumpstey, I.; Ramstadius, C.; Akhtar, T.; Goldstein, I.J.; Winter, H.C. Non-Glycosidically Linked Pseudodisaccharides: Thioethers, Sulfoxides, Sulfones, Ethers, Selenoethers, and Their Binding to Lectins. Eur. J. Org. Chem. 2010, 10, 1951–1970. [Google Scholar] [CrossRef]
- Affeldt, R.F.; Braga, H.C.; Baldassari, L.L.; Luedtke, D.S. Synthesis of selenium-linked neoglycoconjugates and pseudodisaccharides. Tetrahedron 2012, 68, 10470–10475. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J.L.; Fernández-González, M.; Anthony, D.C.; Davis, B.G. Selenenylsulfide-Linked Homogeneous Glycopeptides and Glycoproteins: Synthesis of Human “Hepatic Se Metabolite A”. Angew. Chem. Int. Ed. 2011, 51, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Raics, M.; Timári, I.; Diercks, T.; Szilágyi, L.; Gabius, H.-J.; Kövér, K.E. Selenoglycosides as Lectin Ligands: 77Se-Edited CPMG-HSQMBC NMR Spectroscopy To Monitor Biomedically Relevant Interactions. ChemBioChem 2019, 20, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Llamas, I.; Boutureira, O.; Claridge, T.D.W.; Davis, B.G. Glycosyldiselenides as lectin ligands detectable by NMR in biofluids. Chem. Commun. 2015, 51, 12208–12211. [Google Scholar] [CrossRef]
- Dondoni, A. Triazole: The Keystone in Glycosylated Molecular Architectures Constructed by a Click Reaction. Chem. Asian J. 2007, 2, 700–708. [Google Scholar] [CrossRef]
- Ziegler, T.; Pietrzik, N.; Schips, C. Efficient Synthesis of Glycosylated Asparaginic Acid Building Blocks via Click Chemistry. Synthesis 2008, 4, 519–526. [Google Scholar] [CrossRef]
- Giguere, D.; Bonin, M.-A.; Cloutier, P.; Patnam, R.; St-Pierre, C.; Sato, S.; Roy, R. Synthesis of stable and selective inhibitors of human galectins-1 and -3. Bioorg. Med. Chem. 2008, 16, 7811–7823. [Google Scholar] [CrossRef]
- Adamová, L.; Malinovská, L.; Wimmerová, M. New sensitive detection method for lectin hemagglutination using microscopy. Microsc. Res. Tech. 2014, 77, 841–849. [Google Scholar] [CrossRef]
- Mayer, M.; Meyer, B. Characterization of Ligand Binding by Saturation Transfer Difference NMR Spectroscopy. Angew. Chem. Int. Ed. 1999, 38, 1784–1788. [Google Scholar] [CrossRef]
- Mayer, M.; Meyer, B. Group Epitope Mapping by Saturation Transfer Difference NMR To Identify Segments of a Ligand in Direct Contact with a Protein Receptor. J. Am. Chem. Soc. 2001, 123, 6108–6117. [Google Scholar] [CrossRef]
- Groves, P.; Kövér, K.E.; André, S.; Bandorowicz-Pikuła, J.; Batta, G.; Bruix, M.; Buchet, R.; Canales, Á.; Cañada, F.J.; Gabius, H.-J.; et al. Temperature dependence of ligand-protein complex formation as reflected by saturation transfer difference NMR experiments. J. Magn. Reson. Chem. 2007, 45, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Driguez, H. Thiooligosaccharides in glycobiology. In Glycoscience Synthesis of Substrate Analogs and Mimetics; Springer: Berlin/Heidelberg, Germany, 1997; pp. 85–116. [Google Scholar] [CrossRef]
- Hauber, H.-P.; Schulz, M.; Pforte, A.; Mack, D.; Zabel, P.; Schumacher, U. Inhalation with Fucose and Galactose for Treatment of Pseudomonas Aeruginosa in Cystic Fibrosis Patients. Int. J. Med. Sci. 2008, 5, 371–376. [Google Scholar] [CrossRef] [PubMed]

| Inhibitor | MIC | Potency 2 | Valency | β 3 |
|---|---|---|---|---|
| d-galactose 1 | 6.25 mM | 1 | 1 | 1 |
| Me α-d-Gal | 1.562 mM | 4 | 1 | 4 |
| Compound 3a | 0.781 mM | 8 | 1 | 8 |
| Compound 3b | 0.781 mM | 8 | 1 | 8 |
| Compound 4a | 0.391 mM | 16 | 2 | 8 |
| Compound 4b | 0.781 mM | 8 | 2 | 4 |
| Compound I 4 | 24.41 µM | 256 | 4 | 64 |
| Compound 7a | 24.41 µM | 256 | 4 | 64 |
| Compound 7b | 24.41 µM | 256 | 4 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illyés, T.Z.; Malinovská, L.; Rőth, E.; Tóth, B.; Farkas, B.; Korsák, M.; Wimmerová, M.; Kövér, K.E.; Csávás, M. Synthesis of Tetravalent Thio- and Selenogalactoside-Presenting Galactoclusters and Their Interactions with Bacterial Lectin PA-IL from Pseudomonas aeruginosa. Molecules 2021, 26, 542. https://doi.org/10.3390/molecules26030542
Illyés TZ, Malinovská L, Rőth E, Tóth B, Farkas B, Korsák M, Wimmerová M, Kövér KE, Csávás M. Synthesis of Tetravalent Thio- and Selenogalactoside-Presenting Galactoclusters and Their Interactions with Bacterial Lectin PA-IL from Pseudomonas aeruginosa. Molecules. 2021; 26(3):542. https://doi.org/10.3390/molecules26030542
Chicago/Turabian StyleIllyés, Tünde Zita, Lenka Malinovská, Erzsébet Rőth, Boglárka Tóth, Bence Farkas, Marek Korsák, Michaela Wimmerová, Katalin E. Kövér, and Magdolna Csávás. 2021. "Synthesis of Tetravalent Thio- and Selenogalactoside-Presenting Galactoclusters and Their Interactions with Bacterial Lectin PA-IL from Pseudomonas aeruginosa" Molecules 26, no. 3: 542. https://doi.org/10.3390/molecules26030542
APA StyleIllyés, T. Z., Malinovská, L., Rőth, E., Tóth, B., Farkas, B., Korsák, M., Wimmerová, M., Kövér, K. E., & Csávás, M. (2021). Synthesis of Tetravalent Thio- and Selenogalactoside-Presenting Galactoclusters and Their Interactions with Bacterial Lectin PA-IL from Pseudomonas aeruginosa. Molecules, 26(3), 542. https://doi.org/10.3390/molecules26030542








