2. Results and Discussion
Earlier in the work of Tatlow [
20], it has been shown that the interaction of polyfluorinated aromatic compounds with thiourea, under mild conditions, is a convenient method for the synthesis of diaryl sulphides, but in the case of perfluoro-
m-xylene, polymerization of the latter was observed. It should be noted that the composition of the oligomeric mixture in this reaction has not been studied. It may be assumed that linear oligomers with a low polymerization depth will form predominantly in this reaction under mild conditions with a sufficiently high concentration of the initial perfluoro-
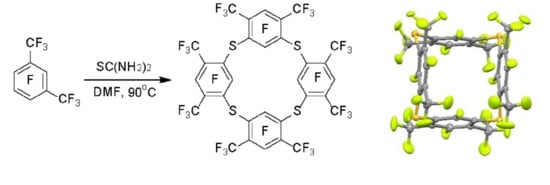
m-xylene. We performed this reaction under conditions favored by cyclo-oligomerization. Indeed, when a mixture of perfluoro-
m-xylene with an excess of thiourea is heated at 80–90 °C in a diluted (c~0.08 mol/L) DMF solution, perfluorinated tetrathiacalix[4]arene
1 is formed as the main product (
Scheme 1).
It is assumed that the interaction of polyfluoroaromatic compounds with thiourea proceeds through the intermediate formation of an isothiouronium derivative of type
2, which then acts as an ArS
− equivalent in the reaction with polyfluoroarene, giving diaryl sulphides or linear oligomers as the products [
20,
21]. Subsequent oligomerization in the case of perfluoro-
m-xylene leads to the intermediate formation of isothiouronium
3 macrocyclization of which gives tetrathiacalixarene
1 (
Scheme 1).
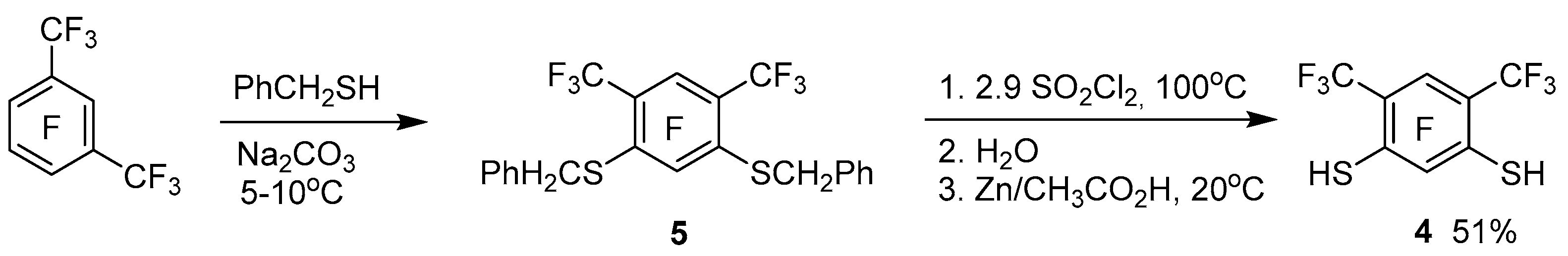
We also obtained tetrathiacalix[4]arene
1 by interaction of perfluoro-
m-xylene with dithiol
4 (
Scheme 1). The latter was synthesized by us from perfluoro-
m-xylene via intermediate formation of bis(benzylthio)benzene
5 according to
Scheme 2. The standard deprotection method [
22] in compound
5, due to the presence of acceptor substituents, does not lead to the formation of dithiol
4. Therefore, based on studies of the reactivity of polyfluorinated arenthiols [
23], for deprotection of the thiobenzyl group in compound
5, it was proposed to use chlorination by SO
2Cl
2, hydrolysis and subsequent reduction by Zn of the resulting product mixture.
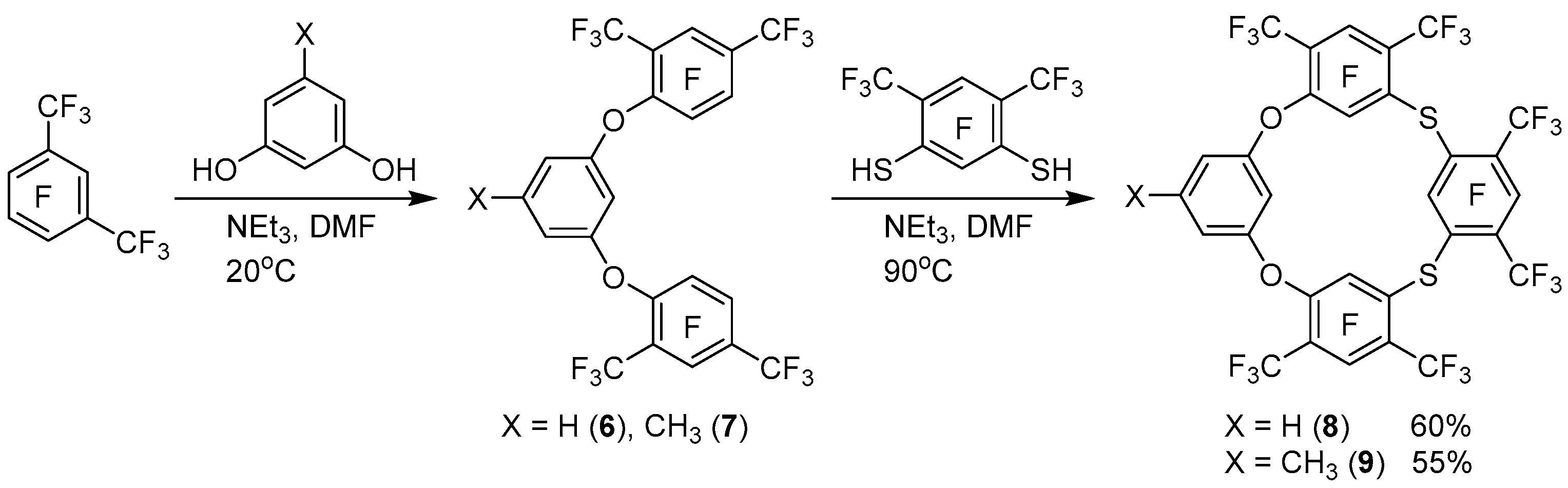
We have previously shown that the reaction of perfluoro-
m-xylene with resorcinol under mild conditions led to the formation of a mixture of polyphenyl ethers with a predominant content of triphenyl ether [
17]. Further heating of this mixture with resorcinol or tetrafluororesorcinol gave polyfluorinated oxacalixarenes of the ABAB or ABAC type. The same approach was used in the reactions of pentafluorobenzonitrile and pentafluoronitrobenzene with various resorcinoles [
18,
19], and the synthesis was also performed without intermediate isolation of triphenyl ethers. We used this approach for the synthesis of dioxadithiacalix[4]arenes. Thus, the interaction of two equivalents of perfluoro-
m-xylene with the equivalent of resorcinol or orcinol under mild conditions and subsequent heating of the reaction mixtures with the equivalent of dithioresorcinol
4 leads to the formation of polyfluorinated dioxadithiacalix[4]arenes
8, 9 with a good yield (
Scheme 3).
At the same time, the interaction of triphenyl ether
6 with thiourea leads to tetraoxadithiacalix[
6]arene
12 and dioxadithiacalix[4]arene
8 as the main and minor products, respectively (
Scheme 4). The intermediate isothiouronium derivative
10 formed in this reaction then reacts successively with another equivalent of triphenyl ether
6 and thiourea giving another isothiouronium derivative
11. Macrocyclization of derivative
11 can take place both on the terminal (main pathway a) and the internal (minor pathway b) perfluoro-
m-xylene fragments to form tetraoxadithiacalix[
6]arene
12 and dioxadithiacalix[4]arene
8, respectively. Intramolecular cyclization of isothiouronium derivative
10 with the formation of dioxatiacalix[
3]arene is unlikely, which can be explained in terms of strain of the intended cycle.
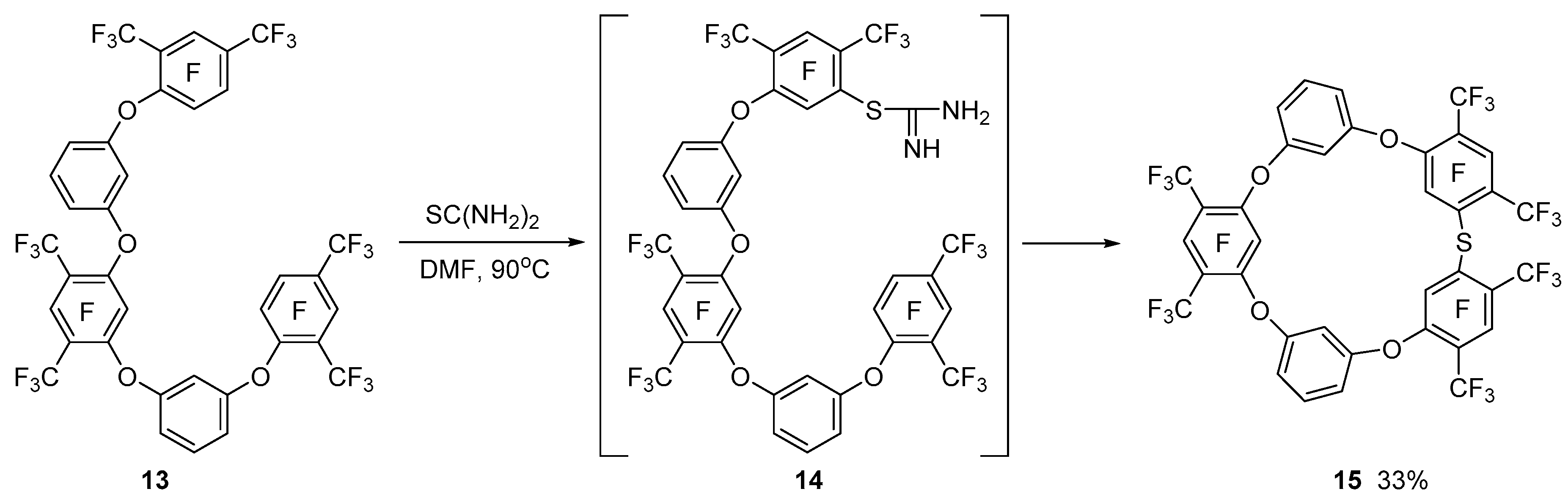
In contrast, the reaction of pentaphenyl ether
13 with thiourea intramolecular macrocyclization of isothiouronium derivative
14 leads to the formation of tetraoxatiacalix[
5]arene
15, which is due to a decrease in transannular strain in the cycle (
Scheme 5).
The structure of thia- and oxathiacalixarenes
1, 8, 9, 12, 15 was determined based on analytical and
1H,
19F,
13C NMR data (
Supplementary Material Figures S1_F–S13_C). The structure of tetrathiacalixarene
1 was also confirmed by X-ray data.
According to X-ray analysis of the single crystal obtained from CH
2Cl
2, tetrathiacalixarene
1 is in the 1,3-alternate conformation, which is typical for tetrathiacalixarenes that do not have substituents in the inner cycle [
13,
15,
24] (
Figure 1a). The sulphur atoms are located in the same plane practically without deviation. The C–S bond length 1.78 Å corresponds to the literature data for tetrathiacalixarenes [
15,
24]. The C–S–C angles are 100.6–100.7°, and the torsion angles around the C–S bonds are 57.8–60.3°. The sulphur atoms are slightly displaced outside from the planes of the aromatic nucleus, the deviation is 0.12–0.21 Å. The opposite aromatic nuclei are located almost parallel to each other, and the dihedral angles are 2.44° and 4.69°, respectively. It should be noted that the difference in the dihedral angles for the tetrathiacalixarenes described in the literature is significantly higher (2–130°) [
15,
24]. Crystallization of tetrathiacalixarene
1 from acetone or acetonitrile leads to the formation of complexes including 1 or 2 solvent molecules (
Figure 1b). In this case, the dihedral angles between the opposite aromatic nuclei increase to 19.2° and 20.1° (
1*2CH
3CN).
One set of three signals in the
19F NMR spectra (
Supplementary Material Figure S3_F) of tetrathiacalixarene
1 without significant broadening in the signal structure at room temperature can indicate both the realization of one symmetric conformation and a very fast conformational interconversion in the NMR time scale. It can be assumed that the presence of fairly large eight-CF
3 groups in the tetrathiacalixarene
1 molecule should shift the equilibrium towards the least sterically hindered 1,3-alternate conformation similar to that determined by the X-ray method for the crystal state. A similar 1,3-alternate conformation was proposed earlier in the analysis of
1H NMR spectra for solutions of tetrathiacalixarene without substituents in the internal macrocycle [
14]. When four volume substituents (OC
2H
5) are introduced, the interconversion becomes difficult, and the equilibrium between all four possible conformations of tetrathiacalixarene according to NMR spectra is fixed [
25].
We have previously observed a noticeable upfield shift of the inner-rim hydrogen and fluorine atoms of the resorcinol fragments in the
1H and
19F NMR spectra of polyfluorinated tetraoxacalixarenes, which is characteristic for this class of compounds [
16,
17,
18,
19]. The value of this upfield shift is solvent dependent, which may be due to implementation of some equilibrium 1,3-alternate conformations characterized by different degrees of magnetic shielding of the inner-rim protons and fluorines of the resorcinol fragments by the neighboring aromatic rings [
19].
In the
1H NMR spectra of dioxadithiacalix[4]arenes
8–9, an upfield shift of the inner-rim protons (H-28) of the resorcinol fragment is also observed, and the value of this shift depends on the solvent (
Supplementary Material Figure S5_H). So, the chemical shift of the hydrogen atom H-28 in CDCl
3 is 5.45 ppm for
8 and 5.08 ppm for
9, and in (CD
3)
2CO 6.25 ppm for
8 (
Figure 2a) and 6.00 ppm for
9. The presence of an upfield shift of the inner-rim protons in the
1H NMR spectra indicates that dioxadithiacalix[4]arenes
8–
9, as well as tetraoxacalixarenes [
26], have a 1,3-alternate conformation in solution. In the
1H NMR spectra of tetraoxadithiacalix[
6]arene
12, a noticeable upfield shift of the inner-rim protons (H-39,42; δ 6.14 ppm in acetone-d
6,
Figure 2b) of the resorcinol fragment is also observed, and it is practically absent in the
1H NMR spectra of tetraoxathiacalixarene
15 (H-33,35; δ 6.92 ppm in acetone-d
6,
Figure 2c). For comparison, the chemical shift of the hydrogen atom, located between two perfluorinated phenoxy fragments, in triphenyl ether
6 (H-2; δ 7.10 ppm in acetone-d
6) and pentaphenyl ether
13 (H-2′; δ 7.13 ppm in acetone-d
6,
Figure 2d) can be used as reference points. It should be noted that, in contrast to tetraoxadithiacalix[
6]arene
12, the
1H NMR spectra of closely related polyfluorinated hexaoxacalix[
6]arenes are lacking for an upfield shift of the inner-rim hydrogen atoms signals [
17].
3. Materials and Methods
3.1. General Methods
Thiourea, resorcinol and orcinol monohydrate were obtained from Aldrich (Milwaukee, WI, USA), AppliChem (Darmstadt, Germany) and FluoroChem (Hadfield, UK), respectively, and used directly without further purification. Dimethylformamide and triethylamine were held over NaOH during a week and distilled immediately before use. Perfluoro-
m-xylene was a ~3:1 mixture with its
para-isomer (according to the
19F NMR data) which is considerably less reactive [
27]. This mixture is a byproduct in the synthesis of octafluorotoluene by interaction of hexafluorobenzene with Teflon chips [
28,
29].
The 19F and 1H NMR spectra were recorded for solutions in CDCl3 and OC(CD3)2 on a Bruker AV300 spectrometer at 282.36 MHz and 300 MHz, respectively, 13C NMR spectra were recorded on a Bruker AV-400 instrument (100.6 MHz). Chemical shifts are given in δ ppm from CCl3F (19F) and TMS (1H, 13C), J values in Hz; C6F6 (−162.9 ppm from CCl3F), CHCl3 (7.24 ppm from TMS), OC(CD3)2 (2.05 ppm from TMS), OC(CD3)2 (29.8 ppm from TMS) were used as internal standards.
The X-ray diffraction experiments for
1 and
1*2CH
3CN were carried out on a Bruker KAPPA APEX II diffractometer with graphite monochromated MoKα (
λ = 0.71073 Å) radiation at 296 K. Experimental data reduction was performed using APEX2 suite [
30]. The structures were solved by direct methods and refined by the full-matrix least-squares technique against F
2 in the anisotropic-isotropic approximation. The H atom positions in
1*2CH
3CN were calculated with the riding model. All calculations were performed using SHELXL-2018/3 [
31]. All CF
3 groups in crystal
1 are disordered over two positions with SOF 0.21–0.74 and some restrictions were applied.
Gas chromatographic–mass spectrometric analysis (GC–MS) was performed on a Hewlett Packard HP 5890 Series II chromatograph coupled with an HP 5971 mass-selective detector (HP-5MS capillary column, 30 m × 0.25 mm, film thickness 0.25 μm; carrier gas helium, flow rate 1 mL/min; oven temperature programming from 50 °C (2 min) to 280 °C at a rate of 10 °/min and finally 5 min at 280 °C; injector temperature 280 °C; ion source temperature 175 °C; electron impact, 70 eV; 1.2 scan/s, a.m.u. range 30–650). HPLC analysis was performed on a Milichrome-A-02 (Econova, Novosibirsk, Russia). Column—2.0 × 75 mm with ProntoSIL-120-5-C18 (BISCHOFF Analysentechnik U.—GERÄTE GMBH, Leonberg, Germany). Temperature of column 30 °C. Eluent: methanole/water, gradient from 4:1 to pure methanole (1500 mcl) and then methanole (1500 mcl). The reference wavelength—260 nm.
The elemental compositions of thiacalixarene 1 and oxathiacalixarenes 8, 9, 12, 15, were determined by classical methods and their molecular weights were determined in acetone solution at 40 °C using a Knauer K-7000 osmometer. The elemental compositions of compounds 4, 5, were determined from the high-resolution mass spectra which were recorded on a Thermo Scientific DFS instrument (electron impact, 70 eV).
The progress of reactions was monitored by TLC on Silica gel 60 F254 plates (Merck, Darmstadt, Germany). Silica gel (0.063–0.200 mm; Merck, Darmstadt, Germany) was used for column chromatography.
3.2. Synthesis of 2,5-difluoro-4,6-bis(trifluoromethyl)benzene-1,3-dithiol 4
3.2.1. 1,3-Bis(benzylthio)-2,5-difluoro-4,6-bis(trifluoromethyl)benzene 5
Na2CO3 (3.11 g, 10.9 mmol) and 18.82 g (58.3 mmol) benzyl mercaptan was added successively to a stirred solution of 10.04 g of a mixture of perfluorinated m- and p-xylenes (~26.3 mmol of the meta-isomer) in 80 mL of dimethylformamide. The solution was stirred for 18 h at 5–10 °C, and then poured into 160 mL of 10% aqueous HCl solution. The precipitate was filtered out and dried over CaCl2. Solid product (12.51 g) contained 85% of dithiol 4, according to HPLC data. Analytical sample was obtained by crystallization from hexane. Mp 62.3–63.7 °C. 19F NMR (CDCl3): δ −116.0 (septet d, 1F, J = 36 Hz, J = 15 Hz, F-5), −93.6 (d, 1F, J = 15 Hz, F-2), −55.5 (d, 6F, J = 36 Hz, 2CF3). 1H NMR (CDCl3): δ 4.10 (s, 2H, CH2), 7.15–7.28 (m, 5H, C6H5). Anal. Calcd for C22H14F8S2: C 53.44; H 2.85; F 30.74; S 12.97%. Found: C 53.85; H 2.79; F 31.18; S 12.80. HRMS m/z: 494.0403 (M+). Calculated: M = 494.0404.
3.2.2. 2,5-Difluoro-4,6-bis(trifluoromethyl)benzene-1,3-dithiol 4
Compound 5 (11.12 g, 19.1 mmol, technical product with an 85% content) and SO2Cl2 (7.52 g, 55.7 mmol) were placed in a glass ampoule. The ampoule was sealed and heated in a metal casing for 16 h at 100–110 °C. The reaction mixture was cooled and poured into 200 mL of water, then 10 mL EtOAc, 40 mL CHCl3 and 9.41 g (88.8 mmol) Na2SO3 were added. After stirring, the organic layer was washed with water (2 × 200 mL) and the solvents were evaporated. The oily product was dissolved in 70 mL of glacial acetic acid, and then Zn (12.62 g, 192.9 mmol) was added by small portions and stirred for 24 h at 20 °C. The reaction mixture was treated by 60 mL of 10% aqueous HCl solution, and the product was extracted with CHCl3 (3 × 25mL) and additionally with EtOAc (3 × 15 mL). The solvents were evaporated, and the product was distilled with steam. Two fractions were obtained: solid (3.51 g) and liquid (1.18 g) with a dithiol 4 content of 87% and 52%, respectively (GC–MS). Pure dithiol 4 (1.86 g, 51% with 98% content according to GC–MS) was obtained by crystallization of solid fraction from hexane. Mp 66.4–67.2 °C. 19F NMR (CDCl3): δ −115.7 (septet d, 1F, J = 27 Hz, J = 15 Hz, F-5), −103.6 (m, 1F, F-2), −57.1 (dd, 6F, J = 27 Hz, J = 8 Hz, 2CF3). 1H NMR (CDCl3): δ 4.36 (quintet, 2H, J = 9.7 Hz, SH). 13C NMR (CDCl3): δ 113.5 (qdd, 2JCF = 33.6 Hz, 2JCF = 15.8 Hz, JCF = 1.7 Hz, C–CF3), 122.2 (qdd, 1JCF= 275.6 Hz, JCF = 2.3 Hz, JCF = 1.5 Hz, CF3), 127.8 (dd, 2JCF = 28.1 Hz, JCF = 3.2 Hz C-SH), 147.9 (dd, 1JCF= 231.0 Hz, JCF = 3.7 Hz C-F), 155.4 (dm, 1JCF= 266.9 Hz, C–F). Anal. Calcd for C8H2F8S2: C 30.58; H 0.64; F 48.37; S 20.41%. Found: C 30.16; H 0.51; F 48.33; S 20.05. HRMS m/z: 313.9472 (M+). Calculated M = 313.9465.
3.3. Synthesis of Polyfluorinated Triphenyl and Pentaphenyl Ethers 6, 13
Solution of trimethylamine (1.60 g, 16 mmol) and resorcinol (0.36 g, 3.3 mmol) in 10 mL of acetone was added dropwise to a stirred solution of 2.20 g of a mixture of perfluorinated m- and p-xylenes (~6 mmol of the meta-isomer) in 15 mL of acetone. The solution was stirred for 20 h at 20 °C, and solvent was evaporated. Column chromatography (eluents petroleum ether (40–70 °C)/CCl4 1:2, 1:3, 1:5) on silica gel gave 1.16 g (1.8 mmol, 60%) of triphenyl ether 6, 0.45 g (0.45 mmol, 27%) of pentaphenyl ether 13 and 0.17 g of polyphenyl ethers.
3.3.1. 1,3-Bis[3,5,6-trifluoro-2,4-bis(trifluoromethyl)phenoxy]benzene 6:
Viscous oil 19F NMR (OC(CD3)2): δ −149.8 (dd, 2F, F-6′), −126.6 (qd, 2F, F-5′), −115.7 (m, 2F, F-3′), −55.4 (t, 6F, 2CF3), −55.3 (d, 6F, 2CF3). 1H NMR (OC(CD3)2): δ 6.97 (dd, 2H, H-4,6), 7.10 (t, H, H-2), 7.41 (t, H, H-5). GC–MS, m/z: 642 (M+).
The
1H and
19F NMR spectra and the mass spectrum of triphenyl ether
6 coincide with the product data obtained from [
17].
3.3.2. 1,3-Bis{3-[3,5,6-trifluoro-2,4-bis(trifluoromethyl)phenoxy]phenoxy}-2,5-difluoro-4,6-bis(trifluoromethyl)benzene 13:
Viscous oil 19F NMR (OC(CD3)2): δ −149.6 (dd, 2F, J = 20 Hz, J = 11 Hz, F-6′), −138.9 (d, 1F, J = 12 Hz, F-2), −126.6 (qd, 2F, J = 23 Hz, J = 20 Hz, F-5′), −115.6 (qqd, 2F, J = 28 Hz, J = 23 Hz, J = 11 Hz, F-3′), −115.2 (septet d, 1F, J = 28 Hz, J = 12 Hz, F-5), −55.5 (t, 6F, J = 23 Hz, 2CF3), −55.4 (d, 6F, J = 28 Hz, 2CF3), −55.3 (d, 6F, J = 28 Hz, 2CF3). 1H NMR (OC(CD3)2): δ 6.94 (dd, 2H, J = 8.2 Hz, J = 2.1 Hz, H-4′), 7.01 (dd, 2H, J = 8.4 Hz, J = 2.1 Hz, H-6′), 7.13 (t, 2H, J = 2.1 Hz, H-2′), 7.39 (t, 2H, J = 8.3 Hz, H-5′). GC–MS, m/z: 499 (M++, M 998).
3.4. Synthesis of Tetrathiacalixarene 1
Method A: Thiourea (0.60 g, 8 mmol) was added to a stirred solution of 1.60 g of a mixture of perfluorinated m- and p-xylenes (~4.1 mmol of the meta-isomer) in 50 mL of dimethylformamide. The solution was stirred for 3 h at 20 °C and 5 h at 90 °C, and then the solvent was evaporated in vacuum. By column chromatography on silica gel using mixture (3:1) CCl4 and CHCl3 as eluent 1.20 g of the solid product was isolated, double-play crystallization of which from CCl4 gave 0.64 g of tetrathiacalixarene 1.
Method B: Triethylamine (0.80 g, 8 mmol) was added to a stirred solution of 0.35 g (1.1 mmol) of dithioresorcinol 4 and 0.80 g of a mixture of perfluorinated m- and p-xylenes (~2 mmol of the meta-isomer) in 70 mL of dimethylformamide. The mixture was stirred for 17 h at room temperature and 30 min at 90 °C then 0.30 g (1 mmol) of dithioresorcinol 4 was introduced and stirred for 20 h at 90 °C. The mixture after evaporation of solvent was treated with 80 mL of ~5% aqueous HCl and then with methylene chloride (3 × 50 mL). The extract was dried over Na2SO4, and evaporation gave 2.30 g of a viscous material. By column chromatography on silica gel using CCl4 and CHCl3 as eluents, 0.68 g of the solid product containing (GC–MS) 93% tetrathiacalixarene 1 was isolated.
5,11,17,23,25,26,27,28-Octafluoro-4,6,10,12,16,18,22,24-octakis(trifluoromethyl)-2,8,14,20-tetrathiapentacyclo[19.3.1.13,7.19,13.115,19]octacosa-1(25),3(28),4,6,9(27),10,12,15(26),16,18,21,23-dodecaene 1:
White solid (0.64 g, 57%). mp 304.6–304.9 °C. 19F NMR ((CD3)2CO): δ −112.1 (septet d, 4F, JF(5)-2CF3(4,6) = 33 Hz, JF(5)-F(28) = 15 Hz, F-5,11,17,23), −91.6 (m, 4F, F-25,26,27,28), −53.5 (dd, 24F, JCF3(4)-F(5) = 33 Hz, JCF3(4)-F(28) = 7 Hz, CF3(4,6,10,12,16,18,22,24)). 13C NMR ((CD3)2CO): δ 122.3 (qd, 2JCF = 32.5 Hz, 2JCF = 14.0 Hz, C–CF3), 122.5 (q, 1JCF = 276.0 Hz, CF3), 127.7 (d, 2JCF = 25.9 Hz, C–S), 155.3 (d, 1JCF = 269.8 Hz, C–F), 158.5 (dd, 1JCF = 244.2 Hz, JCF = 3.0 Hz, C–F). Anal. Calcd for C32F32S4: C, 34.30; F, 54.25; S, 11.44%; M 1120. Found: C, 34.00; F, 54.37; S, 11.48%; M 1120.
Crystal data of 1: C32F32S4, M = 1120.56, monoclinic, space group P21/c, a = 12.5563(14), b = 15.9119(18), c = 18.7987(18) Å, β = 96.635(4)°, V = 3730.7(7) Å3, Z = 4, dcalc = 1.995 g·cm−3, μ = 0.444 mm−1, a total of 67,724 (θmax = 27.58°), 8648 unique (Rint = 0.0541), 6093 [I > 2σ(I)], 837 parameters. GooF = 1.02, R1 = 0.0399, wR2 = 0.0938 [I > 2σ(I)], R1 = 0.0680, wR2 = 0.1136 (all data), max/min diff. peak 0.280/−0.233 e Å−3.
Crystal data of 1*2CH3CN: C32F32S4+2(CH3CN), M = 1202.67, triclinic, space group P-1, a = 10.1087(6), b = 10.1752(6), c = 20.9515(10) Å, α = 82.318(2), β = 81.616(2), γ = 82.275(3)°, V = 2098.3(2) Å3, Z = 2, dcalc = 1.904 g·cm−3, μ = 0.403 mm−1, a total of 67,731 (θmax = 28.01°), 10,126 unique (Rint = 0.0435), 7405 [I > 2σ(I)], 669 parameters. GooF = 1.04, R1 = 0.0550, wR2 = 0.1488 [I > 2σ(I)], R1 = 0.0784, wR2 = 0.1726 (all data), max/min diff. peak 0.810/−0.447 e·Å−3.
3.5. General Procedure for the Synthesis of Dioxadithiacalixarenes 8, 9
Solution of trimethylamine (1.20 g, 12 mmol) and resorcinol (orcinol monohydrate) (2 mmol) in 10 mL of dimethylformamide was added dropwise to a stirred solution of 1.50 g of a mixture of perfluorinated m- and p-xylenes (~4 mmol of the meta-isomer) in 40 mL of dimethylformamide. The solution was stirred for 3 h at 20 °C and 30 min at 90 °C, then 0.63 g (2 mmol) of dithioresorcinol 4 was introduced and the mixture was stirred for 3 h at 90 °C. The solvent was evaporated in vacuum, and by column chromatography on silica gel using CCl4 as eluent, the solid product was isolated. Pure dioxadithiacalixarenes 8, 9, were obtained by crystallization of the solid product from CCl4.
3.5.1. 11,17,23,25,26,27-Hexafluoro-10,12,16,18,22,24-hexakis(trifluoromethyl)-2,8-dioxa-14,20-dithiapentacyclo[19.3.1.13,7.19,13.115,19]octacosa-1(25),3(28),4,6,9(27),10,12,15(26),16,18,21,23-dodecaene 8:
White solid (1.17 g), containing according 19F NMR data dioxadithiacalixarene 8 and tetrathiacalixarene 1 (94:6). mp 225–231 °C. 19F NMR (CDCl3): δ −119.5 (dq, 2F, JF(25)-F(23) = 14 Hz, JF(25)-CF3(22) = 6 Hz, F-25,27), −112.8 (qqd, 2F, JF(11)-CF3(12) = 35 Hz, JF(11)-CF3(10) = 28 Hz, JF(11)-F(27) = 14 Hz, F-11,23), −112.2 (septet d, 1F, JF(17)-2CF3(16,18) = 36 Hz, JF(17)-F(26) = 15 Hz, F-11,23), −96.6 (dm, 1F, JF(26)-F(17) = 15 Hz, F-26), −58.0 (d, 6F, JCF3(10)-F(11) = 28 Hz, CF3(10,24)), −55.3 (dd, 6F, JCF3(12)-F(11) = 36 Hz, JCF3(12)-F(27) = 6 Hz, CF3(12,22)), −55.1 (dd, 6F, JCF3(16)-F(17) = 36 Hz, JCF3(16)-F(26) = 4 Hz, CF3(16,18)). 19F NMR (OC(CD3)2): δ −118.5 (dq, 2F, F-25,27), −113.9 (qqd, 2F, F-11,23), −111.7 (septet d, 1F, F-11,23), −92.6 (dm, 1F, F-26), −55.6 (d, 6F, CF3(10,24)), −53.1 (dd, 6F, CF3(12,22)), −52.7 (dd, 6F, CF3(16,18)). 1H NMR (CDCl3): δ 5.45 (m, 1H, H-28), 7.17 (dd, 2H, J = 8.3 Hz, J = 2.2 Hz, H-4,6), 7.56 (t, 1H, J = 8.3 Hz, H-5). 1H NMR (OC(CD3)2): δ 6.25 (m, 1H, H-28), 7.14 (dd, 2H, H-4,6), 7.55 (t, 1H, H-5). Anal. Calcd for C30H4F24O2S2: C, 39.32; H, 0.44; F, 49.75; S, 7.00%; M 916. Found: C, 38.72; H, 0.41; F, 49.91; S, 6.89%; M 911. GC–MS, m/z: 458 (M++, M 916).
3.5.2. 11,17,23,25,26,27-Hexafluoro-5-methyl-10,12,16,18,22,24-hexakis(trifluoromethyl)-2,8-dioxa- 14,20-dithiapentacyclo[19.3.1.13,7.19,13.115,19]octacosa-1(25),3(28),4,6,9(27),10,12,15(26),16,18,21,23- dodecaene 9:
White solid (1.02 g, 55%). mp 161–165 °C. 19F NMR (CDCl3): δ −119.5 (dq, 2F, JF(25)-F(23) = 14 Hz, JF(25)-CF3(22) = 6 Hz, F-25,27), −112.9 (qqd, 2F, JF(11)-CF3(12) = 36 Hz, JF(11)-CF3(10) = 28 Hz, JF(11)-F(27) = 14 Hz, F-11,23), −112.3 (septet d, 1F, JF(17)-2CF3(16,18) = 36 Hz, JF(17)-F(26) = 15 Hz, F-11,23), −96.6 (dm, 1F, JF(26)-F(17) = 15 Hz, F-26), −57.9 (d, 6F, JCF3(10)-F(11) = 28 Hz, CF3(10,24)), −55.3 (dd, 6F, JCF3(12)-F(11) = 36 Hz, JCF3(12)-F(27) = 6 Hz, CF3(12,22)), −55.1 (dd, 6F, JCF3(16)-F(17) = 36 Hz, JCF3(16)-F(26) = 4 Hz, CF3(16,18)). 19F NMR (OC(CD3)2): δ −118.4 (dq, 2F, F-25,27), −113.9 (qqd, 2F, F-11,23), −111.6 (septet d, 1F, F-11,23), −92.6 (dm, 1F, F-26), −55.6 (d, 6F, CF3(10,24)), −53.1 (dd, 6F, CF3(12,22)), −52.7 (dd, 6F, CF3(16,18)). 1H NMR (CDCl3): δ 2.38 (s, 3H, CH3), 5.08 (m, 1H, H-28), 6.82 (d, 2H, JH(4,6)-H(28) = 4 Hz, H-4,6). 1H NMR (OC(CD3)2): δ 2.40 (s, 3H, CH3), 6.00 (m, 1H, H-28), 6.95 (d, 2H, H-4,6). 13C NMR (OC(CD3)2): δ 21.2 (C(5)-CH3), 97.2 (C-28), 114.1 (C-4,6), 116.3 (qd, 2JCF = 33.0 Hz, 2JCF = 14.9 Hz, C-CF3), 116.5 (qd, 2JCF = 32.5 Hz, 2JCF = 13.2 Hz, C-CF3), 121.9 (qd, 2JCF = 32.4 Hz, 2JCF = 13.9 Hz, C–CF3), 122.0 (q, 1JCF = 274.9 Hz, CF3), 122.6 (q, 1JCF = 276.4 Hz, CF3), 122.8 (q, 1JCF = 275.8 Hz, CF3), 127.3 (d, 2JCF = 24.9 Hz, C–S), 128.4 (d, 2JCF = 17.4 Hz, C–S), 144.2 (C-5), 144.9 (dd, 2JCF = 16.7 Hz, JCF = 5.0 Hz, C(1,9)-0), 152.4 (dd, 1JCF = 249.8 Hz, JCF = 3.7 Hz, C–F), 155.1 (d, 1JCF = 267.8 Hz, C–F), 155.4 (d, 1JCF = 271.8 Hz, C–F), 158.4 (d, 1JCF = 246.9 Hz, C–F), 159.1 (C(3,7)-0). Anal. Calcd for C31H6F24O2S2: C, 40.02; H, 0.65; F, 49.00; S, 6.89%; M 930. Found: C, 40.30; H, 0.98; F, 48.91; S, 6.88%; M 926.
3.6. Synthesis of Tetraoxadithiacalixarene 12
Thiourea (0.76 g, 10 mmol) was added to a stirred solution of 1.27 g (1.9 mmol) of 1,3-bis(2,4-bis(trifluoromethyl)trifluorophenoxy)benzene 6 in 50 mL of dimethylformamide. The solution was stirred for 15 h at 90 °C, and the solvent was evaporated in vacuum. By column chromatography on silica gel using CCl4 as eluent, 1.32 g of the viscous material containing (GC–MS) 91% tetraoxadithiacalixarene 12 and 5% dioxadithiacalixarene 8 was isolated. Double-play crystallization of viscous material from CCl4 gave 0.76 g of tetraoxadithiacalixarene 12*CCl4.
11,17,29,35,37,38,40,41-Octafluoro-10,12,16,18,28,30,34,36-octakis(trifluoromethyl)-2,8,20,26- tetraoxa-14,32-dithiaheptacyclo[31.3.1.13,7.19,13.115,19.121,25.127,31]dotetraconta-1(37),3(42),4,6,9(41),10,12,15 (40),16,18,21(39),22,24,27(38),28,30,33,35-octadecaene 12:
White solid (0.76 g, 47%). mp 291 °C (decomp.). 19F NMR (OC(CD3)2): δ −117.0 (m, 4F, F-37,38,40,41), −112.4 (m, 4F, F-11,17,29,35), −55.8 (d, 12F, JCF3(10)-F(11) = 26 Hz, CF3(10,18,28,36)), −53.1 (d, 12F, JCF3(12)-F(11) = 34 Hz, CF3(12,16,30,34)). 1H NMR (OC(CD3)2): δ 6.14 (m, 2H, H-39,42), 6.85 (dd, 4H, JH(4)-H(5) = 8.4 Hz, JH(4)-H(42) = 2.3 Hz, H-4,6,22,24), 7.38 (t, 2H, JH(5)-H(4,6) = 8.4 Hz, H-5,23). 13C NMR (OC(CD3)2): δ 102.7 (C-39,42), 111.8 (C-4,6,22,24), 116.5 (qd, 2JCF = 33.4 Hz, 2JCF = 15.6 Hz, C–CF3), 118.3 (qd, 2JCF = 32.7 Hz, 2JCF = 13.2 Hz, C–CF3), 121.8 (q, 1JCF = 275.0 Hz, CF3), 122.8 (q, 1JCF = 276.4 Hz, CF3), 128,7 (d, 2JCF = 18.5 Hz, C–S), 132.5 (C-5,23), 144.3 (dd, 2JCF = 17.8 Hz, JCF = 4.8 Hz, C(1,9,19,27)-0), 152.8 (d, 1JCF = 249.1 Hz, C–F), 155.6 (d, 1JCF = 268.9 Hz, C–F), 158.7 (C(3,7,21,25)-0). Anal. Calcd for C44H8F32O4S2*CCl4: C, 37.89; H, 0.57; F, 42.62; Cl, 9.94; S, 4.50%; M 1272. Found: C, 37.80; H, 0.70; F, 42.68; Cl, 9.96; S, 4.79%; M 1272.
3.7. Synthesis of Tetraoxathiacalixarene 15
Thiourea (0.23 g, 3 mmol) was added to a stirred solution of 1.08 g (1.1 mmol) of pentaphenyl ether
13 in 50 mL of dimethylformamide. The solution was stirred for 15 h at 90 °C, and the solvent was evaporated in vacuum. By column chromatography on silica gel using CCl
4 as eluent, 0.99 g of the viscous material containing (GC–MS) 61% tetraoxathiacalixarene
15, 20% pentaphenylether
13, and 17% dimethylformamide was isolated. Double-play crystallization of viscous material from CCl
4 gave 0.36 g of tetraoxathiacalix[
5]arene
15.
11,23,29,31,32,34-Hexafluoro-10,12,22,24,28,30-hexakis(trifluoromethyl)-2,8,14,20-tetraoxa-26- thiahexacyclo[25.3.1.13,7.19,13.115,19.121,25]pentatriaconta-1(31),3(35),4,6,9(34),10,12,15(33),16,18,21(32),22,24,27,29-pentadecaene 15:
White solid (0.36 g, 33%). mp 194 °C (decomp.). 19F NMR (OC(CD3)2): δ −134.2 (d, 1F, JF(34)-F(11) = 12 Hz, F-34), −116.4 (septet d, 1F, JF(11)-CF3(10,12) = 29 Hz, JF(11)-F(34) = 12 Hz, F-11), −116.0 (d, 2F, JF(31)-F(29) = 10 Hz, F-31,32), −113.3 (unresolved m, 2F, F-23,29), −55.9 (d, 6F, J = 27 Hz, 2CF3), −55.0 (d, 6F, J = 29 Hz, 2CF3), −53.2 (d, 6F, J = 30 Hz, 2CF3). 1H NMR (OC(CD3)2): δ 6.92 (m, 1H, H-33,35), 6.94 (dd, 2H, JH(4)-H(5) = 8.4 Hz, JH(4)-H(35) = 1.4 Hz, H-4,18), 7.01 (dd, 2H, JH(6)-H(5) = 8.1 Hz, JH(6)-H(35) = 1.5 Hz, H-6,16), 7.38 (dd, 2H, J = 8.4 Hz, J = 8.1 Hz, H-5,17). 13C NMR (OC(CD3)2): δ 105.7 (C-33,35), 109.0 (qd, 2JCF = 33.1 Hz, 2JCF = 14.5 Hz, C–CF3), 112.9 (C-4,18), 113.4 (C-6,16), 116.5 (qd, 2JCF = 33.1 Hz, 2JCF = 15.4 Hz, C–CF3), 118.1 (qd, 2JCF = 33.1 Hz, 2JCF = 13.0 Hz, C–CF3), 121.7 (q, 1JCF = 274.4 Hz, CF3), 121.8 (q, 1JCF = 274.0 Hz, CF3), 122.0 (q, 1JCF = 276.0 Hz, CF3), 126,7 (d, 2JCF = 20.9 Hz, C–S), 131.3 (C-5,17), 143.7 (dd, 1JCF = 254.9 Hz, JCF = 4.2 Hz, C–F), 144,6 (d, 2JCF = 16.3 Hz, C–O), 144,7 (d, 2JCF = 18.0 Hz, C–O), 153.1 (dd, 1JCF = 251.3.1 Hz, JCF = 2.2 Hz, C–F), 154.3 (d, 1JCF = 264.8 Hz, C–F), 154.7 (d, 1JCF = 267.9 Hz, C–F),157.7 (C-0), 158.4 (C-0). Anal. Calcd for C36H8F24O4S: C, 43.57; H, 0.81; F, 45.94; S, 3.23%; M 992. Found: C, 43.30; H, 0.99; F, 45.82; S, 3.30%; M 994.