Substituent Effects on the Solubility and Electronic Properties of the Cyanine Dye Cy5: Density Functional and Time-Dependent Density Functional Theory Calculations
Abstract
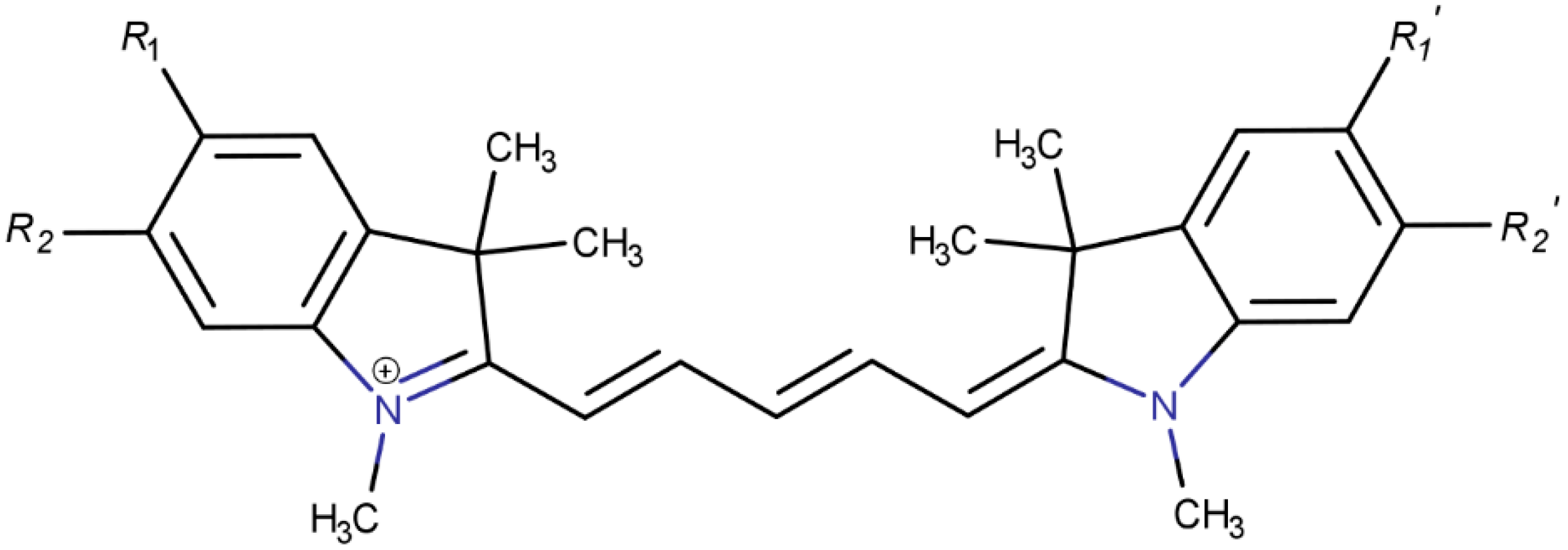
1. Introduction
2. Computational Methods
3. Results
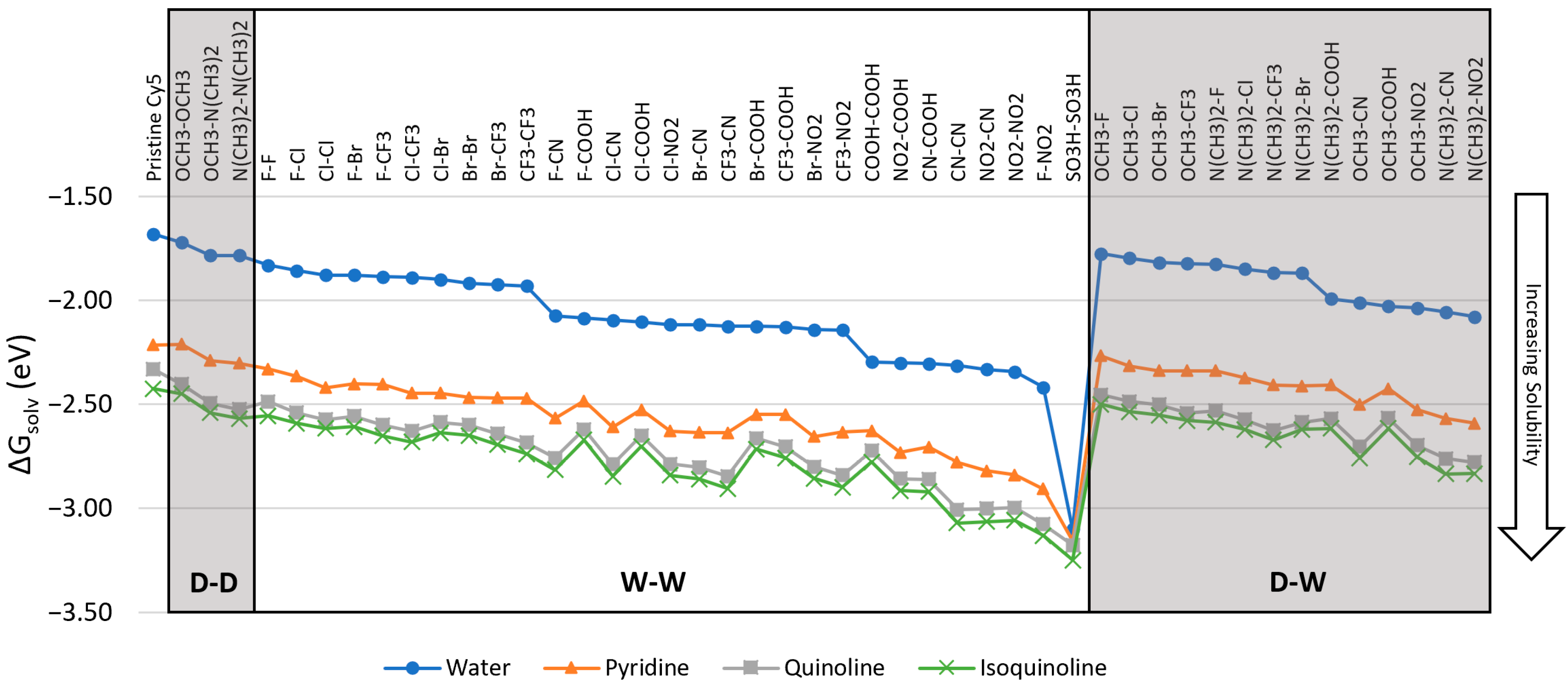
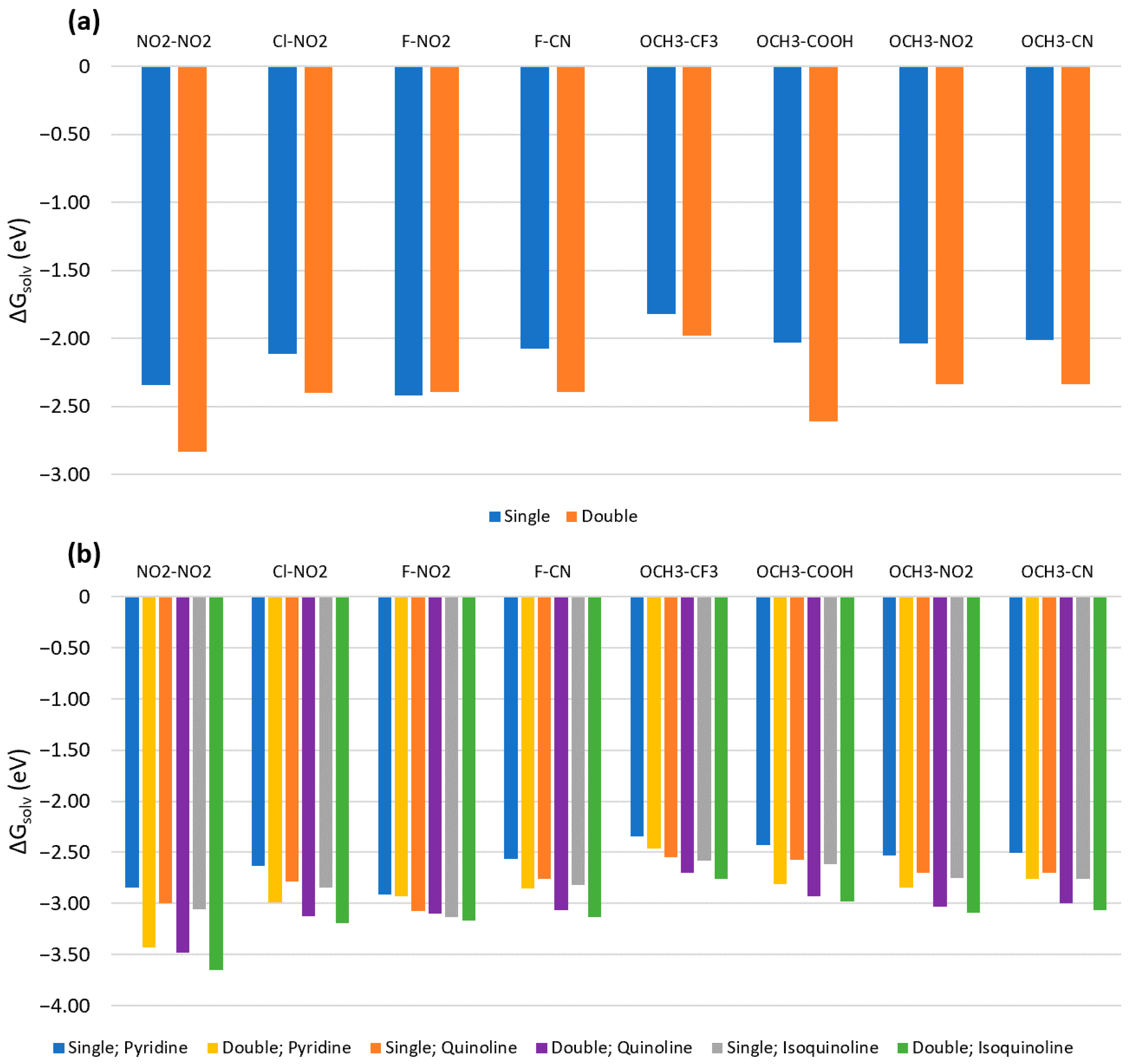
3.1. Solvation Energies
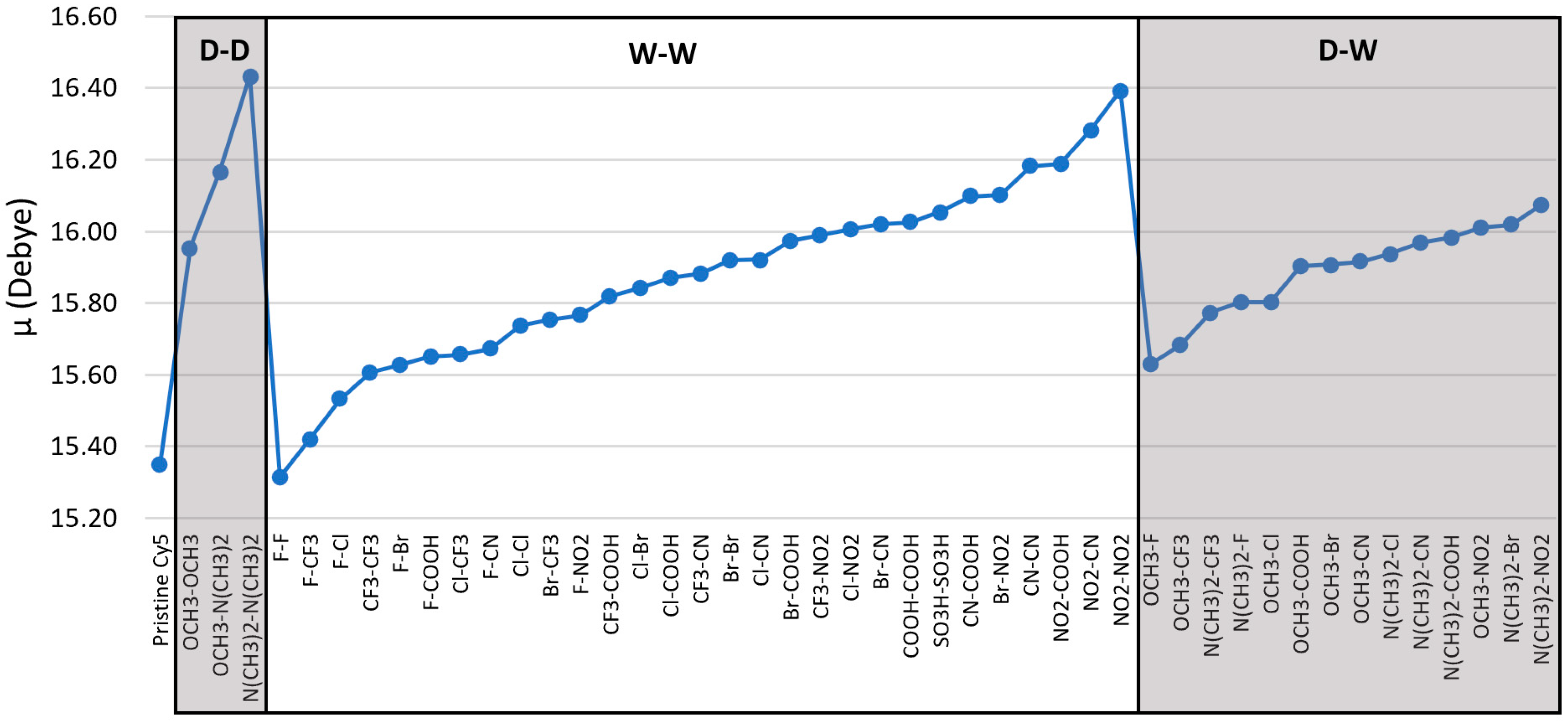
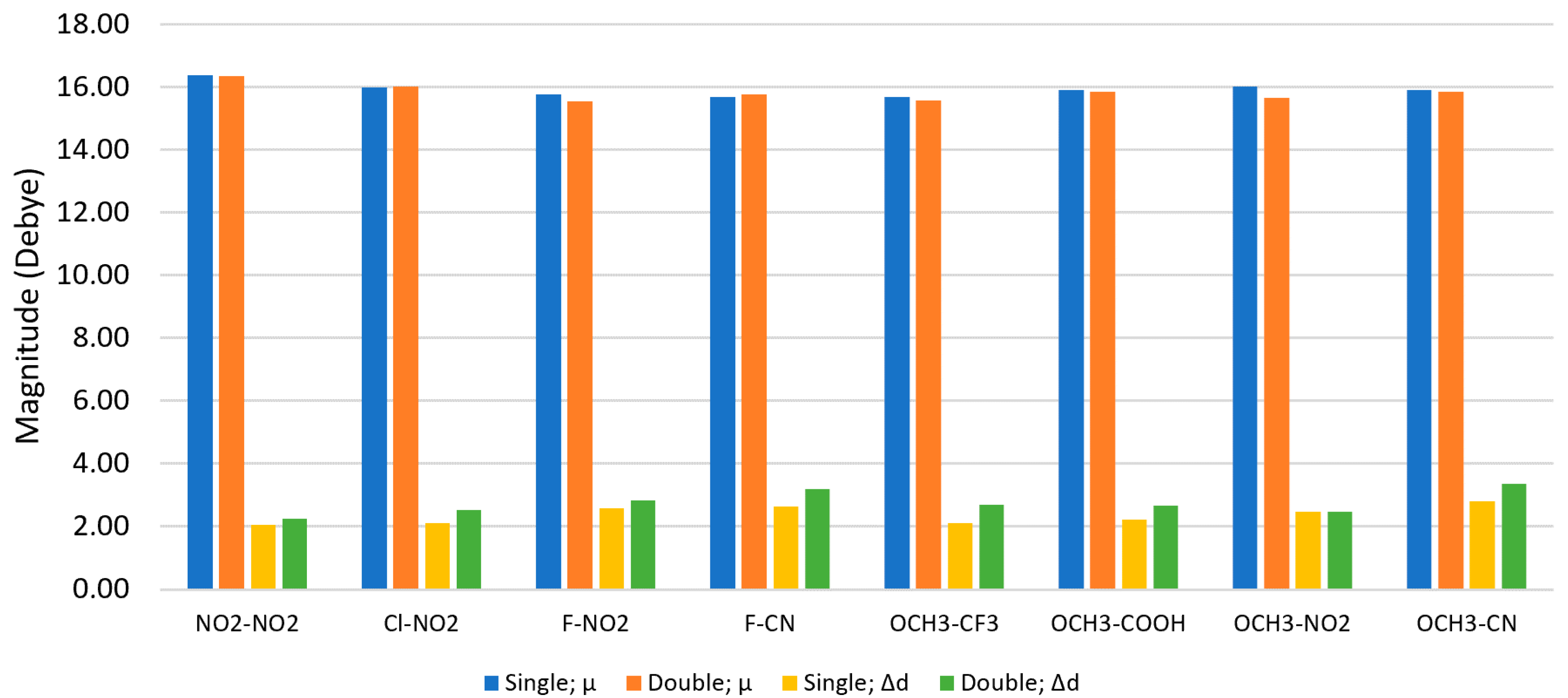
3.2. Dipole Moments
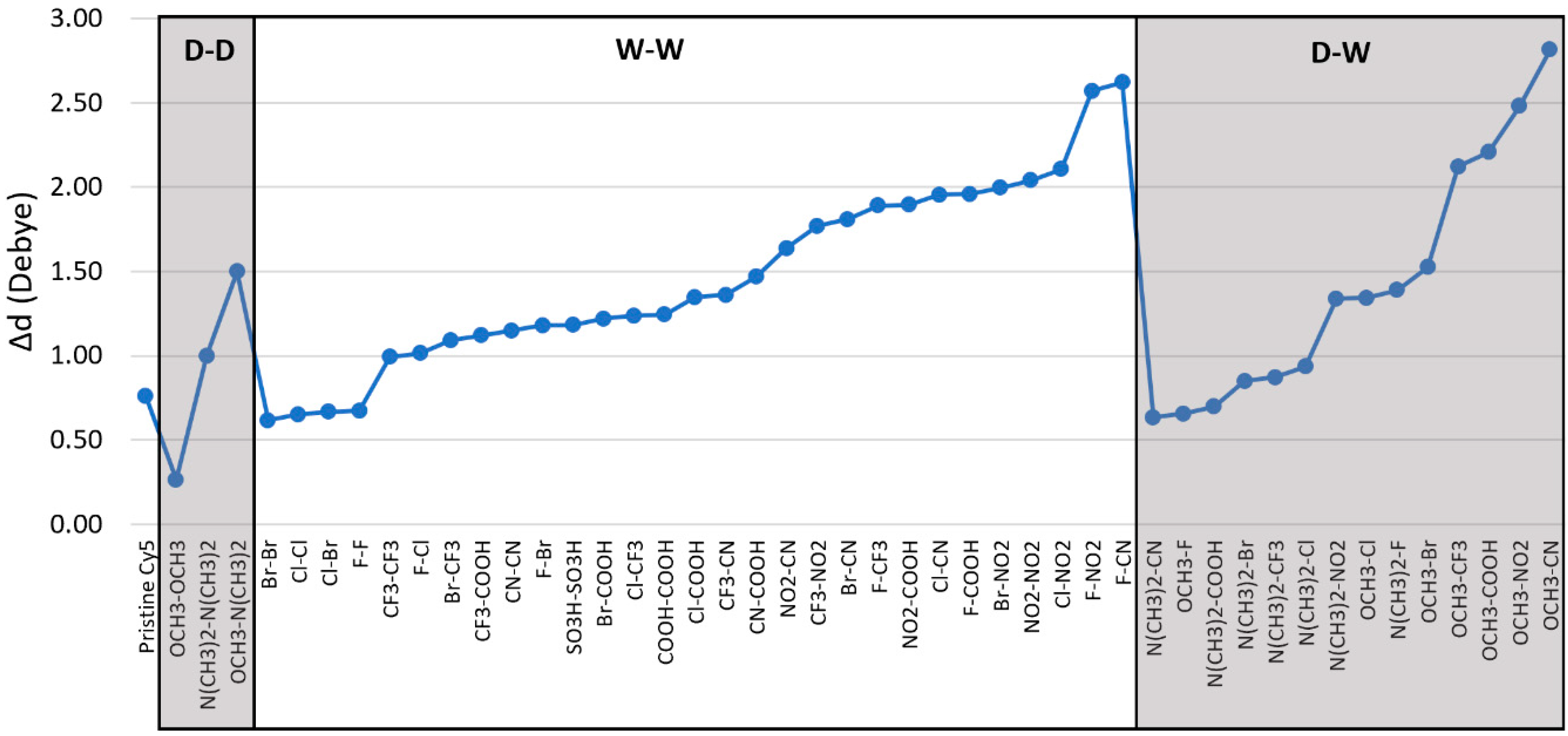
3.3. Double Substituents
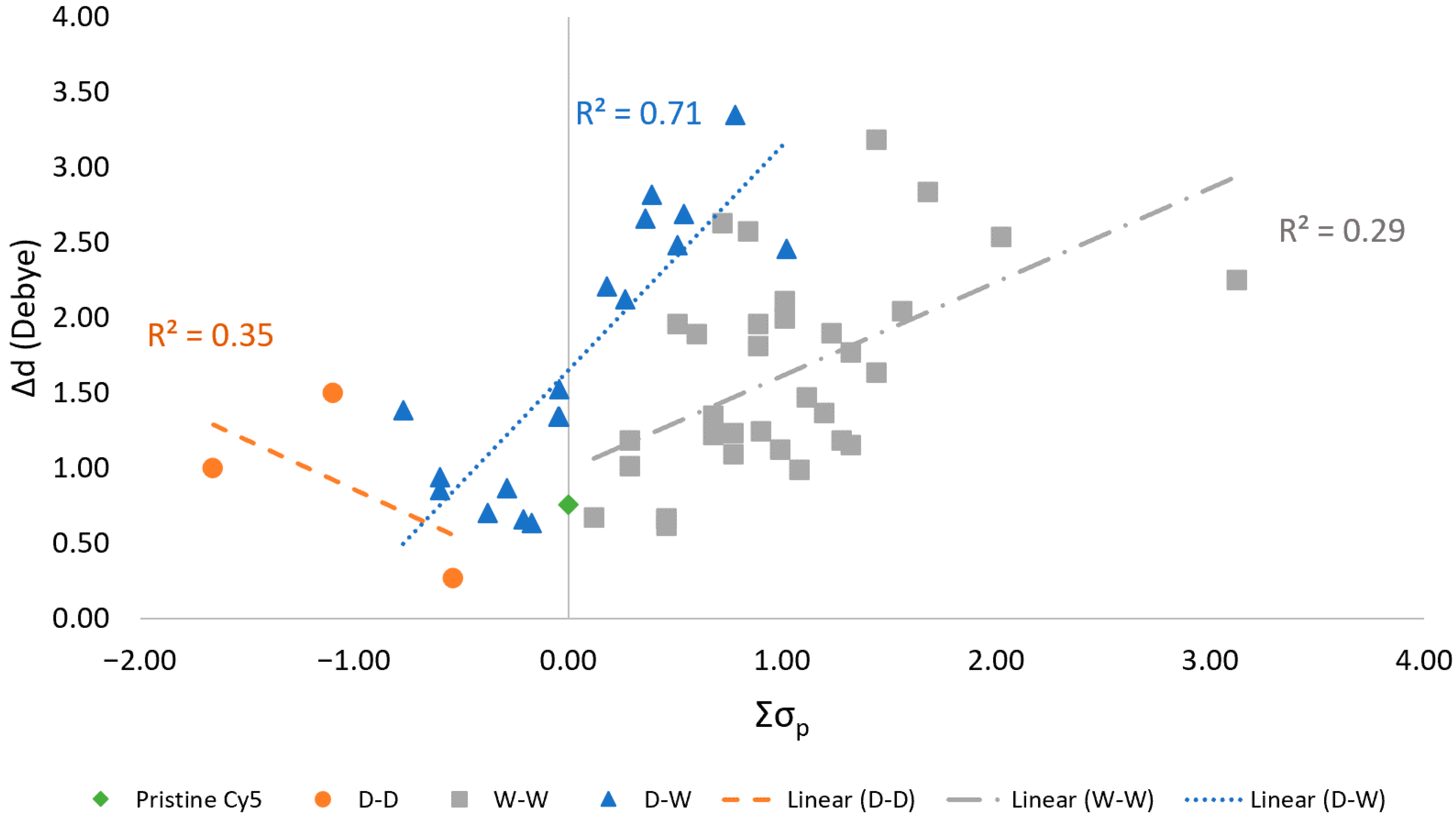
3.4. Relationships with Hammett Constants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Monshouwer, R.; Abrahamsson, M.; Van Mourik, F.; Van Grondelle, R. Superradiance and Exciton Delocalization in Bacterial Photosynthetic Light-Harvesting Systems. J. Phys. Chem. B 1997, 101, 7241–7248. [Google Scholar] [CrossRef]
- Engel, G.S.; Calhoun, T.R.; Read, E.L.; Ahn, T.K.; Mančal, T.; Cheng, Y.C.; Blankenship, R.E.; Fleming, G.R. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 2007, 446, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; Van Grondelle, R.; Govindjee; Scholes, G.D. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Kim, P.; Yoon, M.C.; Sung, J.; Dehm, V.; Chen, Z.; Würthner, F.; Kim, D. Exciton delocalization and dynamics in helical π-stacks of self-assembled perylene bisimides. Chem. Sci. 2013, 4, 388–397. [Google Scholar] [CrossRef]
- Bialas, D.; Zitzler-Kunkel, A.; Kirchner, E.; Schmidt, D.; Würthner, F. Structural and quantum chemical analysis of exciton coupling in homo-and heteroaggregate stacks of merocyanines. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.; Kim, W.; Nowak-Król, A.; Hong, Y.; Kim, D.; Würthner, F. Ultrafast Exciton Delocalization, Localization, and Excimer Formation Dynamics in a Highly Defined Perylene Bisimide Quadruple π-Stack. J. Am. Chem. Soc. 2018, 140, 4253–4258. [Google Scholar] [CrossRef]
- Cannon, B.L.; Kellis, D.L.; Patten, L.K.; Davis, P.H.; Lee, J.; Graugnard, E.; Yurke, B.; Knowlton, W.B. Coherent Exciton Delocalization in a Two-State DNA-Templated Dye Aggregate System. J. Phys. Chem. A 2017, 121, 6905–6916. [Google Scholar] [CrossRef]
- Cannon, B.L.; Patten, L.K.; Kellis, D.L.; Davis, P.H.; Lee, J.; Graugnard, E.; Yurke, B.; Knowlton, W.B. Large Davydov Splitting and Strong Fluorescence Suppression: An Investigation of Exciton Delocalization in DNA-Templated Holliday Junction Dye Aggregates. J. Phys. Chem. A 2018, 122, 2086–2095. [Google Scholar] [CrossRef]
- Jelley, E.E. Spectral absorption and fluorescence of dyes in the molecular state. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- Kasha, M. Energy Transfer Mechanisms and the Molecular Exciton Model for Molecular Aggregates. Radiat. Res. 1963, 20, 55–70. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; Ashraf El-Bayoumi, M. The Exciton Model in Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-aggregates: From serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chemie Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Nüesch, F.; Grätzel, M. H-aggregation and correlated absorption and emission of a merocyanine dye in solution, at the surface and in the solid state. A link between crystal structure and photophysical properties. Chem. Phys. 1995, 193, 1–17. [Google Scholar] [CrossRef]
- Davydov, A.S. The Theory of Molecular Excitons. Sov. Phys. Uspekhi 1964, 7, 393–448. [Google Scholar] [CrossRef]
- Pajusalu, M.; Rätsep, M.; Trinkunas, G.; Freiberg, A. Davydov Splitting of Excitons in Cyclic Bacteriochlorophyll a Nanoaggregates of Bacterial Light-Harvesting Complexes between 4.5 and 263 K. ChemPhysChem 2011, 12, 634–644. [Google Scholar] [CrossRef]
- Collado-Fregoso, E.; Boufflet, P.; Fei, Z.; Gann, E.; Ashraf, S.; Li, Z.; McNeill, C.R.; Durrant, J.R.; Heeney, M.; Ashraf, R.S. Increased Exciton Dipole Moment Translates into Charge-Transfer Excitons in Thiophene-Fluorinated Low-Bandgap Polymers for Organic Photovoltaic Applications. Chem. Mater. 2015, 27, 7934–7944. [Google Scholar] [CrossRef]
- Markov, R.V.; Plekhanov, A.I.; Shelkovnikov, V.V.; Knoester, J. Giant Nonlinear Optical Response of Interacting One-Dimensional Frenkel Excitons in Molecular Aggregates. Phys. Status Solidi 2000, 221, 529–533. [Google Scholar] [CrossRef]
- Cannon, B.L.; Kellis, D.L.; Davis, P.H.; Lee, J.; Kuang, W.; Hughes, W.L.; Graugnard, E.; Yurke, B.; Knowlton, W.B. Excitonic AND Logic Gates on DNA Brick Nanobreadboards. ACS Photonics 2015, 2, 398–404. [Google Scholar] [CrossRef]
- Kellis, D.L.; Sarter, C.; Cannon, B.L.; Davis, P.H.; Graugnard, E.; Lee, J.; Pensack, R.D.; Kolmar, T.; Jäschke, A.; Yurke, B.; et al. An All-Optical Excitonic Switch Operated in the Liquid and Solid Phases. ACS Nano 2019, 13, 2986–2994. [Google Scholar] [CrossRef]
- Abramavicius, D.; Palmieri, B.; Mukamel, S. Extracting single and two-exciton couplings in photosynthetic complexes by coherent two-dimensional electronic spectra. Chem. Phys. 2009, 357, 79–84. [Google Scholar] [CrossRef]
- Markova, L.I.; Malinovskii, V.L.; Patsenker, L.D.; Häner, R. J- vs. H-type assembly: Pentamethine cyanine (Cy5) as a near-IR chiroptical reporter. Chem. Commun. 2013, 49, 5298. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.D.; Kim, Y.C.; Díaz, S.A.; Buckhout-White, S.; Mathur, D.; Medintz, I.L.; Melinger, J.S. Optical Properties of Vibronically Coupled Cy3 Dimers on DNA Scaffolds. J. Phys. Chem. B 2018, 122, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Roos, M.K.; Hemmig, E.A.; Di Antonio, M.; de Vivie-Riedle, R.; Liedl, T. Proximity-Induced H-Aggregation of Cyanine Dyes on DNA-Duplexes. J. Phys. Chem. A 2016, 120, 9941–9947. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.S.; Davis, P.H.; Christy, A.; Kellis, D.L.; Kandadai, N.; Toa, Z.S.D.; Scholes, G.D.; Yurke, B.; Knowlton, W.B.; Pensack, R.D. DNA-Templated Aggregates of Strongly Coupled Cyanine Dyes: Nonradiative Decay Governs Exciton Lifetimes. J. Phys. Chem. Lett. 2019, 10, 2386–2392. [Google Scholar] [CrossRef]
- Misawa, K.; Minoshima, K.; Ono, H.; Kobayashi, T. Giant static dipole moment change on electronic excitation in highly oriented J-aggregates. Chem. Phys. Lett. 1994, 220, 251–256. [Google Scholar] [CrossRef]
- Marder, S.R.; Torruellas, W.E.; Blanchard-Desce, M.; Ricci, V.; Stegeman, G.I.; Gilmour, S.; Brédas, J.L.; Li, J.; Bublitz, G.U.; Boxer, S.G. Large molecular third-order optical nonlinearities in polarized carotenoids. Science (80-) 1997, 276, 1233–1236. [Google Scholar] [CrossRef]
- Garoff, R.A.; Litzinger, E.A.; Connor, R.E.; Fishman, I.; Armitage, B.A. Helical Aggregation of Cyanine Dyes on DNA Templates: Effect of Dye Structure on Formation of Homo-and Heteroaggregates. Langmuir 2002, 18, 6330–6337. [Google Scholar] [CrossRef]
- Stadler, A.L.; Renikuntla, B.R.; Yaron, D.; Fang, A.S.; Armitage, B.A. Substituent effects on the assembly of helical cyanine dye aggregates in the minor groove of a DNA template. Langmuir 2011, 27, 1472–1479. [Google Scholar] [CrossRef]
- Fothergill, J.W.; Hernandez, A.C.; Knowlton, W.B.; Yurke, B.; Li, L. Ab Initio Studies of Exciton Interactions of Cy5 Dyes. J. Phys. Chem. A 2018, 122, 8989–8997. [Google Scholar] [CrossRef]
- Cao, J.; Fan, J.; Sun, W.; Guo, Y.; Wu, H.; Peng, X. The photoprocess effects of an amino group located at different positions along the polymethine chain in indodicarbocyanine dyes. RSC Adv. 2017, 7, 30740–30746. [Google Scholar] [CrossRef]
- Abou-Hatab, S.; Spata, V.A.; Matsika, S. Substituent Effects on the Absorption and Fluorescence Properties of Anthracene. J. Phys. Chem. A 2017, 121, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Naik, L.R.; Kumar, S.; Math, N.N. Steady-state absorption and fluorescence study: Dipole moments of coumarins. Indian J. Pure Appl. Phys. 2006, 44, 292–299. [Google Scholar]
- Bridhkoti, J.P.; Gahlaut, R.; Joshi, H.C.; Pant, S. Effect of positional substitution of amino group on excited state dipole moments of quinoline. J. Lumin. 2011, 131, 1869–1873. [Google Scholar] [CrossRef]
- Jacquemin, D.; Zhao, Y.; Valero, R.; Adamo, C.; Ciofini, I.; Truhlar, D.G. Verdict: Time-dependent density functional theory “not guilty” of large errors for cyanines. J. Chem. Theory Comput. 2012, 8, 1255–1259. [Google Scholar] [CrossRef]
- Jacquemin, D. Excited-State Dipole and Quadrupole Moments: TD-DFT versus CC2. J. Chem. Theory Comput. 2016, 12, 3993–4003. [Google Scholar] [CrossRef]
- Kawauchi, S.; Antonov, L.; Okuno, Y. Prediction of the color of dyes by using time-dependent density functional theory (TD-DFT). Bulg. Chem. Commun. 2014, 46, 228–237. [Google Scholar]
- Laine, M.; Barbosa, N.A.; Wieczorek, R.; Melnikov, M.Y.; Filarowski, A. Calculations of BODIPY dyes in the ground and excited states using the M06-2X and PBE0 functionals. J. Mol. Model. 2016, 22, 1–7. [Google Scholar] [CrossRef]
- García, G.; Adamo, C.; Ciofini, I. Evaluating push-pull dye efficiency using TD-DFT and charge transfer indices. Phys. Chem. Chem. Phys. 2013, 15, 20210–20219. [Google Scholar] [CrossRef]
- Kerkines, I.S.K.; Petsalakis, I.D.; Argitis, P.; Theodorakopoulos, G. Fluorescence properties of organic dyes: Quantum chemical studies on the green/blue neutral and protonated DMA-DPH emitters in polymer matrices. Phys. Chem. Chem. Phys. 2011, 13, 21273–21281. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Yesudas, K. Cationic cyanine dyes: Impact of symmetry-breaking on optical absorption and third-order polarizabilities. Phys. Chem. Chem. Phys. 2013, 15, 19465–19477. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, Y.; Xu, Y.; Xu, F.; Zhang, Q.; Lu, X. Theoretical study of novel porphyrin D-π-A conjugated organic dye sensitizer in solar cells. Mater. Chem. Phys. 2019, 225, 417–425. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A. 03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J. GaussView Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Rauf, S.M.A.; Arvidsson, P.I.; Albericio, F.; Govender, T.; Maguire, G.E.M.; Kruger, H.G.; Honarparvar, B. The effect of N-methylation of amino acids (Ac-X-OMe) on solubility and conformation: A DFT study. Org. Biomol. Chem. 2015, 13, 9993–10006. [Google Scholar] [CrossRef]
- Mananghaya, M.R.; Santos, G.N.; Yu, D.N. Solubility of amide functionalized single wall carbon nanotubes: A quantum mechanical study. J. Mol. Liq. 2017, 242, 1208–1214. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to Isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Budzák, Š.; Medved, M.; Mennucci, B.; Jacquemin, D. Unveiling solvents effect on excited-state polarizabilities with the corrected linear-response model. J. Phys. Chem. A 2014, 118, 5652–5656. [Google Scholar] [CrossRef]
- Selvam, K.; Gandhi, S.; Krishnamurty, S.; Gopalakrishnan, G. Effect of substitution on the excited state photophysical and spectral properties of boron difluoride curcumin complex dye and their derivatives: A time dependent-DFT study. J. Photochem. Photobiol. B Biol. 2019, 199, 111595. [Google Scholar] [CrossRef]
- Heid, E.; Hunt, P.A.; Schröder, C. Evaluating excited state atomic polarizabilities of chromophores. Phys. Chem. Chem. Phys. 2018, 20, 8554–8563. [Google Scholar] [CrossRef] [PubMed]
- Bamgbelu, A.; Wang, J.; Leszczynski, J. TDDFT study of the optical properties of Cy5 and its derivatives. J. Phys. Chem. A 2010, 114, 3551–3555. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Imaizumi, H.; Koyanagi, T.; Zhao, D. Reactivity of sulfonic acid group and estimation of its substituent-effect in T-for-H exchange reaction. J. Radioanal. Nucl. Chem. 2002, 252, 467–472. [Google Scholar] [CrossRef]
- Paschoal, D.; Santos, H.F. Dos Assessing the quantum mechanical level of theory for prediction of linear and nonlinear optical properties of push-pull organic molecules. J. Mol. Model 2013, 19, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Stennett, E.M.S.; Ma, N.; van der Vaart, A.; Levitus, M. Photophysical and Dynamical Properties of Doubly Linked Cy3–DNA Constructs. J. Phys. Chem. B 2014, 118, 152–163. [Google Scholar] [CrossRef]
- Yang, S.K.; Shi, X.; Park, S.; Ha, T.; Zimmerman, S.C. A dendritic single-molecule fluorescent probe that is monovalent, photostable and minimally blinking. Nat. Chem. 2013, 5, 692–697. [Google Scholar] [CrossRef]
- Liu, W.; McGarraugh, H.H.; Smith, B.D. Fluorescent thienothiophene-containing squaraine dyes and threaded supramolecular complexes with tunable wavelengths between 600–800 nm. Molecules 2018, 23, 2229. [Google Scholar] [CrossRef]
- Toutchkine, A.; Nalbant, P.; Hahn, K.M. Facile synthesis of thiol-reactive Cy3 and Cy5 derivatives with enhanced water solubility. Bioconjug. Chem. 2002, 13, 387–391. [Google Scholar] [CrossRef]
- Kang, J.; Kaczmarek, O.; Liebscher, J.; Dähne, L. Prevention of H-aggregates formation in Cy5 labeled macromolecules. Int. J. Polym. Sci. 2010, 2010. [Google Scholar] [CrossRef]
- Markova, L.I.; Terpetschnig, E.A.; Patsenker, L.D. Comparison of a series of hydrophilic squaraine and cyanine dyes for use as biological labels. Dye. Pigment. 2013, 99, 561–570. [Google Scholar] [CrossRef]
- Karaca, S.; Elmaci, N. A theoretical study on the ground and excited state behaviors of TTBC related carbocyanine dyes. J. Mol. Struct. Theochem. 2009, 915, 149–159. [Google Scholar] [CrossRef][Green Version]
| Substituent | σp | Classification |
|---|---|---|
| N(CH3)2 | −0.83 a | Donating |
| OCH3 | −0.27 a | |
| F | 0.06 a | Withdrawing |
| Cl | 0.23 a | |
| Br | 0.23 a | |
| COOH | 0.45 a | |
| CF3 | 0.54 a | |
| SO3H | 0.64 b | |
| CN | 0.66 a | |
| NO2 | 0.78 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biaggne, A.; Knowlton, W.B.; Yurke, B.; Lee, J.; Li, L. Substituent Effects on the Solubility and Electronic Properties of the Cyanine Dye Cy5: Density Functional and Time-Dependent Density Functional Theory Calculations. Molecules 2021, 26, 524. https://doi.org/10.3390/molecules26030524
Biaggne A, Knowlton WB, Yurke B, Lee J, Li L. Substituent Effects on the Solubility and Electronic Properties of the Cyanine Dye Cy5: Density Functional and Time-Dependent Density Functional Theory Calculations. Molecules. 2021; 26(3):524. https://doi.org/10.3390/molecules26030524
Chicago/Turabian StyleBiaggne, Austin, William B. Knowlton, Bernard Yurke, Jeunghoon Lee, and Lan Li. 2021. "Substituent Effects on the Solubility and Electronic Properties of the Cyanine Dye Cy5: Density Functional and Time-Dependent Density Functional Theory Calculations" Molecules 26, no. 3: 524. https://doi.org/10.3390/molecules26030524
APA StyleBiaggne, A., Knowlton, W. B., Yurke, B., Lee, J., & Li, L. (2021). Substituent Effects on the Solubility and Electronic Properties of the Cyanine Dye Cy5: Density Functional and Time-Dependent Density Functional Theory Calculations. Molecules, 26(3), 524. https://doi.org/10.3390/molecules26030524







