Photocatalytic Degradation of Single and Binary Mixture of Brilliant Green and Rhodamine B Dyes by Zinc Sulfide Quantum Dots
Abstract
:1. Introduction
2. Results and Discussions
2.1. Structural and Morphological Studies of ZnS Quantum Dots
2.2. Optical Studies of ZnS Quantum Dots
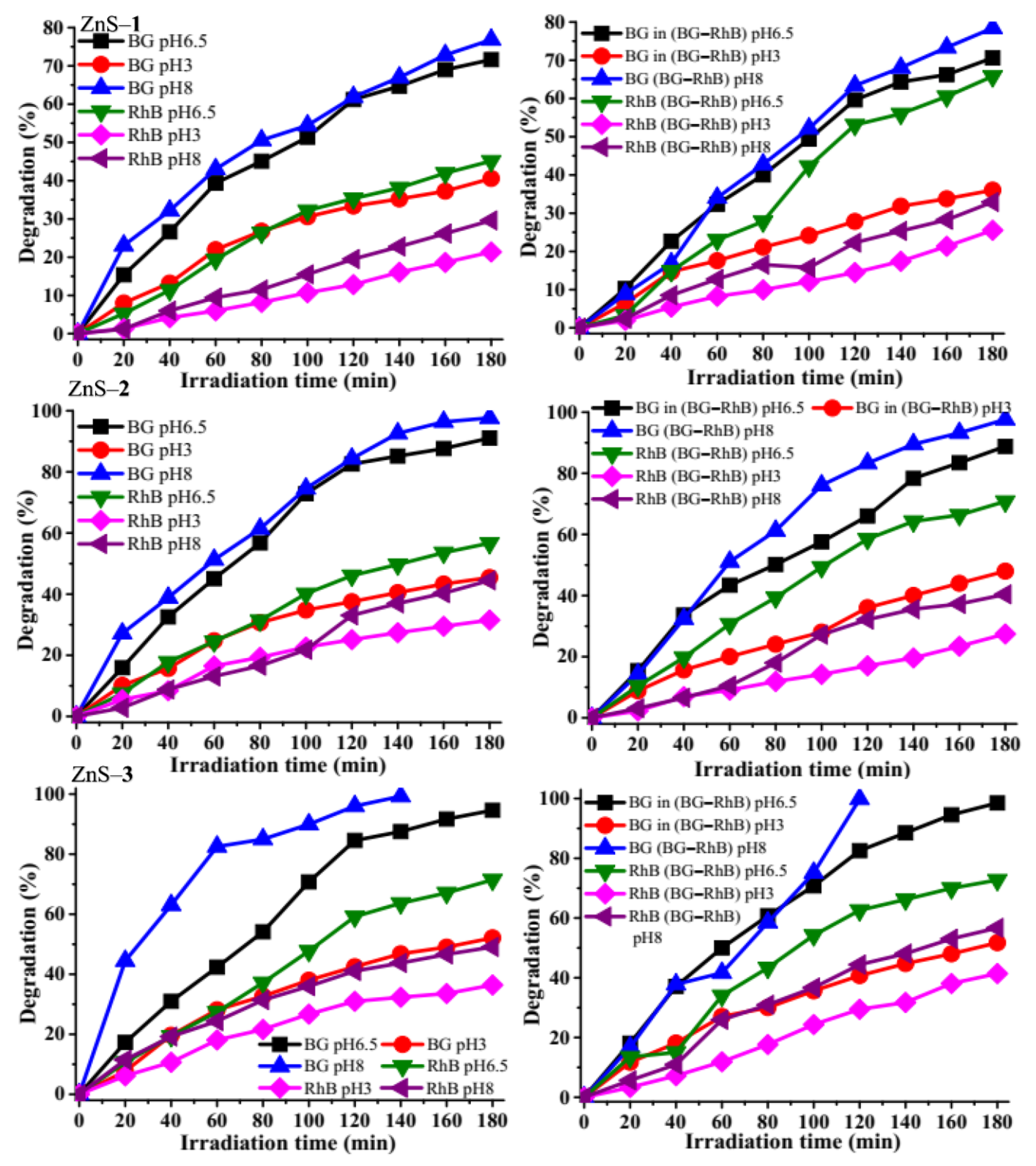
2.3. Photodegradation of Brilliant Green and Rhodamine B Dyes by the ZnS Quantum Dots
2.3.1. Effect of Catalytic Dosage on Photocatalytic Degradation
2.3.2. Effect of Irradiation Time on Photocatalytic Degradation
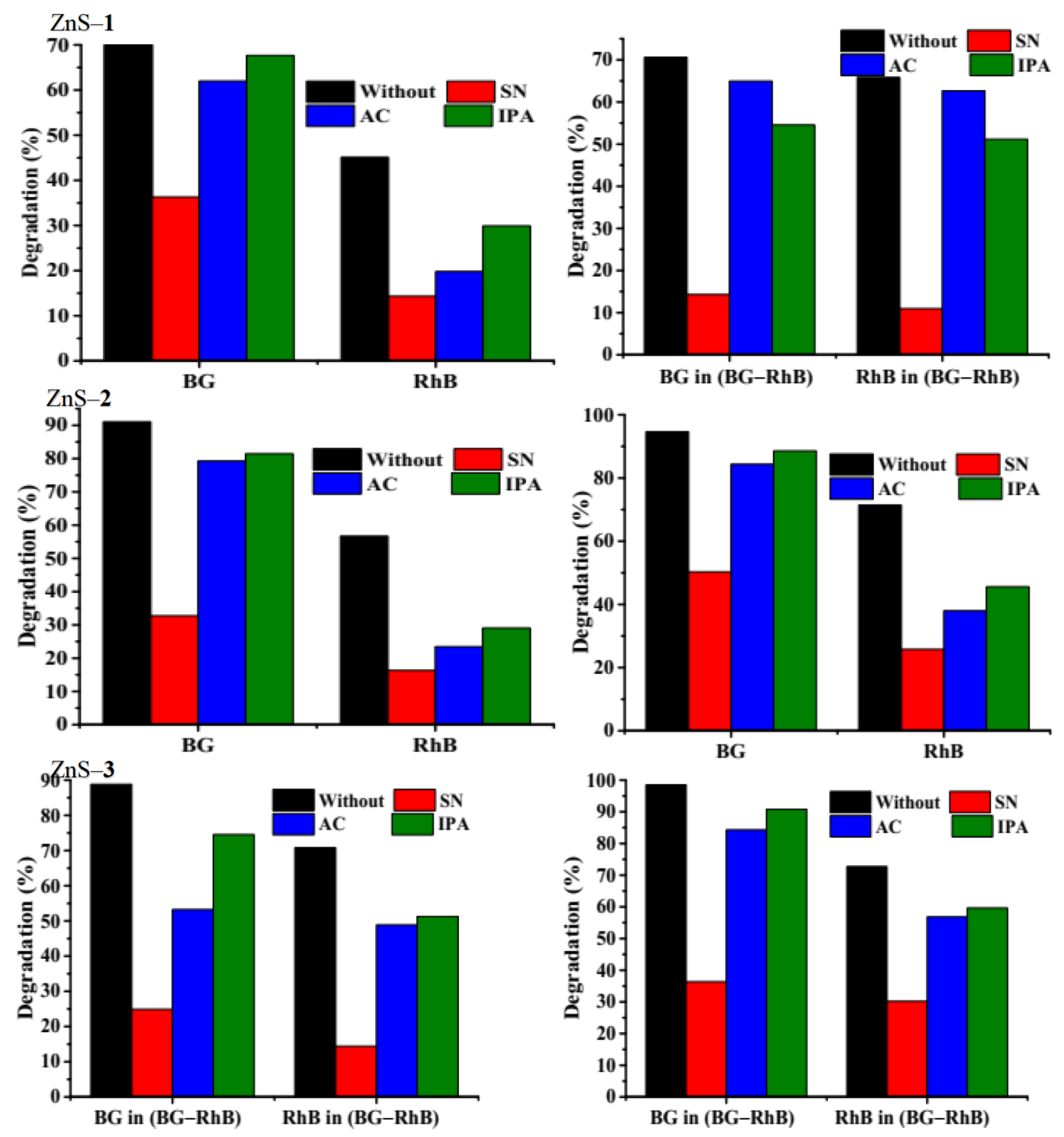
2.3.3. Effect of Scavengers on the Photocatalytic Degradation Efficiency of the Dyes
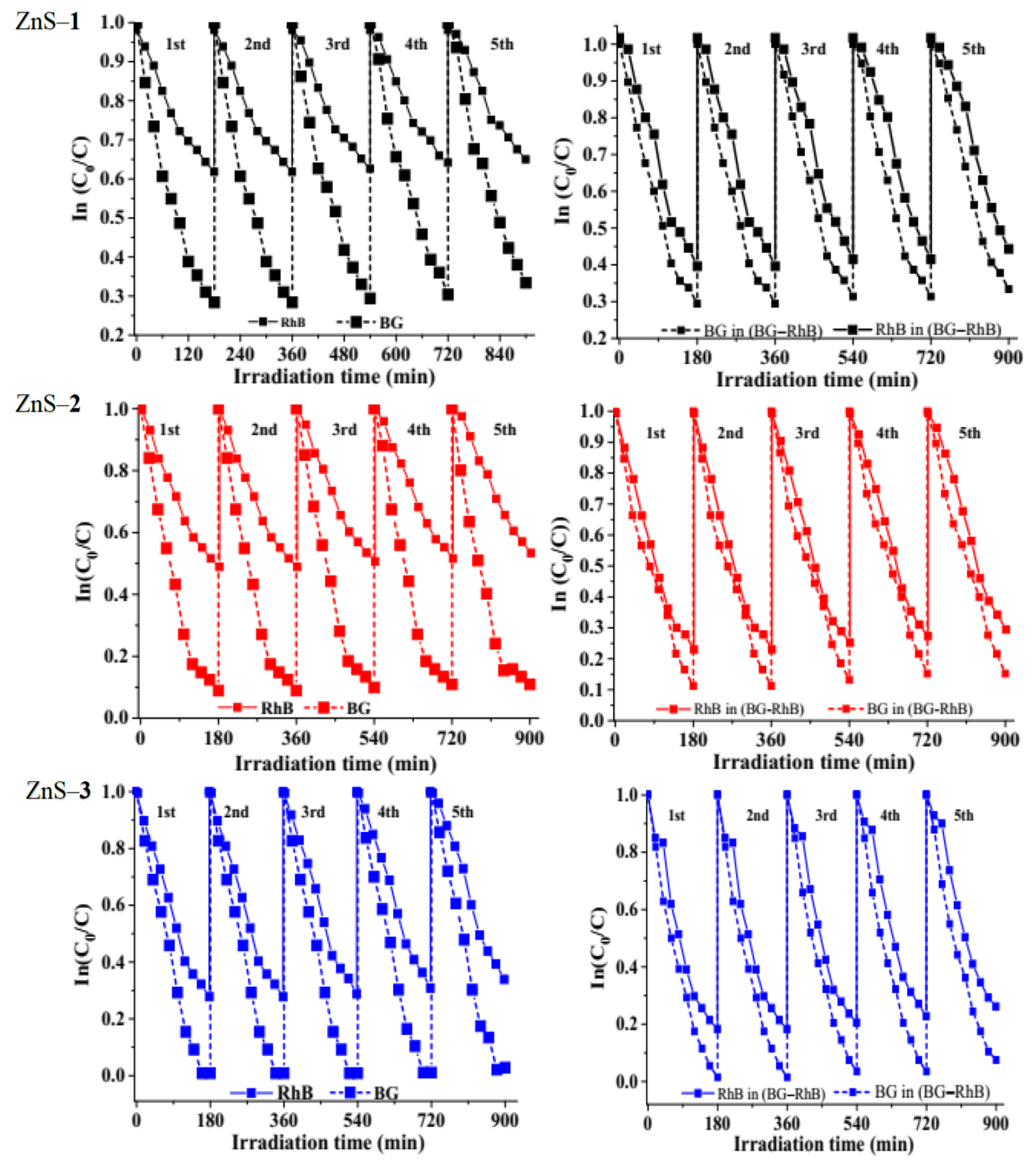
2.3.4. Photostability Studies of the ZnS Quantum Dots
2.3.5. Effect of pH on Photocatalytic Degradation
3. Materials and Methods
3.1. Materials
3.2. Physical Characterization Techniques
3.3. Synthesis of ZnS Quantum Dots
3.4. Photocatalytic Studies of the ZnS Quantum Dots
3.5. Detection of Reactive Oxygen Species Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Electrospinning preparation of NiO/ZnO composite nanofibers for photodegradation of binary mixture of rhodamine B and methylene blue in aqueous solution: Central composite optimization. Appl. Organomet. Chem. 2018, 32, e4335. [Google Scholar] [CrossRef]
- Liu, H.; Guo, W.; Li, Y.; He, S.; He, C. Photocatalytic degradation of sixteen organic dyes by TiO2/WO3-coated magnetic nanoparticles under simulated visible light and solar light. J. Environ. Chem. Eng. 2018, 6, 59–67. [Google Scholar] [CrossRef]
- Wang, W.; Lu, T.; Chen, Y.; Tian, G.; Sharma, V.K.; Zhu, Y.; Zong, L.; Wang, A. Mesoporous silicate/carbon composites derived from dye-loaded palygorskite clay waste for efficient removal of organic contaminants. Sci. Total Environ. 2019, 696, 133955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Y.; Zhu, T.; Wang, A.; Lu, Y.; Lv, L.; Zhang, K.; Li, Z. Enhanced performance, and microbial community analysis of bioelectrochemical system integrated with bio-contact oxidation reactor for treatment of wastewater containing azo dye. Sci. Total Environ. 2018, 634, 616–627. [Google Scholar] [CrossRef]
- Guo, K.; Gao, B.; Tian, X.; Yue, Q.; Zhang, P.; Shen, X.; Xu, X. Synthesis of polyaluminium chloride/papermaking sludge-based organic polymer composites for removal of disperse yellow and reactive blue by flocculation. Chemosphere 2019, 231, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Amanulla, B.; Sannasi, S.; Abubakker, A.K.M.; Ramaraj, S.K. A magnetically recoverable bimetallic Au-FeNPs decorated on g-C3N4 for efficient photocatalytic degradation of organic contaminants. J. Mol. Liq. 2018, 249, 754–763. [Google Scholar] [CrossRef]
- Aziz, A.; Ali, N.; Khan, A.; Bilal, M.; Malik, S.; Ali, N.; Khan, H. Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes. Int. J. Biol. Macromol. 2020, 153, 502–512. [Google Scholar] [CrossRef] [PubMed]
- López-Vinent, N.; Cruz-Alcalde, A.; Romero, L.; Chávez, M.; Marco, P.; Giménez, J.; Esplugas, S. Synergies, radiation and kinetics in photo-Fenton process with UVA-LEDs. J. Hazard. Mater. 2019, 380, 120882. [Google Scholar] [CrossRef]
- Dias, N.C.; Bassin, J.P.; Sant’Anna, G.L., Jr.; Dezotti, M. Ozonation of the dye reactive red 239 and biodegradation of ozonation products in a moving-bed biofilm reactor: Revealing reaction products and degradation pathways. Int. Biodeterior. Biodegrad. 2019, 144, 104742. [Google Scholar] [CrossRef]
- Innocenzi, V.; Prisciandaro, M.; Centofanti, M.; Vegliò, F. Comparison of performances of hydrodynamic cavitation in combined treatments based on hybrid induced advanced fenton process for degradation of azo-dyes. J. Environ. Chem. Eng. 2019, 7, 103171. [Google Scholar] [CrossRef]
- Barrocas, B.; Entradas, T.; Nunes, C.; Monteiro, O. Titanate nanofibers sensitized with ZnS and Ag2S nanoparticles as novel photocatalysts for phenol removal. Appl. Catal. B Environ. 2017, 218, 709–720. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, M.; Wang, C.; Liu, E.; Hu, X.; Fan, J. Defected ZnS/bulk g–C3N4 heterojunction with enhanced photocatalytic activity for dyes oxidation and Cr(VI) reduction. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123861. [Google Scholar] [CrossRef]
- Xiao, J.-H.; Huang, W.-Q.; Hu, Y.-S.; Zeng, F.; Huang, Q.-Y.; Zhou, B.-X.; Pan, A.; Li, K.; Huang, G.-F. Facile in situ synthesis of wurtzite ZnS/ZnO core/shell heterostructure with highly efficient visible-light photocatalytic activity and photostability. J. Phys. D Appl. Phys. 2018, 51, 075501. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Zhang, J.; Zhong, W.; Huang, X. Cu2−xSe/CdS composite photocatalyst with enhanced visible light photocatalysis activity. Appl. Surf. Sci. 2019, 478, 762–769. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K. ZnO nanostructures for photocatalytic dye degradation under visible light irradiation. In Environmental Nanotechnology for Water Purification; Scrivener Publ. LLC: Beverly, MA, USA, 2020; pp. 259–284. [Google Scholar]
- Jiang, Y.; Weiss, E.A. Colloidal quantum dots as photocatalysts for triplet excited state reactions of organic molecules. J. Am. Chem. Soc. 2020, 142, 15219–15229. [Google Scholar] [CrossRef] [PubMed]
- Que, Q.; Xing, Y.; He, Z.; Yang, Y.; Yin, X.; Que, W. Bi2O3/Carbon quantum dots heterostructured photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 220–223. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, H.; Zhang, W.; Cui, Z.; Fu, P.; Liu, M.; Qiao, X.; Pang, X. Size effect of semiconductor quantum dots as photocatalysts for PET-RAFT polymerization. Polym. Chem. 2020, 11, 4961–4967. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhao, J.; Wang, J.; Li, Z. g-C3N4/B doped g-C3N4 quantum dots heterojunction photocatalysts for hydrogen evolution under visible light. Int. J. Hydrogen Energy 2019, 44, 618–628. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, S.; Tan, T.T.Y.; Xiao, F.-X. Graphene quantum dots (GQDs) and its derivatives for multifarious photocatalysis and photoelectrocatalysis. Catal. Today 2018, 315, 171–183. [Google Scholar] [CrossRef]
- Mansur, A.A.; Mansur, H.S.; Ramanery, F.P.; Oliveira, L.C.; Souza, P.P. “Green” colloidal ZnS quantum dots/chitosan nano-photocatalysts for advanced oxidation processes: Study of the photodegradation of organic dye pollutants. Appl. Catal. B Environ. 2014, 158, 269–279. [Google Scholar] [CrossRef]
- Samanta, D.; Basnet, P.; Chanu, T.I.; Chatterjee, S. Biomolecule assisted morphology-controllable synthesis of zinc sulphide nanomaterials for efficient photocatalytic activity under solar irradiation. J. Alloys Compd. 2020, 844, 155810. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, X.-B.; Yuan, H.; Liu, P.-C.; Lei, Y.-B.; Xu, H.; Du, D.-L.; Sun, J.-F.; Feng, Y.-J. Photocatalytic properties of zinc sulfide nanocrystals biofabricated by metal-reducing bacterium Shewanella oneidensis MR-1. J. Hazard. Mater. 2015, 288, 134–139. [Google Scholar] [CrossRef]
- Osuntokun, J.; Ajibade, P.A.; Onwudiwe, D.C. Synthesis and photocatalytic studies of ZnS nanoparticles from heteroleptic complex of Zn (II) 1-cyano-1-carboethoxy-2, −2-ethylenedithiolato diisopropylthiourea and its adducts with N-donor ligands. Superlattices Microstruct. 2016, 100, 605–618. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Umar, A.; Singh, S.; Mehta, S.; Kansal, S.K. Solar light driven enhanced photocatalytic degradation of brilliant green dye based on ZnS quantum dots. Superlattices Microstruct. 2017, 103, 365–375. [Google Scholar]
- Li, Y.-X.; Fu, H.; Wang, P.; Zhao, C.; Liu, W.; Wang, C.-C. Porous tube-like ZnS derived from rod-like ZIF-L for photocatalytic Cr (VI) reduction and organic pollutants degradation. Environ. Pollut. 2020, 256, 113417. [Google Scholar] [CrossRef]
- Jacob, J.M.; Rajan, R.; Tom, T.C.; Kumar, V.S.; Kurup, G.G.; Shanmuganathan, R.; Pugazhendhi, A. Biogenic design of ZnS quantum dots-Insights into their in-vitro cytotoxicity, photocatalysis and biosensing properties. Ceram. Int. 2019, 45, 24193–24201. [Google Scholar] [CrossRef]
- Selvaganapathi, P.; Thirumaran, S.; Ciattini, S. Structural variations in zinc (II) complexes with N, N-di (4-fluorobenzyl) dithiocarbamate and imines: New precursor for zinc sulfide nanoparticles. Polyhedron 2018, 149, 54–65. [Google Scholar] [CrossRef]
- Kashinath, L.; Namratha, K.; Byrappa, K. Sol-gel assisted hydrothermal synthesis and characterization of hybrid ZnS–RGO nanocomposite for efficient photodegradation of dyes. J. Alloys Compd. 2017, 695, 799–809. [Google Scholar] [CrossRef]
- Ghorai, S.; Patra, N.; Pal, A.; Bhattacharyya, D.; Jha, S.N.; Ray, B.; Chatterjee, S.; Ghosh, A.K. Insights into local atomic structure of Fe alloyed ZnS nano crystals: Correlation with structural, optical, magnetic and photocatalyst properties. J. Alloys Compd. 2019, 805, 363–378. [Google Scholar] [CrossRef]
- Oluwalana, A.E.; Ajibade, P.A. Tin sulfide nanoparticles as photocatalysts for the degradation of organic dyes. J. Sulfur Chem. 2021, 1–22. [Google Scholar] [CrossRef]
- Paca, A.M.; Ajibade, P.A. Synthesis and structural studies of iron sulphide nanocomposites from iron (III) dithiocarbamate single source precursors. Mater. Chem. Phys. 2017, 202, 143–150. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Botha, N.L. Synthesis and structural studies of copper sulfide nanocrystals. Res. Phys 2016, 6, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Ajibade, P.A.; Oluwalana, A.E.; Sikakane, B.M.; Singh, M. Structural, photocatalytic and anticancer studies of hexadecylamine capped ZnS nanoparticles. Chem. Phys. Lett. 2020, 755, 137813. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Yan, L. Synthesis and photocatalytic activity of three-dimensional ZnS/CdS composites. Mater. Res. Bull. 2013, 48, 3328–3334. [Google Scholar] [CrossRef]
- Entradas, T.; Cabrita, J.; Barrocas, B.; Nunes, M.; Silvestre, A.J.; Monteiro, O. Synthesis of titanate nanofibers co-sensitized with ZnS and Bi2S3 nanocrystallites and their application on pollutants removal. Mater. Res. Bull. 2015, 72, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.-Q.; Wang, X.; Chen, M.; Xiao, F.; Huang, Y.-M.; Lyu, X.-J. Synthesis of nanoscale zeolitic imidazolate framework-8 (ZIF-8) using reverse micro-emulsion for congo red adsorption. Sep. Purif. Technol. 2021, 260, 118062. [Google Scholar] [CrossRef]
- Sun, J.Q.; Shen, X.P.; Chen, K.M.; Liu, Q.; Liu, W. Low-temperature synthesis of hexagonal ZnS nanoparticles by a facile microwave-assisted single-source method. Solid State Commun. 2008, 147, 501–504. [Google Scholar] [CrossRef]
- Hosseinzadeh, G.; Maghari, A.; Farniya, S.M.F.; Keihan, A.H.; Moosavi-Movahedi, A.A. Interaction of insulin with colloidal ZnS quantum dots functionalized by various surface capping agents. Mater. Sci. Eng. C 2017, 77, 836–845. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; Gupta, A.; Pandey, O. Effect of Zr doping and aging on optical and photocatalytic properties of ZnS nanopowder. Ceram. Int. 2019, 45, 13671–13678. [Google Scholar] [CrossRef]
- Sabaghi, V.; Davar, F.; Fereshteh, Z. ZnS nanoparticles prepared via simple reflux and hydrothermal method: Optical and photocatalytic properties. Ceram. Int. 2018, 44, 7545–7556. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Bunnak, N.; Juntaro, J.; Sain, M.; Manuspiya, H. Synthesis and luminescence properties of ZnS and metal (Mn, Cu)-doped-ZnS ceramic powder. Solid State Sci. 2012, 14, 299–304. [Google Scholar] [CrossRef]
- Green, M. The nature of quantum dot capping ligands. J. Mater. Chem. 2010, 20, 5797–5809. [Google Scholar] [CrossRef]
- Abha, K.; Sumithra, I.; Suji, S.; Anjana, R.; Devi, J.A.; Nebu, J.; Lekha, G.; Aparna, R.; George, S. Dopamine-induced photoluminescence quenching of bovine serum albumin–capped manganese-doped zinc sulphide quantum dots. Anal. Bioanal. Chem. 2020, 412, 5671–5681. [Google Scholar] [CrossRef] [PubMed]
- Hanifehpour, Y.; Soltani, B.; Amani-Ghadim, A.R.; Hedayati, B.; Khomami, B.; Joo, S.W. Synthesis and character-ization of samarium-doped ZnS nanoparticles: A novel visible light responsive photocatalyst. Mater. Res. Bull. 2016, 76, 411–421. [Google Scholar] [CrossRef]
- Dumbrava, A.; Berger, D.; Prodan, G.; Matei, C.; Moscalu, F.; Diacon, A. The influence of triton X-100 surfactant on the morphology and properties of zinc sulfide nanoparticles for applications in azo dyes degradation. Mater. Chem. Phys. 2017, 193, 316–328. [Google Scholar] [CrossRef]
- Wang, C.; Ao, Y.; Wang, P.; Zhang, S.; Qian, J.; Hou, J. A simple method for large-scale preparation of ZnS nano-ribbon film and its photocatalytic activity for dye degradation. Appl. Surf. Sci. 2010, 256, 4125–4128. [Google Scholar] [CrossRef]
- Prasannalakshmi, P.; Shanmugam, N. Fabrication of TiO2/ZnS nanocomposites for solar energy mediated photocatalytic application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 175, 1–10. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Li, Z.; Lou, Z.; Wang, Z.; Dai, Y.; Whangbo, M.-H. Synthesis and characterization of ZnS with controlled amount of S vacancies for photocatalytic H2 production under visible light. Sci. Rep. 2015, 5, 8544. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oluwalana, A.E.; Andrew, F.P. Morphological studies, photocatalytic activity, and electrochemis-try of platinum disulfide nanoparticles from Bis(morpholinyl-4-carbodithioato)-platinum (II). ACS Omega 2020, 5, 27142–27153. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Kumar, T.M.P.K.; Kumar, S.K.A. Visible-light-induced degradation of rhodamine B by nanosized Ag2S–ZnS loaded on cellulose. Photochem. Photobiol. Sci. 2019, 18, 148–154. [Google Scholar] [CrossRef]
- Kaur, J.; Gupta, A.; Pandey, O. Photocatalytic study of ZnS–Ag2S nanocomposites-effect of thioglycerol. Sol. Energy 2018, 176, 678–687. [Google Scholar] [CrossRef]
- Jothibas, M.; Manoharan, C.; Jeyakumar, S.J.; Praveen, P.; Punithavathy, I.K.; Richard, J.P. Synthesis and enhanced photocatalytic property of Ni doped ZnS nanoparticles. Sol. Energy 2018, 159, 434–443. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Chinnadurai, A.; Bharathi, G.; Pazhanivel, T. Magnetically recoverable multifunctional ZnS/Ag/CoFe2O4 nanocomposite for sunlight driven photocatalytic dye degradation and bactericidal application. J. Phys. Chem. Solids 2020, 138, 109231. [Google Scholar] [CrossRef]
- Guo, M.; Song, M.; Li, S.; Yin, Z.; Song, X.; Bu, Y. Facile and economical synthesis of ZnS nanotubes and their superior adsorption performance for organic dyes. Cryst. Eng. Comm. 2017, 19, 2380–2393. [Google Scholar] [CrossRef]
- Sree, G.S.; Botsa, S.M.; Reddy, B.J.M.; Ranjitha, K.V.B. Enhanced UV–Visible triggered photocatalytic degradation of brilliant green by reduced graphene oxide based NiO and CuO ternary nanocomposite and their antimicrobial activity. Arab. J. Chem. 2020, 13, 5137–5150. [Google Scholar] [CrossRef]
- Mishra, S.; Sahu, T.K.; Verma, P.; Kumar, P.; Samanta, S.K. Microwave-assisted catalytic degradation of brilliant green by spinel zinc ferrite sheets. ACS Omega 2019, 4, 10411–10418. [Google Scholar] [CrossRef] [Green Version]
- Ajibade, P.A.; Oluwalana, A.E. Enhanced photocatalytic degradation of ternary dyes by copper sulfide nanoparticles. Nanomaterials 2021, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Tie, L.; Sun, R.; Jiang, H.; Liu, Y.; Xia, Y.; Li, Y.-Y.; Chen, H.; Yu, C.; Dong, S.; Sun, J. Facile fabrication of N-doped ZnS nanomaterials for efficient photocatalytic performance of organic pollutant removal and H2 production. J. Alloys Compd. 2019, 807, 151670. [Google Scholar] [CrossRef]
- Rao, C.V.; Giri, A.S.; Goud, V.V.; Golder, A.K. Studies on pH-dependent color variation and decomposition mechanism of brilliant green dye in Fenton reaction. Int. J. Ind. Chem. 2016, 7, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Le, A.T.; Samsuddin, N.S.B.; Chiam, S.-L.; Pung, S.-Y. Synergistic effect of pH solution and photocorrosion of ZnO particles on the photocatalytic degradation of rhodamine B. Bull. Mater. Sci. 2021, 44, 5. [Google Scholar] [CrossRef]
- Hariprasad, N.; Anju, S.; Yesodharan, E. Sunlight induced removal of rhodamine B from water through semi-conductor photocatalysis: Effects of adsorption, reaction conditions and additives. Res. J. Mater. Sci. 2013, 2320, 6055. [Google Scholar]
- Ajibade, P.A.; Andrew, F.P.; Botha, N.L.; Solomane, N. Synthesis, crystal structures and anticancer studies of morpholinyldithiocarbamato Cu(II) and Zn(II) complexes. Molecules 2020, 25, 3584. [Google Scholar] [CrossRef] [PubMed]
- Oluwalana, A.E.; Ajibade, P.A. Structural, optical and photocatalytic studies of hexadecylamine-capped lead sulfide nanoparticles. Int. J. Ind. Chem. 2020, 11, 249–260. [Google Scholar] [CrossRef]
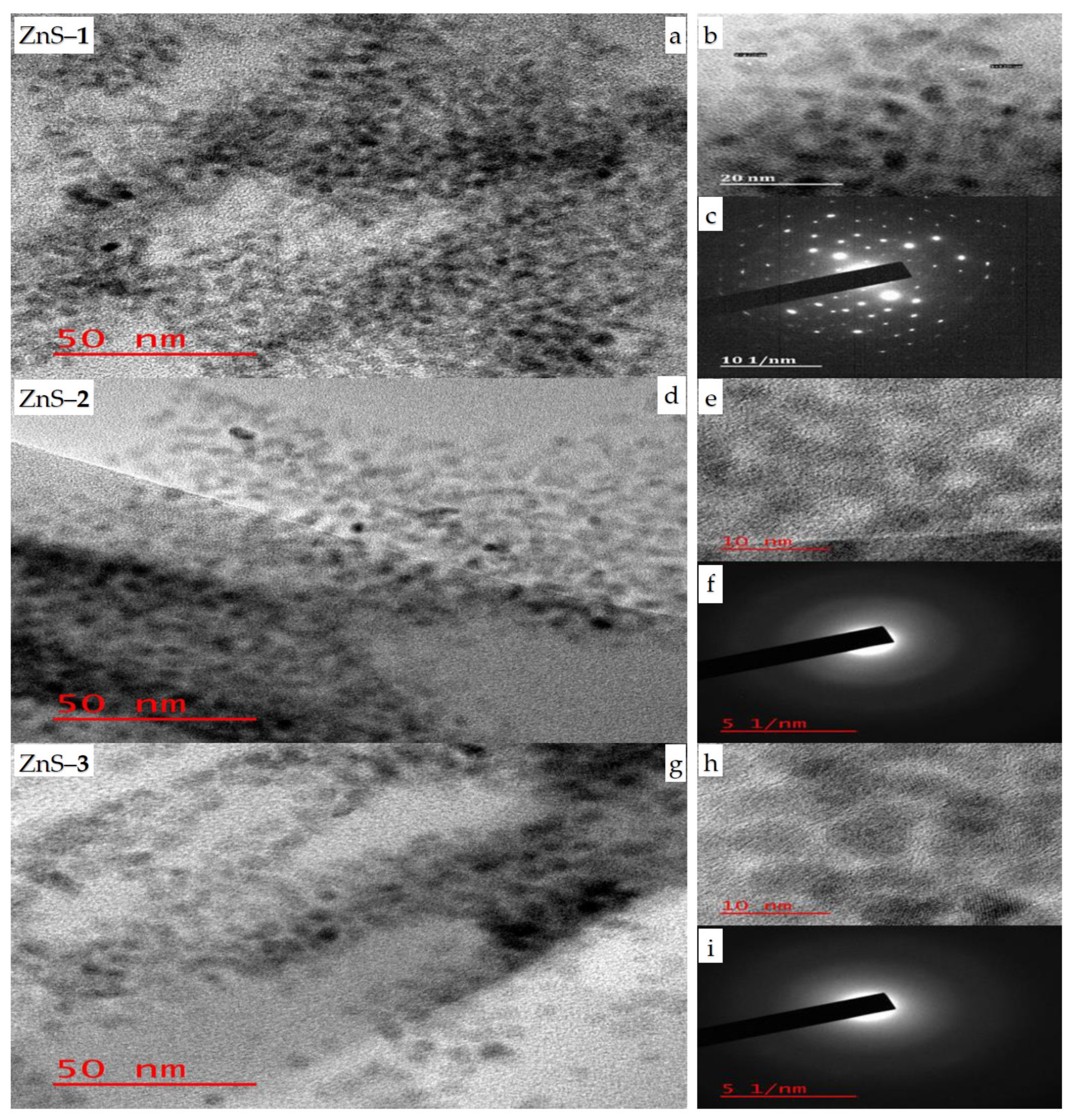
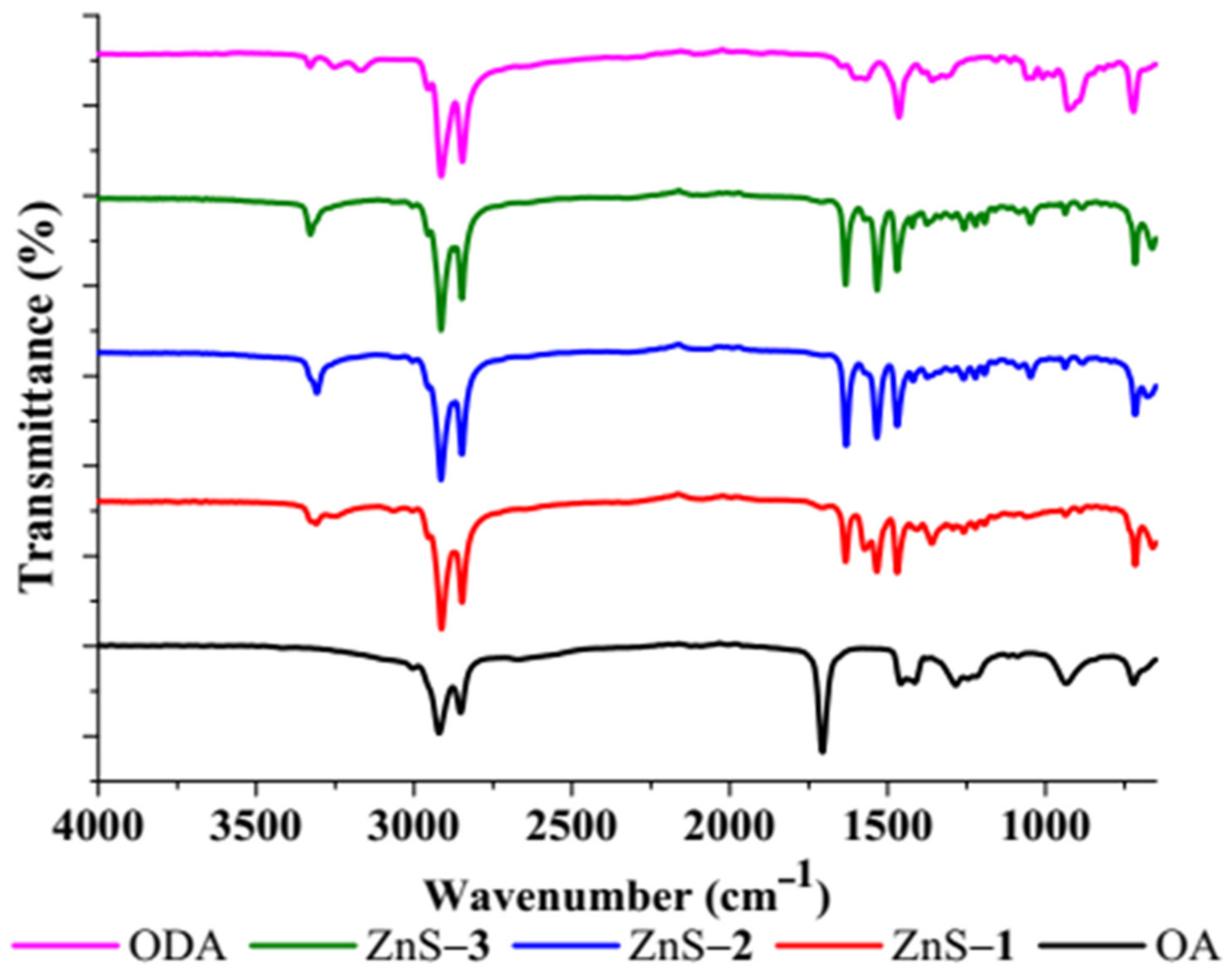
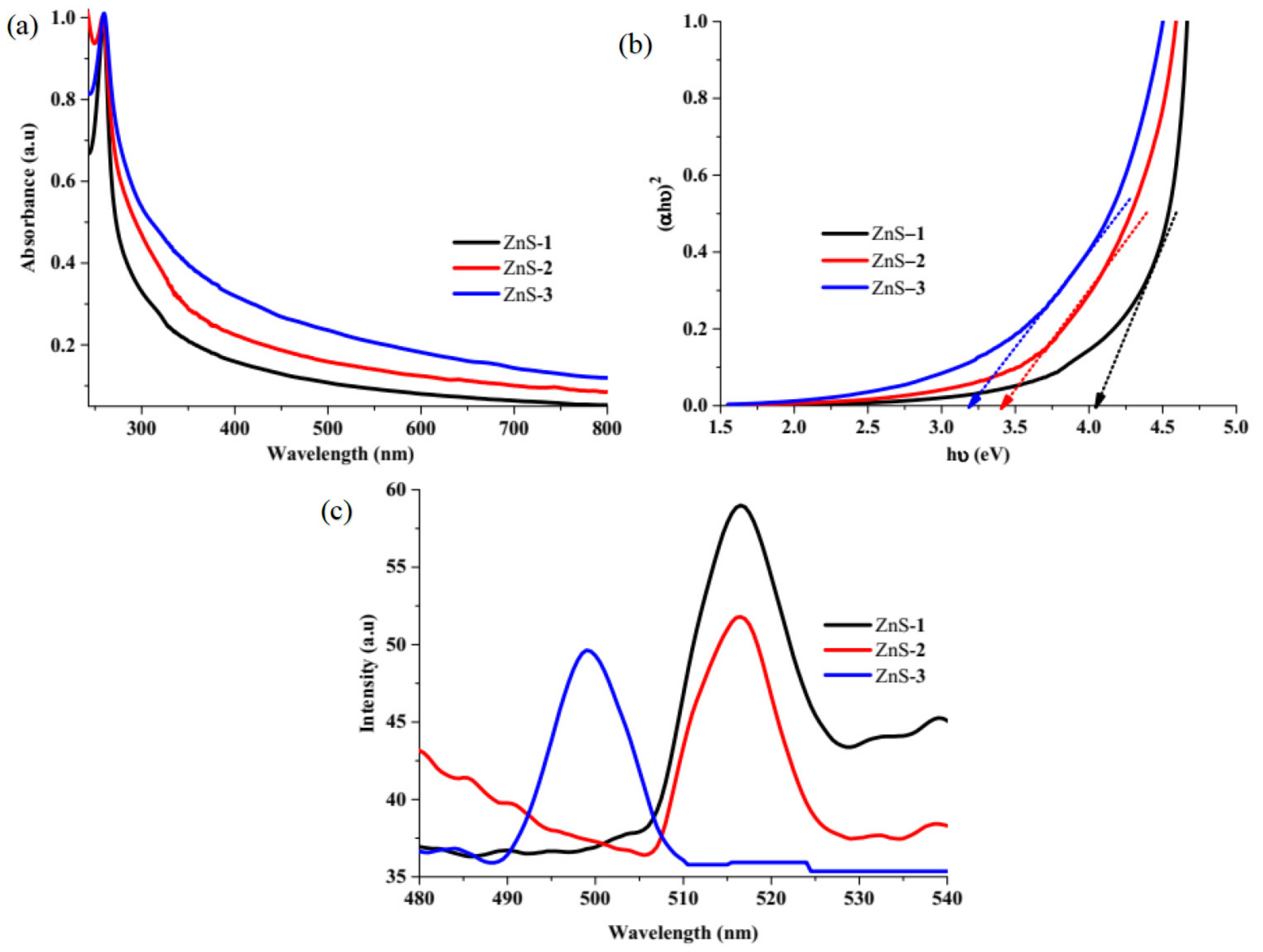
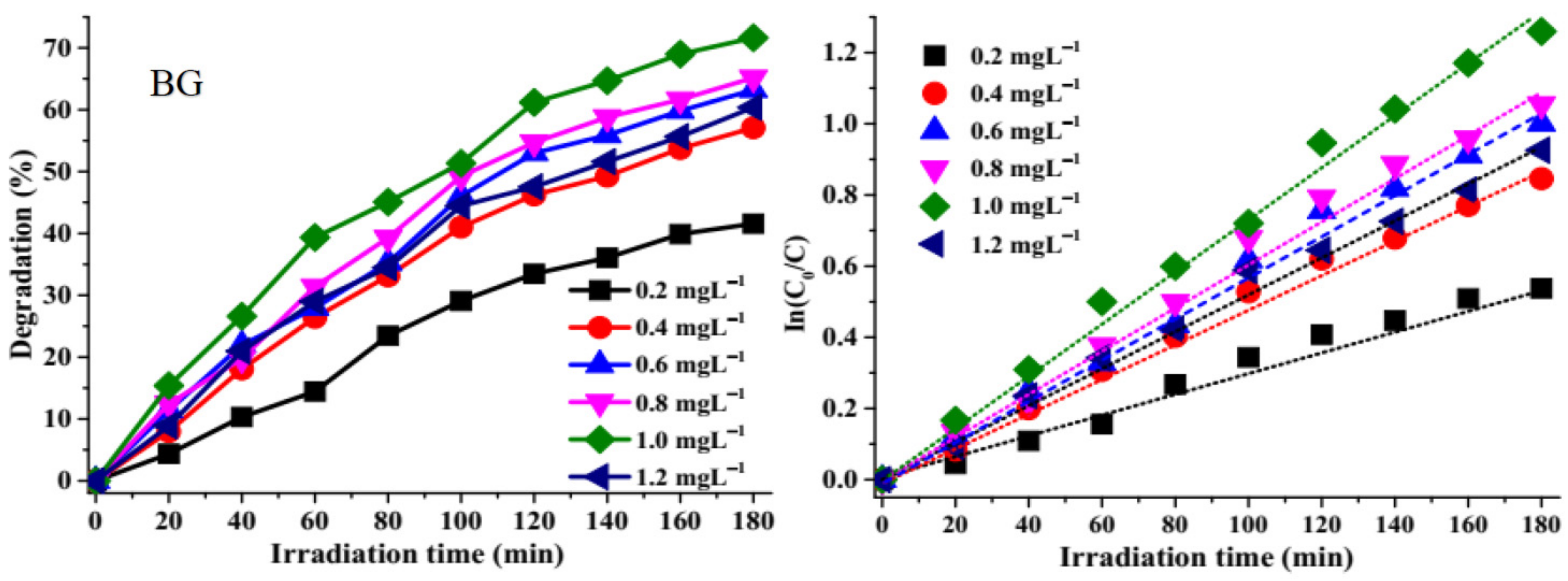
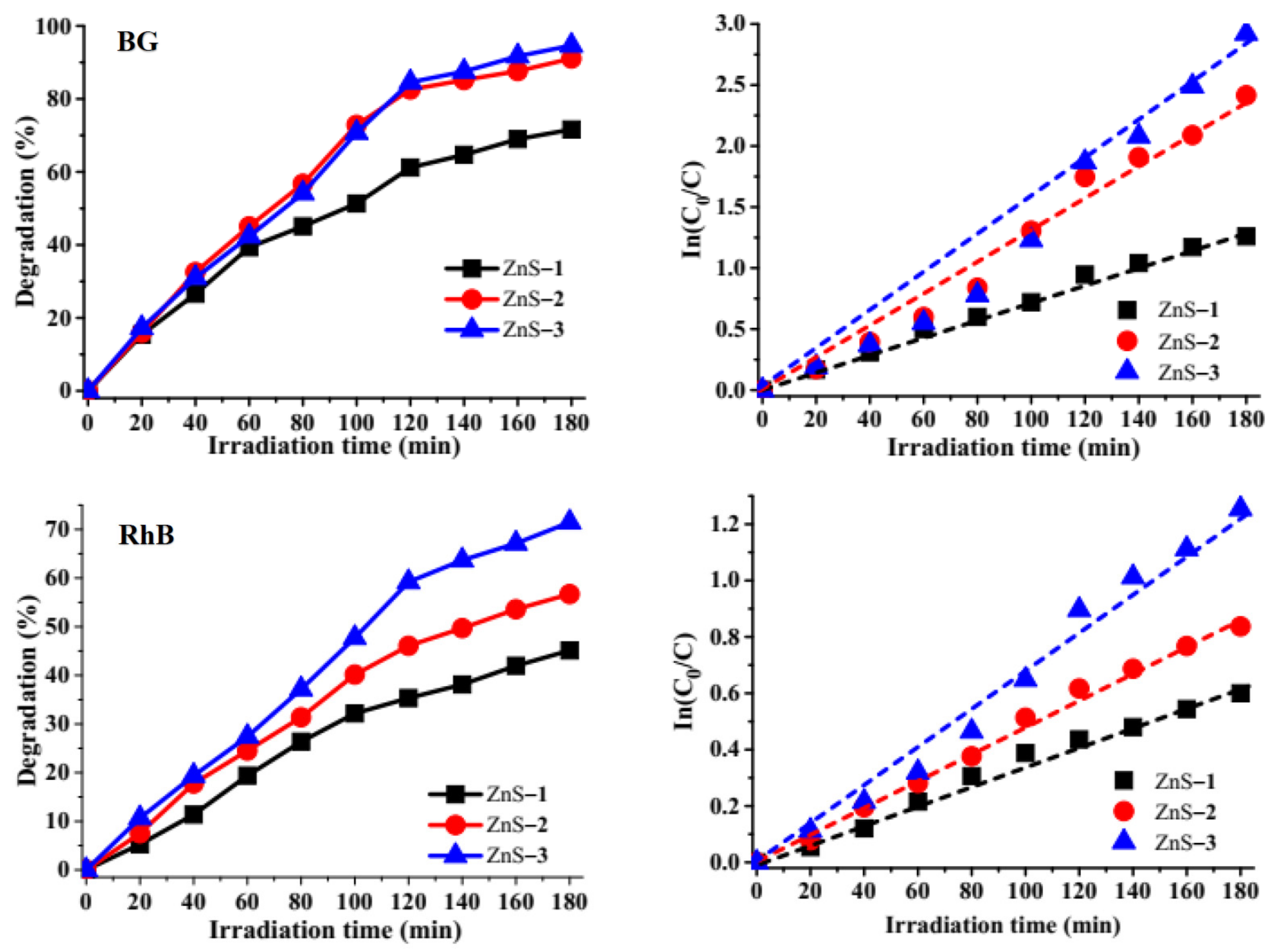
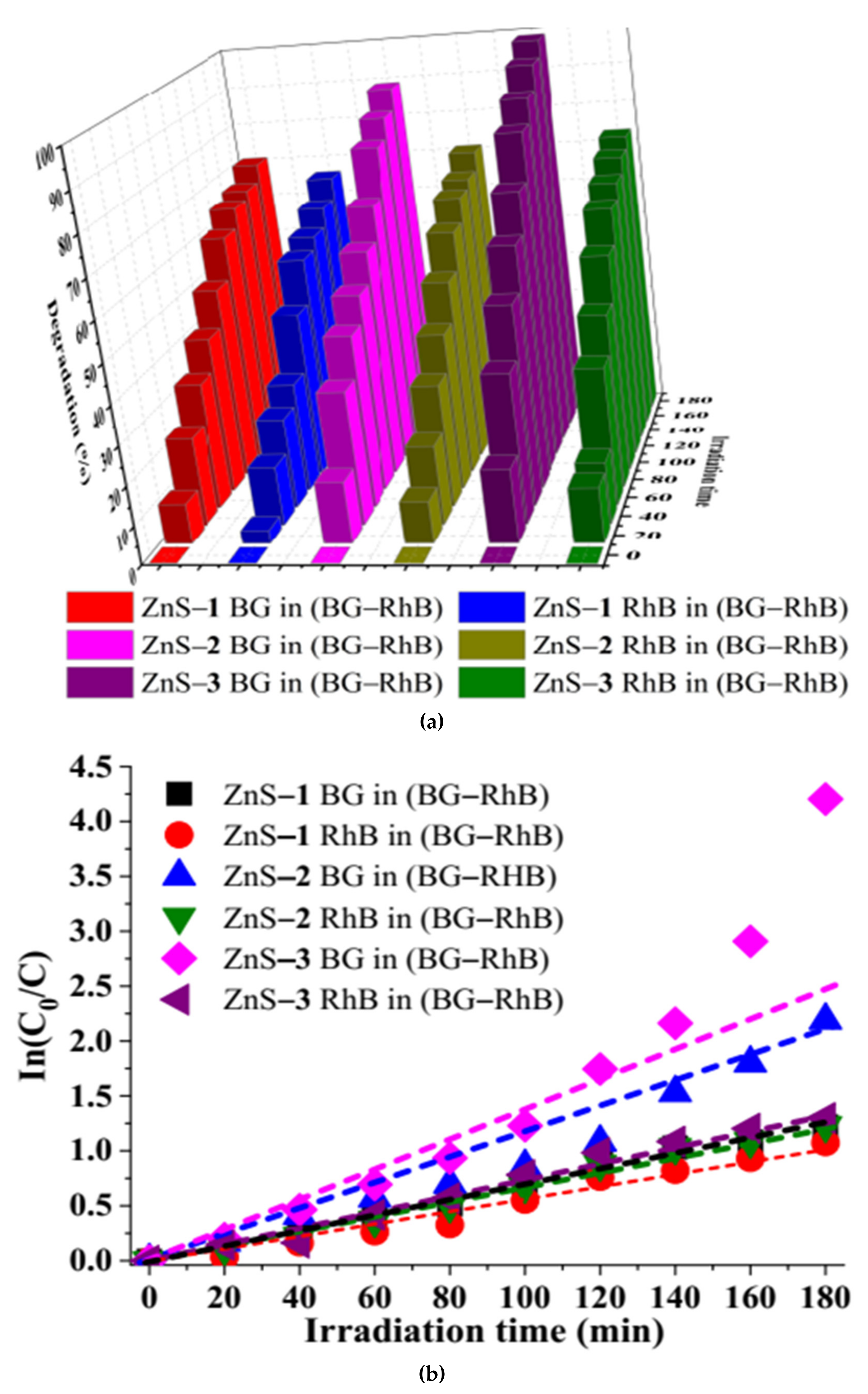
| Dyes | ZnS Photocatalyst | Degradation Efficiency (%) | Rate Constant k (min−1) | R2 |
|---|---|---|---|---|
| BG | ZnS–1 | 71.62 | 0.0071 | 0.994 |
| ZnS–2 | 91.06 | 0.0141 | 0.9854 | |
| ZnS–3 | 94.61 | 0.0245 | 0.9197 | |
| RhB | ZnS–1 | 45.12 | 0.0034 | 0.9894 |
| ZnS–2 | 56.69 | 0.0048 | 0.9948 | |
| ZnS–3 | 71.46 | 0.0073 | 0.9886 | |
| BG in (BG–RhB) | ZnS–1 | 70.79 | 0.0061 | 0.9921 |
| ZnS–2 | 88.81 | 0.0116 | 0.9659 | |
| ZnS–3 | 98.51 | 0.0208 | 0.9045 | |
| RhB in (BG–RhB) | ZnS–1 | 65.81 | 0.0063 | 0.9818 |
| ZnS–2 | 70.60 | 0.0072 | 0.9928 | |
| ZnS–3 | 72.71 | 0.0078 | 0.9855 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajibade, P.A.; Oluwalana, A.E. Photocatalytic Degradation of Single and Binary Mixture of Brilliant Green and Rhodamine B Dyes by Zinc Sulfide Quantum Dots. Molecules 2021, 26, 7686. https://doi.org/10.3390/molecules26247686
Ajibade PA, Oluwalana AE. Photocatalytic Degradation of Single and Binary Mixture of Brilliant Green and Rhodamine B Dyes by Zinc Sulfide Quantum Dots. Molecules. 2021; 26(24):7686. https://doi.org/10.3390/molecules26247686
Chicago/Turabian StyleAjibade, Peter A., and Abimbola E. Oluwalana. 2021. "Photocatalytic Degradation of Single and Binary Mixture of Brilliant Green and Rhodamine B Dyes by Zinc Sulfide Quantum Dots" Molecules 26, no. 24: 7686. https://doi.org/10.3390/molecules26247686
APA StyleAjibade, P. A., & Oluwalana, A. E. (2021). Photocatalytic Degradation of Single and Binary Mixture of Brilliant Green and Rhodamine B Dyes by Zinc Sulfide Quantum Dots. Molecules, 26(24), 7686. https://doi.org/10.3390/molecules26247686






